Summary
Aims
Patients with liver disease may develop hepatic encephalopathy (HE), with cognitive impairment and motor in‐coordination. Rats with HE due to portacaval shunts (PCS) show motor in‐coordination. We hypothesized that in PCS rats: (i) Motor in‐coordination would be due to enhanced GABAergic tone in cerebellum; (ii) increased GABAergic tone would be due to neuroinflammation; (iii) increasing cGMP would reduce neuroinflammation and GABAergic tone and restore motor coordination. To assess these hypotheses, we assessed if (i) treatment with sildenafil reduces neuroinflammation; (ii) reduced neuroinflammation is associated with reduced GABAergic tone and restored motor coordination.
Methods
Rats were treated with sildenafil to increase cGMP. Microglia and astrocytes activation were analyzed by immunohistochemistry, extracellular GABA by microdialysis, and motor coordination in the beam walking.
Results
PCS rats show neuroinflammation in cerebellum, with microglia and astrocytes activation, increased IL‐1b and TNF‐a and reduced YM‐1 and IL‐4. Membrane expression of the GABA transporter GAT1 is reduced, while GAT3 is increased. Extracellular GABA and motor in‐coordination are increased. Sildenafil treatment eliminates neuroinflammation, microglia and astrocytes activation; changes in membrane expression of GABA transporters; and restores motor coordination.
Conclusions
This study supports an interplay between cGMP‐neuroinflammation and GABAergic neurotransmission in impairing motor coordination in PCS rats.
Keywords: cGMP, neuroinflammation; GABAergic neurotransmission; GAT3; hepatic encephalopathy; sildenafil
1. Introduction
Patients with liver cirrhosis may develop hepatic encephalopathy (HE), a neuropsychiatric syndrome, which leads to a wide spectrum of neurological alterations. The earliest form of HE is minimal HE (MHE). Patients with MHE show mild cognitive impairment, psychomotor slowing and motor in‐coordination, which reduce quality of life and life span and increase the risk of falls and hospitalizations. MHE affects several million people and is a serious health, social and economic problem.1 The mechanisms responsible for MHE are beginning to be clarified in animal models. A main animal model of MHE is the rat with portacaval shunt (PCS), which reproduces many cognitive and motor alterations present in patients with MHE.2 PCS rats show neuroinflammation in the cerebral cortex, with increased levels of inflammatory markers, and treatment with ibuprofen reduces neuroinflammation in cortex and restores learning in a Y maze task3 and motor activity.4 PCS rats also show neuroinflammation in hippocampus, associated with altered membrane expression of glutamate and GABA receptors and impaired spatial learning and memory.5 Treating PCS rats with sildenafil, an inhibitor of phosphodiesterase 5 that degrades cGMP, reduces neuroinflammation in hippocampus, normalizes receptors membrane expression, and restores spatial learning5 and learning in the Y maze.6
Sildenafil also reduces neuroinflammation in hippocampus and improves cognitive performance in APP/PS1 transgenic mice model of Alzheimer's disease7 and reduces neuroinflammation and IL‐1β levels in cerebellum in an inflammatory model of demyelination in mice.8 It has not been assessed whether PCS rats show neuroinflammation in cerebellum or whether sildenafil would reduce neuroinflammation in cerebellum and/or improve motor in‐coordination in rats with MHE.
We propose that there is an interplay between neuroinflammation, cGMP, and GABAergic/glutamatergic neurotransmission in the induction of cognitive and motor alterations in MHE (Figure 1).
Figure 1.
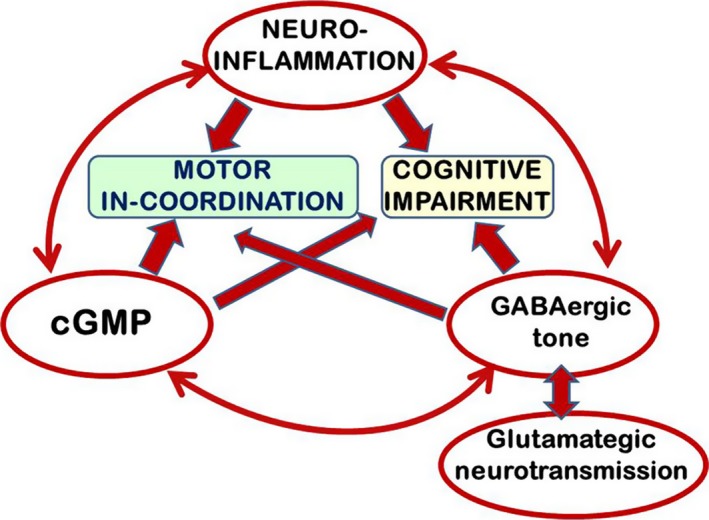
Proposed model for an interplay between neuroinflammation‐cGMP and GABAergic/glutamatergic neurotransmission in the impairment of motor coordination and cognitive function in rats with MHE
PCS rats show impaired motor coordination, which is modulated mainly by GABAergic neurotransmission in cerebellum. Enhanced GABAergic tone impairs motor coordination.9, 10 We hypothesized that in PCS rats: (i) Motor in‐coordination would be due to enhanced GABAergic tone; (ii) increased GABAergic tone would be due to neuroinflammation in cerebellum; (iii) increasing cGMP levels would reduce neuroinflammation and GABAergic tone and restore motor coordination.
To assess these hypotheses, we put the questions if: (i) PCS rats show neuroinflammation in cerebellum; (ii) treatment with sildenafil reduces this neuroinflammation; (iii) reduction in neuroinflammation is associated with reduced GABAergic tone and restoration of motor coordination. We used control and PCS rats, treated or not chronically with sildenafil to enhance cGMP levels. To analyze neuroinflammation, we measured astrocyte and microglia activation, the pro‐inflammatory markers IL‐1b and TNF‐a and the antiinflammatory markers YM‐1 and IL‐4. To evaluate GABAergic tone, we measured extracellular GABA by in vivo microdialysis and membrane expression of GABA transporters and receptors. We also measured motor coordination in the beam walking.
