Summary
Aims
Neuroinflammation contributed to the pathogenesis of multiple system atrophy (MSA). We aimed to detect the correlation between inflammatory mediators, such as Klotho (Klt), vitamin D (25(OH)D) and homocysteine (Hcy), and disease severity among MSA patients.
Methods
A total of 53 MSA patients, 65 PD patients, and 62 normal subjects were recruited in our cross‐sectional study. Serum Klotho (Klt), vitamin D (25(OH)D), and homocysteine (Hcy) levels were measured. Several scales were undertaken to assess the motor/nonmotor function and cognitive impairment of MSA.
Results
Decreased Serum Klt and 25(OH)D levels and increased Hcy levels were found in patients with MSA, compared with healthy controls. These results were more pronounced in male patients. The three biomarkers also displayed differences between MSA and PD subgroups based on genders. Interestingly, Klt, 25(OH)D and Hcy levels associated with cognition impairment, motor dysfunction, mood/cardiovascular disorder among MSA patients. In addition, the combination of Klt, 25(OH)D and Hcy had a better diagnostic ability for distinguishing MSA patients from healthy subjects, as well as distinguishing male MSA patients from male PD patients.
Conclusion
This study suggested that Klt, 25(OH)D and Hcy levels could be a potential predictor for MSA severity evaluation.
Keywords: homocysteine, Klotho, multiple system atrophy, neuroinflammation, vitamin D
Highlights.
MSA patients, particularly in male patients, had significantly lower Serum Klt and 25(OH)D levels compared with normal subjects.
MSA patients, especially in male patients, had significantly higher Hcy serum levels compared with normal subjects.
Significant correlations were observed between serum Klt, 25(OH)D and Hcy levels and the severity of MSA.
1. INTRODUCTION
Multiple system atrophy (MSA) is a progressing neurodegenerative disease,1 which is clinically characterized by autonomic failure, parkinsonism, and cerebellar ataxia.2, 3 MSA included three subtypes: autonomic and urinary dysfunction (MSA‐A), cerebellar dysfunction (MSA‐C), and parkinsonism (MSA‐P).4 Parkinson's disease (PD) and multiple system atrophy (MSA) are both motor dysfunction and α‐synuclein‐related neurodegenerative diseases. PD and MSA seem similar in the initial phase of the diseases; however, disease prevalence and treatment of dopamine differs considerably.5 Therefore, identifying biomarkers that could differentiate between PD and MSA would be very useful.
Several lines of evidence demonstrated that inflammatory responses play an essential role in neurodegenerative diseases.6, 7 It has been shown that neurodegeneration in the pathogenesis of PD and AD is mediated by some inflammatory mediators such as TNFα, IL‐1β, and IL‐6.8, 9 Recent studies have also shown that upregulation of these proinflammatory cytokines causes microglial activation, which leads to dopaminergic neurons degeneration and blood‐brain barrier (BBB) dysfunction.10, 11 Klotho (Klt) is a transmembrane molecule that is predominantly expressed in the kidneys and brain.12 Loss of Klt accelerates human aging, neural degeneration, cognitive impairment, and synapses deficiency in the hippocampus. However, its brain function remains unclear.13 Accumulating evidence demonstrated that Klt is functionally related to anti‐inflammation14, 15 and exerts protective effects against age‐related diseases such as PD.13, 16 Another important feature of Klt is that it, together with 1,25‐dihydroxy25(OH)D (1,25‐(OH)2D), contributes to calcium (Ca)‐phosphorus (P) metabolism.17, 18, 19 Previous reports have shown that 25(OH)D is associated with inflammation and may involve in the pathogenesis of PD.20, 21, 22, 23 Although both Klt and 25(OH)D are correlated with neuroinflammation14, 15, 24 and age‐related diseases,13, 16, 22, 23 the association of Klt and 25(OH)D with MSA severity has not been systemically elucidated. Homocysteine (Hcy) is a key product at the intersection of cysteine and methionine and is associated with neuroinflammation and cognitive dysfunction.25, 26 Several studies have indicated that some cytokines in the peripheral blood can be regarded as biomarkers to evaluate PD severity.25, 27
Klt, 25(OH)D and Hcy are not only associated with inflammation and oxidative stress14, 24, 27 but also play important roles in the pathogenesis of various neurodegenerative diseases.15, 16, 21, 25, 28, 29 To our knowledge, the independent and combined effects of Klt, 25(OH)D and Hcy have not been investigated in assessing MSA patients yet. This study was performed to explore whether the cytokines, Klt, 25(OH)D, and Hcy are related to MSA outcomes and severity. The purpose of our study was (i) to compare serum Klt/25(OH)D/Hcy levels among PD patients, MSA patients, and healthy subjects; (ii) to explore the correlation between serum Klt/25(OH)D/Hcy levels and motor/nonmotor dysfunction, cognitive impairment in MSA patients; (iii) to identify the diagnostic ability of the serum of Klt, 25(OH)D, Hcy levels to discriminate MSA patients from healthy subjects, as well as distinguishing MSA patients from PD patients.
