Summary
Aims
A successful cephalosomatic anastomosis (“head transplant”) requires, among others, the ability to control long‐term immune rejection and avoidance of ischemic events during the head transference phase. We developed a bicephalic model of head transplantation to study these aspects.
Methods and Results
The thoracic aorta and superior vena cava of a donor rat were anastomosed with the carotid artery and extracorporeal veins of a recipient rat by vascular grafts. Before thoracotomy in the donor rat, the axillary artery and vein of the donor were connected to the carotid and the extracranial vein of the third rat through a silicone tube. The silicone tube was passed through a peristaltic pump to ensure donor brain tissue blood supply. There is no ischemia reperfusion injury in donor brain tissue analyzed by electroencephalogram. Postoperative donor has pain reflex and corneal reflex.
Conclusions
Peristaltic pump application can guarantee the blood supply of donor brain tissue per unit time, while the application of temperature change device to the silicone tube can protect the brain tissue hypothermia, postoperative experimental data show that there is no brain tissue ischemia during the whole operation. The application of vascular grafting can also provide the possibility of long‐term survival of the model.
Keywords: head transplantation, immune rejection, ischemia reperfusion injury, rat model
1. Introduction
On May 21, 1908, Charles Guthrie in the USA succeeded in grafting one dog's head onto the side of another's neck, creating the world's first artificially bicephalic dog. However, the grafted head suffered irreversible ischemic damage. On February 24, 1954, Vladimir Demikhov in the Soviet Union succeeded in producing dogs with two viable heads, and in 1959, Zhao in China followed suit. Similar procedures were later reported by other groups. In 1970, Robert White, a US neurosurgeon based in Cleveland, beheaded a monkey and attached the head of another monkey, with full brain preservation over >7 days (historical review in 1). These experiments helped test neural preservation techniques, above all hypothermia, in the absence of blood flow to the brain. Their goal was not long‐term survival of such preparations: at the time, there was no effective anti‐immune rejection protocol.
Over the past few years, our group renewed the efforts toward the first human cephalosomatic anastomosis (CSA; “head transplant”).2, 3, 4 A CSA calls for the availability of appropriate technologies to reconnect the spinal cord, to preserve neural function during the critical stage of head detachment and reattachment onto the new body in the absence of blood supply, and to keep in check immune rejection. Spinal cord reconnection has been recently demonstrated,5 and neural preservation had already been achieved by past pioneers. However, the problem of immune rejection has not yet been studied sufficiently in this context: immune rejection would be fatal to the patient, if not properly addressed. Prof White specifically noticed “no evidence of cellular changes compatible with a hyper‐rejection reaction in cerebral tissue,” which bodes well for the surgery, but the possibility of long‐term rejection remains a critical issue.6 We thus developed several animal CSA models, including a full head transplant in mice, with the goal of testing both neuroprotective techniques (e.g., hypothermia) and antirejection immunological protocols.7, 8 Here, we improve on the bicephalic preparations employed by past pioneers (see above). This article describes the technique employed, and we discuss its implications.
2. Materials and Methods
The experimental protocol was approved by The Animal Care and Use Committee of The Second Affiliated Hospital of Harbin Medical University, Harbin, PRC.
2.1. Animals
Sixty male Wistar rats with different weights were studied (500 g: N=15; 300 g: N=30; 100 g. N=15; 50 g: N=15) (study rats, donor rats, recipient rats: Figure 1B). The study rat's and donor rat's weight ratio of 1/6 had the goal of avoiding circulatory overload (cardiac output of adult rats: ca 180 mL min−1 kg−1, the blood supply of brain tissue being about 25% of cardiac output). All animals were obtained from the animal center of Harbin Medical University. Rats were injected with 3% sodium pentobarbital intraperitoneally (50 mg/kg); barbiturate‐induced respiratory depression was counteracted by atropine sulfate (0.1 mg IM). Anticoagulation was maintained by subcutaneous injection of heparin sodium (100U/100 g). Hypotensive episodes were reversed with norepinephrine injections (5 mg/kg).