2. Methods
2.1. Portacaval anastomosis
Male Wistar rats (220‐240 g) were subjected to portacaval anastomosis as described by Lee and Fisher.11 The experiments were approved by the Ethics Committee for Animal Experimentation of our Center and performed according to the guidelines of the European Commission (20/63/EU) for care and management of experimental animals.
2.2. Treatment with sildenafil
Rats were distributed in four groups: two groups of Sham, with (SM+SIL) or without (SM) sildenafil and two groups of PCS, with (PCS+SIL) or without (PCS) sildenafil. This drug was administered (50 mg/L) in the drinking water, ad libitum, as in,6 beginning 2 weeks after PCS surgery.
2.3. Motor coordination in the beam walking
Motor coordination was assessed using the beam walking test. The number of foot faults (slips) is recorded as a measure of in‐coordination as described in.12 A fault was defined as any foot slip off the top surface of the beam or any limb use on the side of the beam.
2.4. Ammonia determination in blood
Blood (20 μL) was taken from the tail vein. Blood ammonia was measured immediately after blood collection with the Ammonia Test Kit II for the PocketChemBA system (Arkay, Inc., Kyoto, Japan).
2.5. In vivo microdialysis
Rats were anesthetized using isoflurane, and a microdialysis guide was implanted in cerebellum as in.13 After 48 hours, a microdialysis probe was implanted in the freely moving rat. Probes were perfused (3 μL/min) with artificial cerebrospinal fluid (in mmol/L): NaCl, 145; KCl, 3.0; CaCl2, 2.26; buffered at pH 7.4 with 2 mmol/L phosphate. After a 2‐ to 3‐hour stabilization period, samples were collected every 30 minutes. Samples were stored at −80°C until analysis of cGMP or GABA levels.
2.6. Determination of cGMP
cGMP was measured using the BIOTRAK cGMP enzyme immunoassay kit from Amersham (Amersham, Buckinghamshire, UK) using 50 μL of dialysate.
2.7. GABA determination
GABA concentration was measured by HPLC as described in.14
Membrane surface expression of GAT‐1 and GAT‐3 transporters by cross‐linking with BS3 in cerebellar slices was analyzed as described by Boudreau and Wolf.15 Cerebellum was dissected, and transversal slices (400 μm) were obtained and added to tubes containing ice‐cold Krebs buffer with or without 2 mmol/L bis[sulfosuccinimidyl] suberate (BS3) (Pierce, Rockford, IL, USA) and incubated for 30 minutes at 4°C. Cross‐linking was terminated by adding 100 mmol/L glycine (10 minutes, 4°C). The slices were homogenized by sonication for 20 seconds. Samples treated with or without BS3 were analyzed by Western blot using anti‐GAT‐3 or anti‐GAT‐1 (1:1000; Abcam, Cambridge, UK). The surface expression of transporters was calculated as the difference between the intensity of the bands without BS3 (total protein) and with BS3 (nonmembrane protein).
2.8. Brain immunohistochemistry
At week 4 after PCS surgery, the rats were anaesthetized with sodium pentobarbital and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4). Brains were removed and postfixed in the same fixative solution for 24 hours at 4°C. Then, the samples were placed inside histology cassettes and processed for permanent paraffin embedding on a Leica ASP 300 tissue processor (Leica Microsystems , Nussloch, Germany). Five‐micrometer thick, paraffin‐embedded sections (5 μm) were cut and mounted on coated slide glass. The tissue sections were then processed with the Envision Flex+kit (DAKO, Santa Clara, CA, USA) blocking endogenous peroxidase activity for 5 minutes and then incubating with primary antibody. The reaction was visualized by Envision Flex+horseradish peroxidase for 20 minutes and finally diaminobenzidine for 10 minutes. Sections were counterstained with Mayer's hematoxylin (DAKO S3309; Ready to use) for 5 minutes.
The primary antibodies used were as follows: anti‐Iba1 (Wako, 019‐19741; 1:300 for 30 minutes), anti‐GFAP (DAKO, IR524; ready to use for 20 minutes; 1:2000 for 45 minutes), anti‐IL‐1β (Abcam, ab9722, 1:100 for 30 minutes).
Immunohistochemical quantification was performed using Image (National Institutes of Health, Bethesda, MD, USA) (1.48v). For analysis of microglial activation and of the areas stained by GFAP antibody, the area of interest was selected. Using Auto Local Threshold and analyze particles functions, the intensity thresholds and size filter were applied.
To measure area and perimeter of microglia, the Bernsen method was used and 2000‐20000 size filter was applied. For each rat, at least 30‐40 cells were quantified. The result was expressed as percentage respect to control.
For GFAP staining, no size filter was applied. For each rat, at least 10 fields (56×) were quantified. The result was expressed as percentage of control rats.
IL‐1b‐positive cells were manually counted using ImageJ. For each rat, at least 10 fields (40×) were quantified and results were expressed as percentage of control rats.
2.9. Analysis of protein contents in cerebellum by western blot
Rats were sacrificed by decapitation. Cerebellum was dissected, homogenized, and were subjected to electrophoresis and immunoblotting as in.16 Primary antibodies were against IL‐4 (1:2000) and YM‐1 (1:500) from Abcam, IL‐1β and TNF‐α (1:500) from R&D SYSTEMS, Minneapolis, MN, USA; secondary antibodies were anti‐rabbit or anti‐goat IgG (1:2000 or 1:4000)‐conjugated with alkaline phosphatase (Sigma, St. Louis, MO, USA). The images were captured using the ScanJet 5300C (Las Rozas, Madrid, Spain) and band intensities quantified.