2. MATERIALS AND METHODS
2.1. Patients and study design
This cross‐sectional study was performed in accordance with the Declaration of Helsinki and good clinical practice guideline. From August 2014 to December 2016, we enrolled 53 MSA patients (30 males and 23 females) and 65 PD patients (38 males and 27 females) who were enrolled from the First Affiliated Hospital of Sun Yat‐sen University, Guangzhou, P.R. China. The patients fulfilled the consensus criteria for the clinical diagnosis of MSA and the UK PD Society Brain Bank criteria (UK‐PDSBB) for diagnosis, respectively.2, 30, 31 Additionally, 62 healthy control subjects (34 males and 28 females) were recruited from the outpatient population of the same hospital, according to clinical practice.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat‐sen University. All subjects agreed and signed informed consent for the measurement of serum Klt, 25(OH)D and Hcy levels. Disease severity, the motor/nonmotor function, and cognitive function were evaluated by Hoehn and Yahr Staging Scale (H&Y), Webster Scale, PD Sleep Scale (PDSS), the Unified MSA Rating Scale (UMSARS), NMS Scale (NMSS), the Schwab & England Activities of Daily Living (ADL) Scale, Mini‐Mental State Examination (MMSE). The patients (i) who took medication that could influence vitamin D metabolism; (ii) who were diagnosis of familial Parkinsonism, Lewy bodies dementia, cerebral vascular disease, and cerebrovascular sequelae were excluded from our study. MSA and PD groups were divided into two subgroups based on gender. For additional details, please refer to the Supporting Information.
2.2. Laboratory measurement
Venous blood samples for Klt, 25(OH)D and Hcy measurements were obtained at 8 am after an overnight fasting. Blood sample (3 mL) was collected from the all the subjects. The serum was separated via centrifugation for laboratory analysis. The serum levels of Klt were detected by R&D Systems ELISA kits (USA). The 25(OH)D levels were determined using competitive chemiluminescence immunoassays (Dia‐Sorin, Saluggia, Italy).32 The serum levels of Hcy were measured by routine laboratory tests, as previously described.25
2.3. Statistical analysis
All continuous data including age, scores on the UMSARS, NMSS, PDSS, MMSE, and Webster tests; and Klt, 25(OH)D and Hcy levels, were given as means and standard deviation (SD); group mean values were compared by the Kruskal‐Wallis test and Mann‐Whitney U test for non‐normally distributed data or Student's t test for normally distributed data. One‐way ANOVA followed by post hoc analysis and Bonferroni's corrections was used to assess differences in serum Klt, 25(OH)D and Hcy levels among three groups. We compared the differences in Klt/25(OH)D/Hcy levels between these gender subgroups by Mann‐Whitney U test (the data were not normally distributed) and Student's t test (the data were normally distributed). Correlations between the clinical characteristics and serum cytokines levels were performed using Spearman's rank correlation coefficient (r s). The predictive value of Klt, 25(OH)D and Hcy and their combination for MSA was calculated by receiver operating characteristic (ROC) curves. Differences were deemed significant at P<.05. Statistical analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The SPSS 13.0 have made the automatic corrections during statistical analyses of One‐way ANOVA followed by Bonferroni's comparison post hoc analysis, given the corrected P′ (P×3) in Table 3. The corrected P′ value more than .05 was defined as “no significant differences” among the three groups (controls, MSA, and PD). For additional details, please refer to the Supporting Information.
Table 3.