Figure 1.
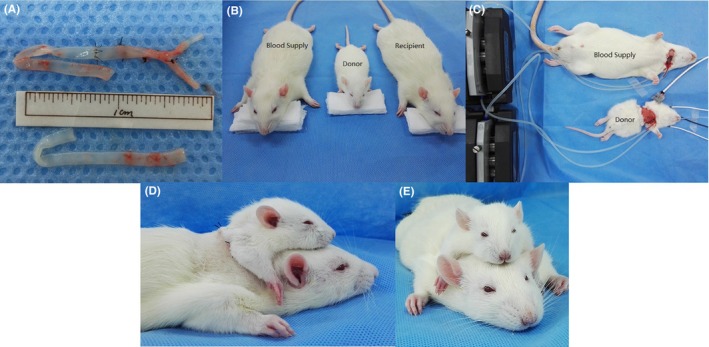
(A) Transplantation blood vessels; (B) preoperative rats; (C) donor and blood supply rat; (D, E) after transplantation
2.2. Equipment
EEG, EKG, and blood oxygen saturation were monitored with a MP150 multiconductive physiological recorder (Biopac, Goleta, CA, USA). A small animal ventilator was employed (ACLV8D,Gene&I, PRC) and set to provide a respiratory rate of 55/min, a tidal volume of 3 mL/100 g, and a respiration ratio of 1.5. A BT100‐2J peristaltic pump (Longer, BaoDing, China) maintained the blood supply of the study and donor rat. Intraoperative euthermia was achieved with an ALCHTP animal constant temperature system (ACLBIO, PRC). Mild hypothermia (31±2°C) was kept via a HHS4 constant temperature water bath (FuWei, Harbin, China). All surgery was conducted under magnification (Operating microscope SXP‐1C, Shanghai Medical Optical Instruments CO, Shanghai, PRC) with microneurosurgical instruments purchased with other surgical instrumentation from the Shanghai Instrument Company (Shanghai, PRC). Intraoperative hemostasis was achieved with bipolar coagulation (GN160, Aesculap, Melsungen, Germany).
2.3. Preoperative preparation (100 g and 500 g Rats)
Both carotid arteries and common iliac arteries were removed from 500 g and 100 g rats before the actual surgery, followed by ligation of the branches of the internal and external iliac arteries and subsequent microsurgical anastomosis (Figure 1A). The blood vessel diameter was roughly equal for both donors and recipients. This ensemble was bathed in a heparin/saline solution. Iliac arteries would guarantee recycling of the donor's blood from the superior venae cavae and spare further damage to the recipient.
2.4. Preparation of the blood supply of the study rat (300 g)
After successful induction of anesthesia in the 300 g rats, a transverse incision of the neck was carried out, followed by isolation of the soft tissues and muscles and full exposure of the carotid artery, external jugular vein, and trachea. At this point, tracheal intubation was performed and the animal connected to the ventilator. The carotid artery and external jugular vein were, respectively, ligated at the distal end; silicone tubes were inserted into the proximal ends and connected to the donor rat's axillary artery and vein (Figure 1C). These tubes went through the peristaltic pump and the constant temperature water bath, where mild hypothermia (31±2°C) was induced for brain protection.
2.5. Preparation of the donor rat (50 g)
50‐g rats were anesthetized, and their trachea intubated and connected to the ventilator. An annular incision was performed in the middle of the sternum, followed by isolation of the pectoralis major and minor, which fully exposed the axillary artery and vein. As described above, the silicone tubing from the study rat was inserted into the donor rat's axillary artery, while the tube from the study rat's jugular vein was inserted through the axillary vein into the superior vena cava; the wall of the silicone tube was perforated, similar to deep venous catheters. Subsequently, both sternum and ribs were split up, while protecting the heart and lungs; after separating the thymus, the superior vena cava and thoracic aorta were fully exposed. The peristaltic pump was opened; the thoracic aorta and the superior vena cava were ligated and separated. The heart and lung were removed, dividing the esophagus, trachea, and spine; hemostasis was achieved whenever appropriate. The previously prepared blood vessels were anastomosed, the internal iliac artery and external iliac artery to the superior vena cava and the carotid artery to the thoracic aorta.