2.10. Statistical analysis
Results are expressed as mean±SEM. Data were analyzed by one‐way analysis of variance (ANOVA) followed by Tukey's and Dunnet Post hoc tests. P<.05 is considered to indicate statistically significant differences.
3. Results and Discussion
PCS rats show reduced (P<.05) levels of extracellular cGMP in cerebellum (250±41 pmol/L) compared to control rats (441±48 pmol/L). Sildenafil treatment increased the levels of extracellular cGMP in PCS rats to 401±58 pmol/L, which was not different from control rats.
Ammonia levels were 30±6 μmol/L in control rats and were increased (P<.001) in PCS rats to 194±18 μmol/L. Treatment with sildenafil did not affect ammonia levels, which remained at 28±3 μmol/L and 200±16 μmol/L in control and PCS rats, respectively.
PCS rats show motor in‐coordination in the beam walking. They performed more slips (2.0±0.3, P<.05) than control rats (1.0±0.2). Treatment with sildenafil restores motor coordination in PCS rats, which performed 1.4±0.4, not different from control rats (Figure 2).
Figure 2.
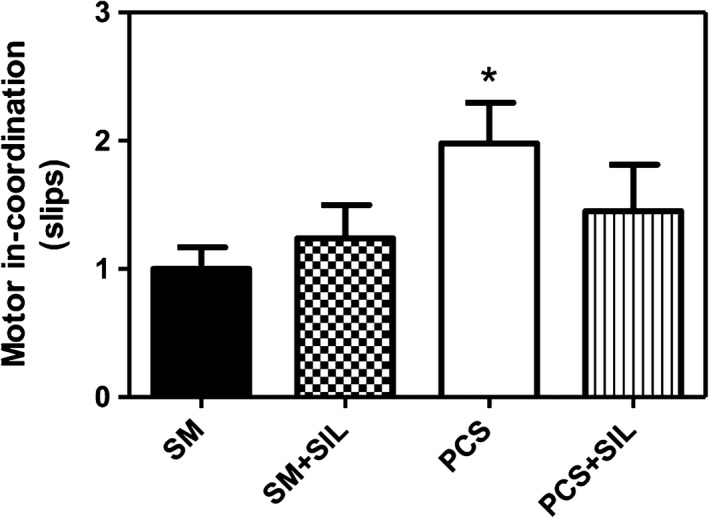
Sildenafil restores motor coordination in PCS rats. Control (SM) and PCS rats treated with tap water or sildenafil (SIL) were subjected to the beam walking test. The number of slips is shown as a measure of in‐coordination. Values are the mean±SEM of 16 sham and PCS rats and 14 sham and PCS rats treated with sildenafil. Values significantly different from controls are indicated by asterisks. *P<.05
PCS rats show increased concentration of extracellular GABA in cerebellum, reaching 18±3 nmol/L (P<.05) compared to 11±1 nmol/L in control rats. Treatment with sildenafil reduced extracellular GABA in PCS rats to 13.8±0.7 nmol/L, which was not different from control rats (Figure 3B).
Figure 3.
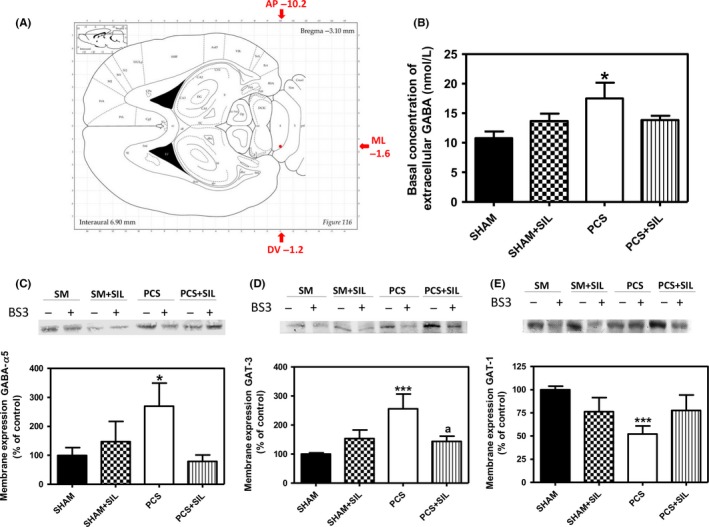
Sildenafil reduces GABAergic tone in cerebellum of PCS rats. Microdialysis guide was implanted in cerebellum as described in methods, in the position indicated in (A) and extracellular GABA concentration was measured by microdialysis in freely moving rats (B). Values are the mean±SEM of 14 sham and PCS rats and eight sham and PCS rats treated with sildenafil. Membrane expression of the alpha 5 subunit of GABAA receptor (C) and of the GABA transporters GAT3 (D) and GAT1 (E) was analyzed using the BS3 cross‐linker procedure as described in methods. Samples, incubated in the absence or presence of BS3, were subjected to Western blotting using the corresponding antibodies. Samples in the absence of BS3 represent the total amount of each protein. Samples in the presence of BS3 represent the nonmembrane fraction. The intensities of the bands were quantified, and membrane expression was calculated as the difference of intensity between samples without and with BS3. Values are expressed as percentage of control rats and are the mean±SEM of five rats per group in (C) and 9‐13 rats per group in (D) and (E). Values significantly different from control rats are indicated by asterisks and from PCS rats by a. *P<.05;***P<.001; a P<.05
PCS rats also show increased membrane expression of the alpha 5 subunit of the GABAA receptor, which increased to 269%±80% of control rats (P<.05). Treatment with sildenafil reduced membrane expression of the alpha 5 subunit in PCS rats to 79%±22% of control rats (Figure 3C).
We also analyzed the membrane expression of the GABA transporters GAT3 (Figure 3D) and GAT1 (Figure 3E). PCS rats show increased membrane expression of GAT3 to 256%±51% of control rats (P<.001) and reduced membrane expression of GAT1, to 51%±8% of control rats (P<.001). Treatment with sildenafil normalized membrane expression of GAT3 to 143%±18% and of GAT1 to 78%±13% of control rats (Figure 3D,E).