Comparison of age, Klt, 25(OH)D, and Hcy levels among PD and MSA patients and normal subjects
| Variable | MSA (mean) | PD | Control | t Value | P‐value | MSA/PD | MSA/Control |
|---|---|---|---|---|---|---|---|
| P | P | ||||||
| Age | 59.7±10.1 | 61.5±7.97 | 53.4±9.08 | 11.3 | 0.000c | 0.093 | 0.287 |
| Klt | 2.41±1.51 | 2.23±1.94 | 3.06±1.28 | 2.28 | 0.003b | 0.080 | <0.001c |
| 25(OH)D | 39.9±15.7 | 35.2±12.1 | 62.7±19.6 | 13.1 | 0.042a | 0.192 | 0.007b |
| Hcy | 15.2±5.95 | 14.3±5.76 | 10.4±3.09 | 13.4 | 0.002b | 0.865 | 0.005b |
The comparison among MSA and PD patients and normal subjects (one‐way ANOVA analysis); MSA vs PD (Bonferroni's post hoc analysis); MSA vs Control (Bonferroni's post hoc analysis).
P<0.05.
P<0.01.
P<0.001.
3. RESULTS
3.1. Characteristics of subjects
Tables 1 and 2 presented the demographic and clinical features of the subjects. We enrolled 53 MSA [30 males (56.6%) and 23 females (43.4%)], 65 PD patients [38 males (58.5%) and 27 (41.5%) females], and 62 healthy subjects [34 males (54.8%) and 28 females (45.2%)] in this cross‐sectional study (Table 1). There was no difference in age between the MSA patients and normal subjects (59.7±10.1 vs 53.4±9.08, P=.287, Table 3) or between MSA and PD patients (59.7±10.1 vs 61.5±7.97, P=.093, Table 3). In addition, when MSA patients divided into three subtypes (MSA‐A, MSA‐C, and MSA‐P), we also found no statistically significant differences in age among these three groups.
Table 1.
Demographic, clinical parameters in the MSA and PD patients
| Clinical parameters | MSA | Healthy subjects | PD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Min | Max | Mean (SD) | Min | Max | Mean (SD) | Min | Max | ||
| Gender (n) | Male n (%) | 30 (56.6) | / | / | 34 (54.8) | / | / | 38 (58.5) | / | / |
| Female n (%) | 23 (43.4) | / | / | 28 (45.2) | / | / | 27 (41.5) | / | / | |
| Age (y) | 59.7 (10.1) | 36 | 80 | 53.4 (9.08) | 41 | 80 | 61.5 (7.97) | 42 | 85 | |
| H&Y | 3.85 (1.59) | 1 | 5 | / | / | / | 2.91 (1.82) | 1 | 5 | |
| PDSS | 119 (9.48) | 89 | 145 | / | / | / | 106 (12.9) | 75 | 139 | |
| UMSARS | 38.9 (13.1) | 27 | 69 | / | / | / | / | / | / | |
| UMSARS (I) | 13.7 (4.05) | 9 | 24 | / | / | / | / | / | / | |
| UMSARS (II) | 19.2 (8.64) | 10 | 39 | / | / | / | / | / | / | |
| UMSARS (IV) | 2.56 (0.50) | 1 | 5 | / | / | / | / | / | / | |
| NMSS (total) | 89.4 (23.5) | 29 | 138 | / | / | / | 75.1 (52.1) | 10 | 157 | |
| Cardiovascular | 6.06 (4.90) | 0 | 15 | / | / | / | 8.75 (4.69) | 0 | 18 | |
| Sleep/fatigue | 15.2 (5.58) | 0 | 28 | / | / | / | 15.2 (7.91) | 0 | 39 | |
| Mood | 17.5 (8.61) | 3 | 34 | / | / | / | 16.3 (10.7) | 0 | 45 | |
| Perceptual problem | 1.87 (3.49) | 0 | 17 | / | / | / | 1.47 (5.36) | 0 | 15 | |
| Attention/memory | 9.27 (5.69) | 0 | 25 | / | / | / | 7.51 (8.11) | 0 | 27 | |
| Gastrointestinal | 8.35 (5.70) | 0 | 19 | / | / | / | 9.65 (5.37) | 0 | 26 | |
| Urinary | 9.30 (6.06) | 0 | 28 | / | / | / | 8.33 (3.92) | 0 | 30 | |
| Sexual function | 4.45 (7.23) | 0 | 25 | / | / | / | 3.40 (5.61) | 0 | 22 | |
| Miscellaneous | 7.67 (16.3) | 0 | 20 | / | / | / | 6.81 (6.25) | 0 | 18 | |
| MMSE | 25.7 (3.27) | 15 | 30 | / | / | / | 25.3 (4.10) | 13 | 30 | |
| Schwab & England | 72.8 (19.0) | 0 | 90 | 81.7 (11.4) | 35 | 90 | ||||
| Webster | 13.8 (3.57) | 5 | 31 | 15.8 (8.39) | 6 | 90 | ||||
| Daily dose of l‐Dopa (mg) (MSA‐P) | 262 (52.4) | 227 | 282 | / | / | / | 273 (69.3) | 241 | 318 | |
| Disease duration | 5.15 (5.43) | 0.5 | 16 | / | / | / | 4.62 (4.28) | 0.5 | 14 | |
SD, standard deviation; UMSARS, Unified Multiple system atrophy Rating Scale; PDSS, PD Sleep Scale; Webster, Webster Scale; Schwab & England, the Schwab & England Activities of Daily Living Scale; H&Y, the modified Hoehn and Yahr staging scale; MMSE, mini‐mental state examination; NMSS, nonmotor symptoms scale.