2.6. Preparation of the recipient rat (300 g)
300‐g rats were anesthetized, and their trachea intubated and connected to the ventilator. A transverse incision of the neck was carried out, followed by isolation of the soft tissues and muscles, with full exposure and distal ligation of the carotid artery and external jugular vein; a vascular clip was applied to the proximal end, and the vessels were cut. The back of the neck was longitudinally incised and soft tissues and muscles separated down to the plane between the posterior and anterior neck, to allow insertion of the transplanted blood vessels.
2.7. Transplantation
The donor rat was transferred to the posterior neck of the recipient rat. The two previously stitched vessels were transferred along the channel to the anterior neck. Donor rats were firmly sutured to the back of the recipient rats. Finally, two graft vessels were anastomosed with the two vessels of the recipient. Until establishment of full cross‐circulation between donor and recipient, their respective circulations were not interrupted. Thereafter, the silicone tubes were pulled out and the donor's axillary artery and vein ligated (Figures 1D and 1E).
2.8. Monitoring
Continuous monitoring of the donor's brain EEG was carried out for signs of ischemia pre‐, intra‐, and postoperatively (and waves). EEG can detect signs of impending ischemia at a very early stage. EKG monitoring was enacted to look for signs of circulatory overload or arrhythmias due to the anomalous circulatory load on the heart of the recipient. Blood oxygen saturation of the donor rat was also monitored with a clip on the rat's finger or ear.
3. Results
Fourteen groups (triads) survived for an average of >36 hours; one triad was accidentally lost during anesthesia. Postoperatively, there was no sign of peripheral ischemia or other vascular anomaly, or tissue necrosis in the transplanted donor rat (Figures 1D and 1E). Blood oxygen saturation remained normal, signaling (Figure 2). Postoperatively, rats exhibited pain and corneal reflexes. No circulatory overload or other anomalies were seen on EKG in both donor and recipient; baroreceptor stability was not compromised (Figures 3 and 4). No sign of ischemia was ever detected on EEG at any time (Figure 5). All rats were euthanized after about 36 hours.
Figure 2.
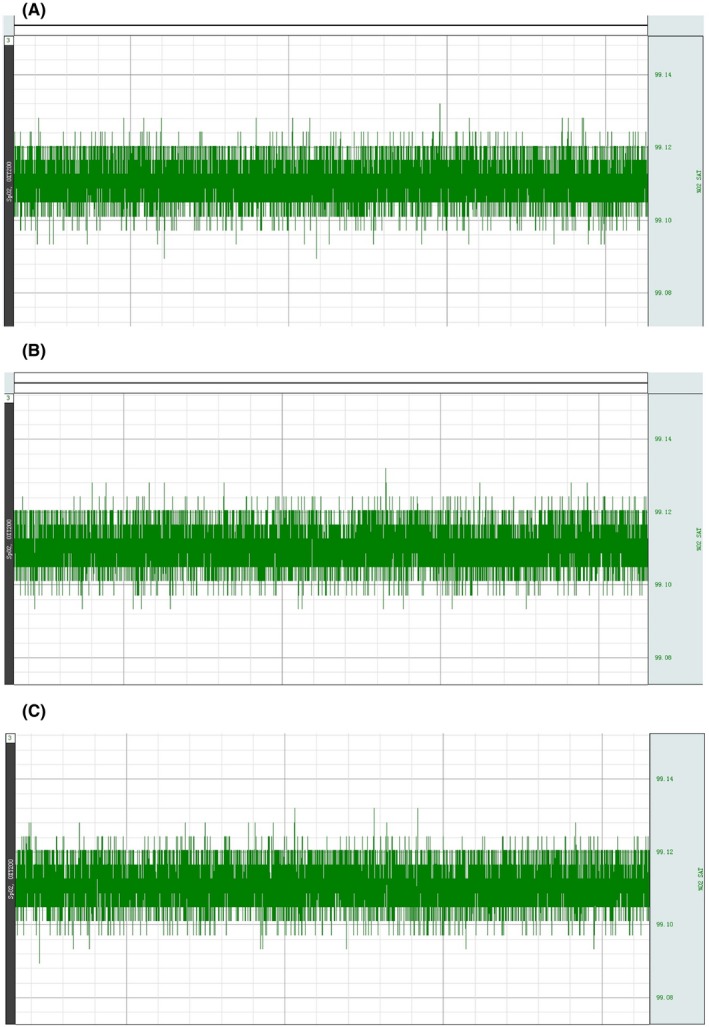
Donor blood's oxygen levels (preoperative, intraoperative, and postoperative)
Figure 3.