To assess the presence of neuroinflammation, we analyzed by immunohistochemistry activation of microglia and astrocytes and the levels of relevant pro‐ and antiinflammatory cytokines. As a marker of microglia, we analyzed Iba‐1, a 17‐kDa actin‐binding protein that is constitutively expressed in all microglia.17 PCS rats show activated microglia in cerebellum, as reflected by the ameboid, less ramified morphology (Figure 4A) and the reduced perimeter, which decreased to 76%±4% (P<.05) of control rats (Figure 4C).
Figure 4.
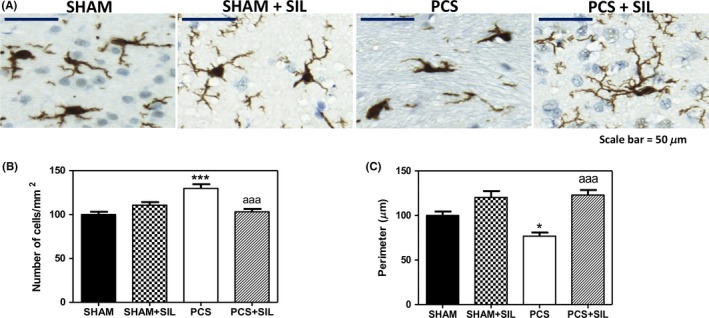
Sildenafil reduces microglial activation in cerebellum of PCS rats. Immunohistochemistry was performed as indicated in methods using antibody against IBA1 (A). The number of microglial cells (B) and their Perimeter (C) were quantified. Values are the mean±SEM of four rats per group. Values significantly different from controls are indicated by asterisks and from PCS rats by a. *P<.05; ***P<.001; aaa P<.005. Scale bar (A)=50 μm
Moreover, the number of microglial cells was increased to 130%±5% (P<.001) of control rats (Figure 4B). Treatment with sildenafil normalized the morphology of microglial cells (Figure 4A) and its perimeter (to 123%±6% of control rats, Figure 4C) and number (to 103%±3% of control rats, Figure 4B).
In addition to parenchymal microglia, Iba1 can recognize perivascular, meningeal, and choroid plexus macrophages but not resident monocytes or inflammatory monocytes.18, 19 Macrophages can be easily distinguished from microglia by the distinctive ramified microglial morphology. The data shown in Figure 4B correspond specifically to microglia; no macrophages were counted. The data in Figure 4B therefore suggest increased proliferation of microglia in PCS rats, but not in PCS rats treated with sildenafil.
Figure 4C shows that sildenafil appears to not only “renormalize” the perimeter of microglia but has also a tendency to even “reverse” the direction of the effects of PCS. The perimeter in PCS rats treated with sildenafil is significantly (P<.05) larger than in sham rats. Also in sham rats treated with sildenafil, there is a tendency to increase the microglial perimeter compared to untreated sham rats. We believe that this effect is due to the fact that sham rats have suffered a sham surgery, which induces a mild inflammatory effect resulting in a slight neuroinflammation compared to naïve (nonoperated) rats. This is reflected for example in an increased content of the inducible form of nitric oxide synthase in cerebral cortex in sham rats compared to nonoperated rats.20 This mild neuroinflammation in sham rats would be associated with a slight reduction in microglial perimeter which would be improved by treatment with sildenafil.
PCS rats also show activated astrocytes in cerebellum (Figure 5), reflected in an increase in the area covered by GFAP staining, that increases (P<.01) to 121%±5% of control rats (Figure 5C). Treatment with sildenafil reduced astrocytes activation in PCS rats and the area covered by GFAP staining decreased (P<.001) to 91%±3% of control rats (Figure 5C). The values in PCS rats treated with sildenafil are not significantly different from control rats treated or not with sildenafil.
Figure 5.
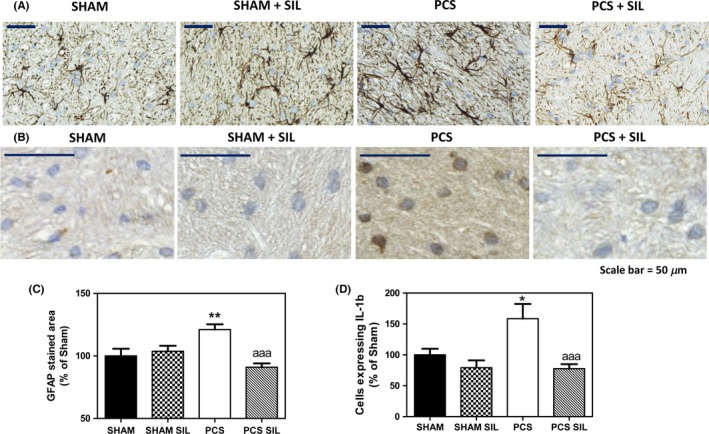
Sildenafil reduces astrocyte activation and the number of IL‐1b‐positive cells in cerebellum of PCS rats. Immunohistochemistry was performed using antibody against GFAP (A) or IL‐1b (B). The area stained by GFAP antibody (C) and the number of IL‐1b‐positive cells (D) were quantified. Values are the mean±SEM of four rats per group. Values significantly different from controls are indicated by asterisks and from PCS rats by a. *P<=0.05; **P<.01; “aaa” P<.005. Scale bar (A)=50 μm
We also analyzed by immunohistochemistry the number of cells expressing IL‐1b, which was increased in PCS rats (P<.05) to 159%±24% of control rats and was reduced (P<.001) by treatment with sildenafil to 76%±7% of control rats (Figure 5B,D).