Table 2.
Demographic, clinical parameters in MSA‐P, MSA‐C, and MSA‐A patients
| Clinical parameters | MSA‐P | MSA‐C | MSA‐A | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Gender (n) | Male n (%) | 6 (31.6) | 10 (40.0) | 3 (33.3) |
| Female n (%) | 13 (68.4) | 15 (60.0) | 6 (66.7) | |
| Age (y) | 59.5 (10.1) | 58.3 (10.1) | 64.1 (9.57) | |
| H&Y | 2.85 (0.62) | 3.59 (0.73) | 3.91 (0.92) | |
| PDSS | 128 (9.68) | 125 (16.7) | 122 (7.36) | |
| UMSARS | 32.2 (4.59) | 45.5 (12.9) | 35.9 (12.0) | |
| UMSARS (I) | 11.5 (3.59) | 17.4 (5.71) | 15.2 (3.91) | |
| UMSARS (II) | 17.6 (5.98) | 25.2 (5.99) | 20.5 (9.26) | |
| UMSARS (VI) | 2.69 (0.72) | 3.75 (0.52) | 4.18 (3.39) | |
| NMSS (total) | 88.3 (20.4) | 70.4 (16.2) | 72.4 (25.4) | |
| Cardiovascular | 7.18 (4.90) | 7.39 (5.18) | 13.3 (5.93) | |
| Sleep/fatigue | 16.2 (5.91) | 12.6 (6.42) | 16.4 (7.91) | |
| Mood | 15.7 (6.27) | 13.5 (6.91) | 12.6 (6.72) | |
| Perceptual problem | 2.95 (4.26) | 2.58 (3.52) | 1.66 (1.39) | |
| Attention/memory | 9.59 (4.59) | 8.28 (4.31) | 6.53 (8.90) | |
| Gastrointestinal | 11.26 (4.92) | 9.97 (4.57) | 8.13 (5.42) | |
| Urinary | 8.51 (5.92) | 9.11 (5.18) | 12.8 (9.62) | |
| Sexual function | 3.54 (6.83) | 3.79 (6.21) | 3.52 (6.61) | |
| Miscellaneous | 9.68 (4.29) | 6.87 (4.76) | 5.95 (3.25) | |
| MMSE | 26.8 (4.04) | 25.5 (3.62) | 25.7 (3.10) | |
| Schwab & England | 62.6 (29.3) | 66.3 (31.5) | 51.7 (25.6) | |
| Webster | 13.3 (3.88) | 13.1 (2.59) | 12.8 (2.63) | |
SD, standard deviation; UMSARS, Unified Multiple system atrophy Rating Scale; PDSS, PD Sleep Scale; Webster, Webster Scale; Schwab & England, the Schwab & England Activities of Daily Living Scale; H&Y, the modified Hoehn and Yahr staging scale; MMSE, mini‐mental state examination; NMSS, nonmotor symptoms scale.
3.2. Comparisons of Klt/25(OH)D/Hcy between MSA/PD patients and normal subjects
There were significant differences in serum Klt, 25(OH)D and Hcy among the PD and MSA groups and normal subjects (**P=.003 for Klt, *P=.042 for 25(OH)D, **P=.002 for Hcy, Table 3). MSA patients had significantly higher serum Hcy level than controls (15.2±5.95 vs 10.4±3.09, **P=.005, Table 3). Additionally, the Klt and 25(OH)D levels exhibited a downward trend in MSA, when compared to the healthy subjects (2.41±1.51 vs 3.06±1.28, ***P<.001 for Klt; 39.9±15.7 vs 62.7±19.6, **P<.007 for 25(OH)D; Table 3). However, no significant differences were observed in Klt, 25(OH)D or Hcy levels among MSA and PD patients (Table 3).