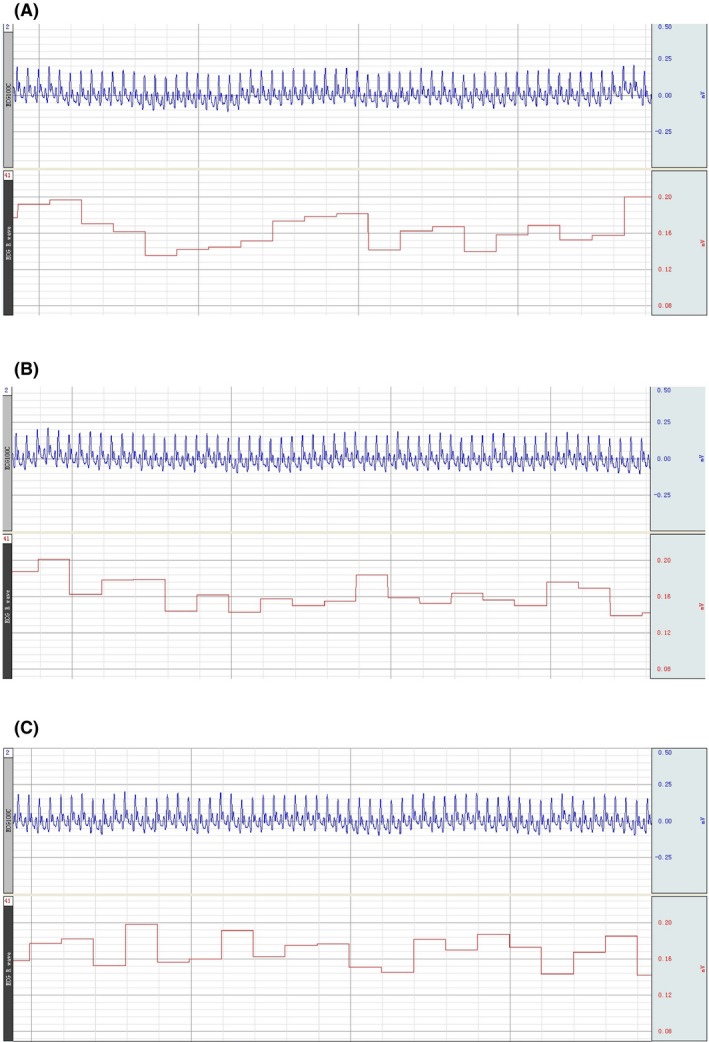
Blood supply rat preoperative ECG; the circulation was established for 2 h; the circulation was established for 4 h
Figure 4.