We have recently shown that IL‐1b and TNF‐a induction in the hippocampus of PCS rats is neuronal.5, 21 However, in cerebellum, we found an induction of IL‐1b mainly in astrocytes. IL‐1b is expressed in multiple cell types in the brain. Its expression is particularly high during neuroinflammation being rapidly synthesized and released, primarily by microglia and astrocytes.22, 23 TNF‐a and IL‐1b are expressed in hippocampal neurons in vivo in response to lesions24 or to pneumococcal meningitis.25 In situ hybridization studies show that in murine pneumococcal meningitis, TNF‐a mRNA was first upregulated in astroglial cells but at 18‐24 hours was strongly increased in hippocampal neurons.25 A similar process would occur in hippocampus of rats with HE due to PCS, leading to increased expression of IL‐1b and TNF‐a in neurons. However, this process seems to be specific for hippocampus and we do not observe increased levels of IL‐1b or TNF‐a in cerebellar neurons.
The content of IL‐1b in cerebellum, analyzed by Western blot, was also increased in PCS rats (P<.01) to 127%±8% of control rats and was reduced by treatment with sildenafil to 110%±11% of control rats (Figure 6A). A similar increase was found for TNF‐a, which increased in PCS rats (P<.05) to 132%±13% of control rats and was reduced by treatment with sildenafil to 102%±12% of control rats (Figure 6B).
Figure 6.
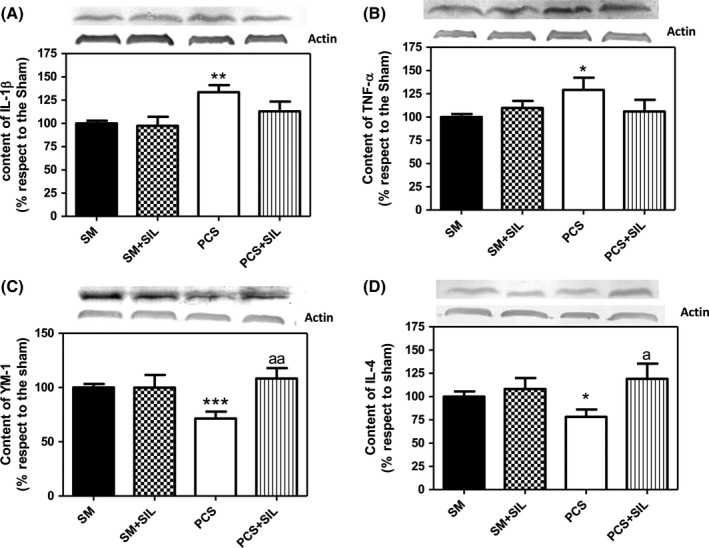
PCS rats show increased content of pro‐inflammatory markers IL‐1b and TNF‐a and reduced content of antiinflammatory YM‐1 and IL‐4 in cerebellum. Sildenafil normalizes their content. Cerebellar homogenates from control (SM) and PCS rats treated with tap water or sildenafil (SIL) were subjected to electrophoresis and Western blot using antibodies against IL‐1b (A); TNF‐a (B), YM‐1 (C), or IL‐4 (D). Representative images of the blots are shown for each protein. Values are the mean±SEM of 10‐12 rats per group. Values significantly different from control rats are indicated by asterisks. Values significantly different from hyperammonemic rats are indicated by “a.” *P<.05; **P<.01; ***P<.001; “a” P<.05; “aa” P<.01
In contrast, the contents of the antiinflammatory markers YM‐1 and IL‐4 were reduced in cerebellum of PCS rats to 72%±6% (P<.001) and 78%±8% (P<.05) of control rats, respectively (Figure 6C,D). Treatment with sildenafil normalized the contents of YM‐1 and IL‐4 in PCS rats to 108%±10% and 119%±16% of control rats, respectively (Figure 6C,D).
The results reported show that PCS rats show reduced levels of cGMP, associated with neuroinflammation in cerebellum, as indicated by the activation of microglia and astrocytes, associated with increased levels of the pro‐inflammatory markers IL‐1b and TNF‐a and reduced levels of the antiinflammatory markers YM‐1 and IL‐4. This neuroinflammation is associated with increased levels of extracellular GABA and of membrane expression of the alpha 5 subunit of GABAA receptors.
The increased membrane expression of the alpha 5 subunit of GABAA receptors would increase the response to extracellular GABA, contributing together with the increased concentration of extracellular GABA, to enhanced GABAergic tone. Neuroinflammation would contribute to increase extracellular GABA by altering the membrane expression of the GABA transporters GAT3 and GAT1, as discussed below.
Increased GABAergic tone in cerebellum would be responsible for motor in‐coordination in PCS rats. Motor coordination is mainly modulated in cerebellum by GABAergic neurotransmission. Increased GABAergic tone in cerebellum impairs motor coordination.9 Mice lacking the GABA transporter subtype 1 (GAT1) show increased extracellular GABA and reduced motor coordination.26 Also, rats developmentally exposed to polychlorinated biphenyls show motor in‐coordination which correlates with extracellular GABA levels in cerebellum.10
The changes in motor coordination observed in PCS rats would be a consequence of altered GABAergic neurotransmission in cerebellum. The improvement of motor coordination in PCS rats treated with sildenafil would be therefore due to the reduction of GABAergic tone in cerebellum.
PCS rats show both inflammation and hyperammonemia. Rats with hyperammonemia show increased GABAergic tone in cerebellum, with increased extracellular GABA which is associated with motor in‐coordination. Treating hyperammonemic rats with pregnenolone sulfate, which reduces GABAergic tone27 or with sulforaphane, which reduces neuroinflammation28 normalizes GABAergic tone and motor coordination, further supporting that motor in‐coordination is a consequence of increased GABAergic tone.
Extracellular cGMP levels are reduced in cerebellum of PCS rats and, as shown here and in,6 are normalized by treatment with sildenafil. Also, in hyperammonemic rats, increased GABAergic tone alters glutamatergic neurotransmission, impairing the glutamate‐nitric oxide‐cGMP pathway in cerebellum,29 which contributes to impair learning in the Y maze. Restoring cGMP levels with sildenafil6 or reducing GABAergic tone with bicuculline29 restored the glutamate‐nitric oxide‐cGMP pathway and learning in the Y maze.
We show here that sildenafil increases cGMP in PCS rats, which is associated with reduction in neuroinflammation and normalization of neurotransmission and motor coordination.
Altogether, the new data reported and these previous data support the hypothesis summarized in Figure 1 proposing an interplay between neuroinflammation, cGMP, and GABAergic/glutamatergic neurotransmission in the impairment of cognitive and motor function in rats with MHE. This interplay could also occur in general in most pathological situations associated with neuroinflammation.
This interplay would occur in different brain areas with different relative relevance of each player, resulting in different types of neurological alterations. In hippocampus, glutamatergic neurotransmission would be more relevant and this interplay would impair mainly spatial learning.6 In cerebellum, as shown here, GABAergic neurotransmission would be more relevant and would impair motor coordination and learning in the Y maze.6
We show here that modulating cGMP levels in cerebellum by sildenafil treatment eliminates neuroinflammation and normalizes GABAergic tone and motor coordination, further supporting the interplay hypothesis proposed in Figure 1.
The mechanisms by which cGMP modulates neuroinflammation are not known in detail. Transgenic Tg APP/PS1 mice, a model for Alzheimer's disease, show increased IL‐1β in hippocampus and cognitive impairment. Treatment with sildenafil normalizes IL‐1β levels and restores cognitive function. Both effects are prevented by an inhibitor of cGMP‐dependent protein kinase (PKG), indicating that activation of PKG by cGMP mediates the effects of sildenafil on neuroinflammation.7 These authors propose that in Tg mice phosphorylation of CREB by PKG is reduced and its recovery by sildenafil mediates the reduction of IL‐1β levels.7
cGMP may also modulate IL‐1b levels by modulating NF‐kB. IL‐1β transcription is mainly modulated by NF‐kB.30 cGMP may reduce activation of NF‐kB31 by a mechanism mediated by increased HSP70 protein and of its binding to IkB, the inhibitor of NF‐kB translocation to the nucleus and activation.32 Sildenafil would reduce IL‐1β levels by increasing cGMP, which would reduce transcriptional activity of NF‐kB.
However, the results reported here indicate more complex effects of cGMP on neuroinflammation. We show that sildenafil reduces IL‐1b and TNF‐a levels, but also reverse activation of microglia and astrocytes. This should be mediated by more complex mechanisms. Also, a recent report suggests that intra‐ and extra‐cellular cGMP may play different roles in modulating neuroinflammation. Hyperammonemia induces astrocytes and microglia activation in hippocampus, increases IL‐1b and TNF‐a, and alters NMDA and AMPA receptors membrane expression and impairs spatial working and reference memory.33 Continuous intracerebral administration of extracellular cGMP normalized astrocytes activation, IL‐1b levels, membrane expression of NMDA receptors and working memory, but not microglia activation, TNF‐a levels, membrane expression of AMPA receptors, and reference memory.33 In contrast, in PCS rats, sildenafil that increased both intra‐ and extra‐cellular cGMP normalized all above parameters.6 Further studies to clarify in detail the mechanisms by which cGMP modulates neuroinflammation are necessary. These studies may provide new therapeutic targets to treat neuroinflammation.
We show here that in cerebellum of PCS rats, neuroinflammation is associated with enhanced GABAergic neurotransmission due both to increased membrane expression of GABAA receptor and increased extracellular GABA concentration. Treatment with sildenafil reduces neuroinflammation and normalizes GABAergic neurotransmission. This supports the idea proposed in Figure 1 of an interplay between cGMP‐neuroinflammation‐GABAergic neurotransmission.
In hippocampus, IL‐1b or lipopolysaccharide enhances GABAergic neurotransmission by enhancing membrane expression of GABAA receptors.34, 35, 36 This is prevented by co‐incubation with the IL‐1 receptor antagonist IL‐1Ra.34 It seems therefore that IL‐1b would play a main role in increasing membrane expression of GABAA receptors, but the underlying mechanisms remain unknown.
We show here that PCS rats also show increased membrane expression of GABAA receptors in cerebellum, associated with increased IL‐1b levels. Sildenafil normalizes IL‐1b levels and membrane expression of GABAA receptor. This suggests that sildenafil would reduce IL‐1b levels and this, in turn, would eliminate the enhanced membrane expression of the GABAA receptor, which would return to normal values.
We show here that in PCS there is a second factor enhancing GABAergic neurotransmission, an increase in extracellular GABA concentration, which would be also a consequence of neuroinflammation and would be mediated by altered membrane expression of the GABA transporters GAT1 and GAT3. Under normal conditions, both GAT1 and GAT3 take up GABA from the extracellular fluid into the cells. The reduced membrane expression of GAT1 in PCS rats would therefore contribute to increase extracellular GABA.
The function of GAT3 is reversed under different conditions, leading to GABA release through it from cells to the extracellular fluid. GABA transport gains its driving force from the concentration gradient of Na+ between intracellular and extracellular compartments.37 Situations that increase intracellular Na+ to levels enough to change this gradient may reverse the function of GAT3 leading to GABA release. For example, uptake of certain levels of glutamate by astrocytic transporters triggers the release of GABA through GAT3, likely by elevating the intracellular Na+ concentration in astrocytes.38, 39 Reversal of GAT3 function and GABA release through it has been also reported in activated astrocytes of a mouse model of Alzheimer's disease.40 A similar GAT3‐mediated release of GABA has been suggested in activated astrocytes in cerebellum of hyperammonemic rats.30 Neuroinflammation would therefore contribute to increased release of GABA through GAT3 in activated astrocytes also in PCS rats. Membrane expression of GAT3 is increased in cerebellum of PCS rats, which would further potentiate GABA release and extracellular GABA increase. Sildenafil reduces neuroinflammation, eliminating astrocytes activation and the enhancement of GAT3 membrane expression. This would also return GAT3 function to uptake instead of release of GABA, thus normalizing extracellular GABA.