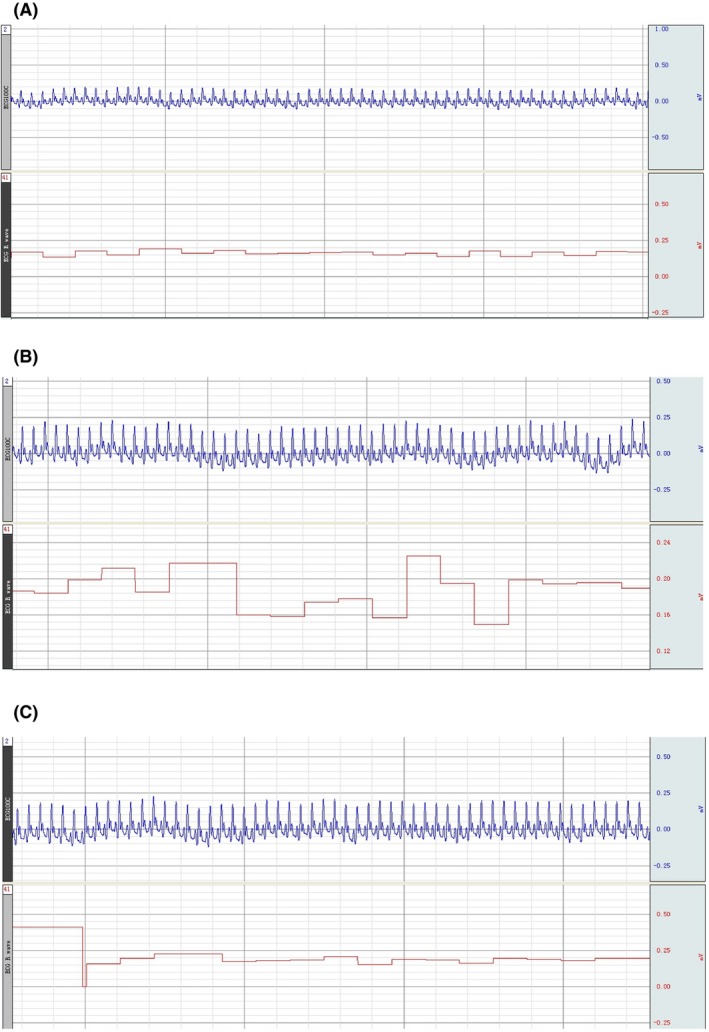
Recipient rat preoperative ECG; postoperative 2 h; postoperative 4 h
Figure 5.
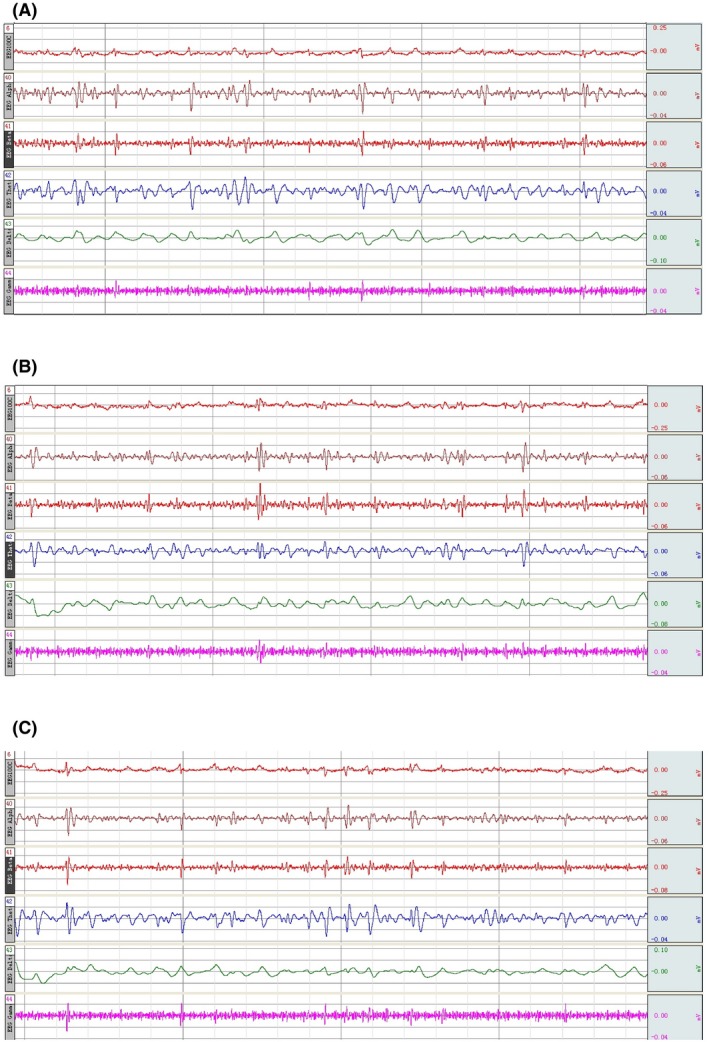
The donor EEG in preoperative, intraoperative, and postoperative
4. Discussion
We designed a bicephalic model of head transplantation in a rat model, in which the donor's mid‐sternum is split open and the thoracic aorta and superior vena anastomosed with the recipient. A peristaltic pump is used throughout the surgery to ensure adequate blood supply to the donor's brain until the donor is connected to the recipient. The whole operation process preserves the carotid arteries, jugular veins, and vertebral arteries. As such, this might represent an alternative to head detachment at neck level. No sign of ischemia or cardiovascular overload was observed in this initial study. Actually this model is an extended version of the Demikhov's model, in that the section is carried out in the chest rather than in the neck. The arteriovenous ratio of 2:1 increased the safety factor. At the same time, compared with the mouse head transplantation model we previous designed, vascular grafts have a lower risk of vascular accidents than silicone tubes.7, 8, 9 We have also improved the cross‐circulation setup by adding a peristaltic pump between the silicone tubes to quantify the blood flow per unit time and adjust the blood flow appropriately according to the peripheral circulation. These improvements extend the survival time of the model and further reduce the ischemia of donor brain.
In our experiment, the donor head was first connected to a rat's blood supply, by peristaltic pumps, and only thereafter separated donor head from the donor body. In this state, the donor head was maintained for up to 6 hours, with no obvious abnormalities in the donor's EEG or corneal reflexes. Following nociceptive stimuli, the donor head exhibited substantial movement.
We speculate that the method used in this experiment can provide a new idea for the short‐term preservation of central nervous system transplantation.