Increased extracellular GABA in cerebellum would be responsible for motor in‐coordination in PCS rats. Reducing extracellular GABA and GABAergic tone by modulating GABAA receptors with the antagonist bicuculline or the neurosteroid pregnenolone sulfate restores motor coordination in hyperammonemic rats.29, 31 Sildenafil normalizes GABAergic tone, likely as a consequence of reducing neuroinflammation. This would be responsible for restoration of motor coordination in PCS rats by sildenafil.
4. Conclusions
In summary, this work shows that PCS rats show reduced cGMP associated with neuroinflammation in cerebellum, with activation of microglia and astrocytes, associated with increased levels of IL‐1b and TNF‐a and reduced levels of the antiinflammatory markers YM‐1 and IL‐4. This neuroinflammation is associated with increased levels of extracellular GABA and of membrane expression of the alpha 5 subunit of GABAA receptors, resulting in increased GABAergic tone which impairs motor coordination. Increased extracellular GABA would be a consequence of the reduced membrane expression of the GAT1 transporter and increased membrane expression and reversal of the function of GAT3. Treatment with sildenafil increases cGMP levels; eliminates neuroinflammation, activation of microglia, and astrocytes; changes in inflammatory markers and in membrane expression of GABA receptor and transporters and restores motor coordination. These results support an interplay between cGMP‐neuroinflammation and GABAergic neurotransmission in the impairment of motor coordination in PCS rats.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
Supported by Ministerio de Ciencia e Innovación (SAF2014‐51851‐R), Generalitat Valenciana (PROMETEO‐2009‐027, PROMETEOII/2014/033) and co‐funded with European Regional Development Funds (ERDF).
Agusti A, Hernández‐Rabaza V, Balzano T, et al. Sildenafil reduces neuroinflammation in cerebellum, restores GABAergic tone, and improves motor in‐coordination in rats with hepatic encephalopathy. CNS Neurosci Ther. 2017;23:386–394. 10.1111/cns.12688
References
- 1. Felipo V. Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci. 2013;14:851‐858. [DOI] [PubMed] [Google Scholar]
- 2. Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT. Group Authors: ISHEN Commission Expt Models HE. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009;29:783‐788. [DOI] [PubMed] [Google Scholar]
- 3. Cauli O, Rodrigo R, Piedrafita B, Boix J, Felipo V. Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with porto‐caval shunts. Hepatology. 2007;46:514‐519. [DOI] [PubMed] [Google Scholar]
- 4. Cauli O, Rodrigo R, Piedrafita B, Llansola M, Mansouri MT, Felipo V. Neuroinflammation contributes to hypokinesia in rats with hepatic encephalopathy. Ibuprofen restores its motor activity. J Neurosci Res. 2009;87:1369‐1374. [DOI] [PubMed] [Google Scholar]
- 5. Hernandez‐Rabaza V, Agusti A, Cabrera‐Pastor A, et al. Sildenafil reduces neuroinflammation and restores spatial learning in rats with hepatic encephalopathy. Underlying mechanisms. J Neuroinflammation. 2015;12:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erceg S, Monfort P, Hernández‐Viadel M, Rodrigo R, Montoliu C, Felipo V. Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunt. Hepatology. 2005;45:2‐10. [DOI] [PubMed] [Google Scholar]
- 7. Zhang J, Guo J, Zhao X, et al. Phosphodiesterase‐5 inhibitor sildenafil prevents neuroinflammation, lowers beta‐amyloid levels and improves cognitive performance in APP/PS1 transgenic mice. Behav Brain Res. 2013;250:230‐237. [DOI] [PubMed] [Google Scholar]
- 8. Raposo C, Nunes AK, Luna RL, Araújo SM, da Cruz‐Höfling MA, Peixoto CA. Sildenafil (Viagra) protective effects on neuroinflammation: the role of iNOS/NO system in an inflammatory demyelination model. Mediators Inflamm. 2013;00:321460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol‐induced motor impairment caused by increased extrasynaptic GABAA. receptor activity. Nat Neurosci. 2005;8:339‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boix J, Cauli O, Felipo V. Developmental exposure to polychlorinated biphenyls 52, 138 or 180 affects differentially learning or motor coordination in adult rats. Mechanisms involved. Neuroscience. 2010;167:994‐1003. [DOI] [PubMed] [Google Scholar]
- 11. Lee SH, Fisher B. Portacaval shunt in the rat. Surgery. 1961;50:668‐672. [PubMed] [Google Scholar]
- 12. Jover R, Rodrigo R, Felipo V, et al. Brain edema and inflammatory activation in bile duct ligated rats with diet‐induced hyperammonemia: a model of hepatic encephalopathy in cirrhosis. Hepatology. 2006;43:1257‐1266. [DOI] [PubMed] [Google Scholar]
- 13. Monfort P, Corbalán R, Martinez L, López‐Talavera J, Córdoba J, Felipo V. Altered content and modulation of soluble guanylate cyclase in the cerebellum of rats with portacaval anastomosis. Neuroscience. 2001;104:1119‐1125. [DOI] [PubMed] [Google Scholar]
- 14. Canales JJ, Elayadi A, Errami M, Llansola M, Cauli O, Felipo V. Chronic hyperammonemia alters motor and neurochemical responses to activation of group I metabotropic glutamate receptors in the nucleus accumbens in rats in vivo. Neurobiol Dis. 2003;14:380‐390. [DOI] [PubMed] [Google Scholar]
- 15. Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144‐9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Felipo V, Miñana MD, Azorín I, Grisolía S. Induction of rat brain tubulin following ammonium ingestion. J Neurochem. 1988;51:1041‐1045. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin‐binding proteins Coronin‐1a and IBA‐1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55:687‐700. [DOI] [PubMed] [Google Scholar]
- 18. Toku K, Tanaka J, Fujikata S. Distinctions between microglial cells and peripheral macrophages with regard to adhesive activities and morphology. J Neurosci Res. 1999;57:855‐865. [PubMed] [Google Scholar]
- 19. Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227‐1235. [DOI] [PubMed] [Google Scholar]
- 20. Rodrigo R, Erceg S, Rodriguez‐Diaz J, et al. Glutamate‐induced activation of nitric oxide synthase is impaired in cerebral cortex in vivo in rats with chronic liver failure. J Neurochem. 2007;102:51‐64. [DOI] [PubMed] [Google Scholar]
- 21. Dadsetan S, Balzano T, Forteza J, et al. Reducing peripheral inflammation with infliximab reduces neuroinflammation and improves cognition in rats with hepatic encephalopathy. Front Mol Neurosci. 2016;9:106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986;164:594‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pearson VL, Rothwell NJ, Toulmond S. Excitotoxic brain damage in the rat induces interleukin‐1beta protein in microglia and astrocytes: correlation with the progression of cell death. Glia. 1999;25:311‐323. [PubMed] [Google Scholar]
- 24. Tchélingérian JL, Le Saux F, Jacque C. Identification and topography of neuronal cell populations expressing TNF alpha and IL‐1 alpha in response to hippocampal lesion. J Neurosci Res. 1996;43:99‐106. [DOI] [PubMed] [Google Scholar]
- 25. Izadpanah K, Freyer D, Weber JR, Braun JS. Brain parenchymal TNF‐α and IL‐1β induction in experimental pneumococcal meningitis. J Neuroimmunol. 2014;276:104‐111. [DOI] [PubMed] [Google Scholar]
- 26. Sirad F, Hlaing S, Kovanecz I, et al. Sildenafil promotes smooth muscle preservation and ameliorates fibrosis through modulation of extracellular matrix and tissue growth factor gene expression after bilateral cavernosal nerve resection in the rat. J Sex Med. 2011;8:1048‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oger S, Behr‐Roussel D, Gorny D, et al. Signalling pathways involved in sildenafil‐induced relaxation of human bladder dome smooth muscle. Br J Pharmacol. 2010;160:1135‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiu CS, Brickley S, Jensen K, et al. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA‐induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234‐3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzalez‐Usano A, Cauli O, Agusti A, Felipo V. Pregnenolone sulphate restores the glutamate‐nitric oxide‐cGMP pathway and extracellular GABA in cerebellum and learning and motor coordination in hyperammonemic rats. ACS Chem Neurosci. 2014;5:100‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hernandez‐Rabaza V, Cabrera‐Pastor A, Taoro‐Gonzalez L, et al. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT‐3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J Neuroinflammation. 2016;13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cauli O, Mansouri MT, Agusti A, Felipo V. Hyperammonemia increases GABAergic tone in cerebellum but decreases it in rat cortex. Gastroenterology. 2009;136:1359‐1367. [DOI] [PubMed] [Google Scholar]
- 32. Cogswell JP, Godlevski MM, Wisely GB, et al. NF‐kappa B regulates IL‐1 beta transcription through a consensus NF‐kappa B binding site and a nonconsensus CRE‐like site. J Immunol. 1994;153:712‐723. [PubMed] [Google Scholar]
- 33. Kiemer AK, Vollmar AM, Bilzer M, Gerwig T, Gerbes AL. Atrial natriuretic peptide reduces expression of TNF‐alpha mRNA during reperfusion of the rat liver upon decreased activation of NF‐kappaB and AP‐1. J Hepatol. 2000;33:236‐246. [DOI] [PubMed] [Google Scholar]
- 34. Kiemer AK, Gerbes AL, Bilzer M, Vollmar AM. The atrial natriuretic peptide and cGMP: novel activators of the heat shock response in rat livers. Hepatology. 2002;35:88‐94. [DOI] [PubMed] [Google Scholar]
- 35. Cabrera‐Pastor A, Hernandez‐Rabaza V, Taoro‐Gonzalez L, Balzano T, Llansola M, Felipo V. In vivo administration of extracellular cGMP normalizes TNF‐a and membrane expression of AMPA receptors in hippocampus and spatial reference memory but not IL‐1b, NMDA receptors in membrane and working memory in hyperammonemic rats. Brain Behav Immun. 2016;57:360‐370. [DOI] [PubMed] [Google Scholar]
- 36. Hellstrom IC, Danik M, Luheshi GN, Williams S. Chronic LPS exposure produces changes in intrinsic membrane properties and a sustained IL‐beta‐dependent increase in GABAergic inhibition in hippocampal CA1 pyramidal neurons. Hippocampus. 2005;15:656‐664. [DOI] [PubMed] [Google Scholar]
- 37. Serantes R, Arnalich F, Figueroa M, et al. Interleukin‐1beta enhances GABAA receptor cell‐surface expression by a phosphatidylinositol 3‐kinase/Akt pathway: relevance to sepsis‐associated encephalopathy. J Biol Chem. 2006;281:14632‐14643. [DOI] [PubMed] [Google Scholar]
- 38. Wang DS, Zurek AA, Lecker I, et al. Memory deficits induced by inflammation are regulated by α5‐subunit‐containing GABAA receptors. Cell Rep. 2012;2:488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanner BI. Structure and function of sodium‐coupled GABA and glutamate transporters. J Membr Biol. 2006;213:89‐100. [DOI] [PubMed] [Google Scholar]
- 40. Héja L, Barabás P, Nyitrai G, et al. Glutamate uptake triggers transporter‐mediated GABA release from astrocytes. PLoS ONE. 2009;4:e7153. [DOI] [PMC free article] [PubMed] [Google Scholar]