In sum, this model will facilitate current efforts toward the first human head transplantation.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (81470425), the funds for Wu Liande Foundation of HMU (Wld‐qn1414) and the funds for Harbin Science and Technology Bureau (2014RFXYJ023).
Li P‐W, Zhao X, Zhao Y‐L, et al. A cross‐circulated bicephalic model of head transplantation. CNS Neurosci Ther. 2017;23:535–541. 10.1111/cns.12700
The first two authors contributed equally to this work.
References
- 1. Ren X. The age of head transplants. CNS Neurosci Ther. 2016;22:257‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ren X, Canavero S. Editorial. Human head transplantation. Where do we stand and a call to arms. Surg Neurol Int. 2016;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ren X, Laugel MC. The next frontier in composite tissue allotransplantation. CNS Neurosci Ther. 2013;19:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canavero S. HEAVEN: the head anastomosis venture: project outline for the first human head transplantation with spinal linkage (GEMINI). Surgical Neurology Int. 2013;S335‐S342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canavero S, Ren X. Houston, GEMINI has landed: spinal cord fusion achieved. Surg Neurol Int. 2016;7:S626‐S628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarr MG, Behrns KE. The Science of head transplantation and spinal cord reconstruction. Surgery. 2016;160:3‐4.27117945 [Google Scholar]
- 7. Ren X, Song Y, Ye YJ. Allogeneic head and body reconstruction: mouse model. CNS Neurosci Ther. 2014;20:1056‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ren XP, Ye YJ, Li PW, Shen ZL, Han KC, Song Y. Head transplantation in mouse model. CNS Neurosci Ther. 2015;21:615‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ren X, Orlova EV, Maevsky EI, Bonicalzi V, Canavero S. Brain protection during cephalosomatic anastomosis. Surgery. 2016;160:5‐10. [DOI] [PubMed] [Google Scholar]