After dividing the data by gender, the male and female MSA patients showed significantly higher serum level of Hcy, compared to those of the male/female healthy subjects (19.2±4.92 vs 12.6±3.80, ***P<.001 for male; 15.6±5.53 vs 11.8±3.25, **P=.002 for female, Table 4, Figure 1C). Interestingly, significantly lower plasma Klt levels were observed in MSA male/female patients compared to male/female healthy subjects (2.29±1.07 vs 3.19±1.10, **P=.007 for male; 2.53±1.26 vs 3.01±1.56, *P=.029 for female, Table 4, Figure 1A). Furthermore, the male and female MSA patients exhibited lower serum level of 25(OH)D, compared to normal male/female patients (34.2±14.3 vs 68.2±16.5, **P=.005 for male; 40.1±12.9 vs 61.8±15.4, **P=.009 for female, Table 4, Figure 1B). Additionally, compared with female patients, decreased serum levels of 25(OH)D were observed in male MSA patients (34.2±14.3 vs 40.1±12.9, **P=.005, Table 4); while the male MSA patients exhibited higher serum levels of Hcy compared to female patients with MSA (19.2±4.92 vs 15.6±5.53, **P=.003, Table 4). However, the male and female MSA patients exhibited similar trend for the serum levels of Klt (Table 4).
Table 4.
Comparison of Klt, 25(OH)D, and Hcy between MSA patients and healthy controls based on gender
| Variable | MSA (mean±SD) | Control (mean±SD) | MSA vs control | MSA (male) vs (female) | |||
|---|---|---|---|---|---|---|---|
| Value | P | Value | P | ||||
| Klt | Male | 2.29±1.07 | 3.19±1.10 | −3.86 | 0.007d , a | 0.98 | 0.438 |
| Female | 2.53±1.26 | 3.01±1.56 | −2.54 | 0.029c , b | |||
| 25(OH)D | Male | 34.2±14.3 | 68.2±16.5 | −2.78 | 0.005d , b | 2.86 | 0.005d , b |
| Female | 40.1±12.9 | 61.8±15.4 | −2.12 | 0.009d , a | |||
| Hcy | Male | 19.2±4.92 | 12.6±3.80 | 3.57 | <0.001e , a | 3.24 | 0.003d , b |
| Female | 15.6±5.53 | 11.8±3.25 | 3.02 | 0.002d , b | |||
Student's t test.
Mann‐Whitney U test.
P<0.05.
P<0.01.
P<0.001.
Figure 1.
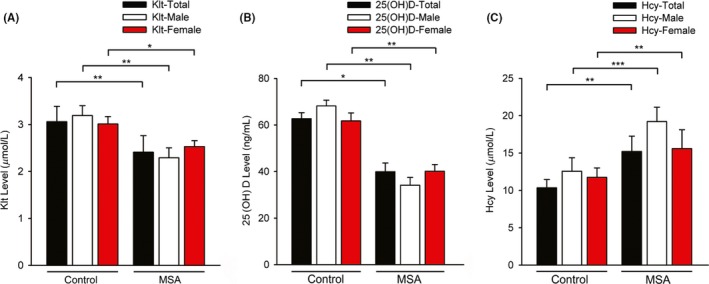
Comparison of Klt, 25(OH)D, and Hcy levels between MSA patients and healthy controls, according to gender. (A) Comparison of Klt level between control and MSA groups. MSA (male) vs control (male), **P=0.007; MSA (female) vs control (female), *P=0.029. (B) Comparison of 25(OH)D level between control and MSA groups. MSA (male) vs control (male), **P=0.005; MSA (female) vs control (female), **P=0.009. (C) Comparison of Hcy levels between control and MSA groups. **MSA (male) vs control (male), ***P<0.001; MSA (female) vs control (female), **P=0.002; MSA (male) vs MSA (female), **P=0.003
3.3. Comparison of Klt/25(OH)D/Hcy between MSA and PD patients based on genders
When focusing on the differences between MSA and PD patients, we found serum Klt, 25(OH)D and Hcy levels in male MSA patients were significantly higher than those in male PD patients (2.29±1.07 vs 1.71±1.05, *P=.044 for Klt; 34.2±14.3 vs 29.1±15.0, *P=.047 for 25(OH)D; 19.2±4.92 vs 15.2±5.48, **P=.002 for Hcy), while Klt, 25(OH)D, and Hcy levels displayed no significant differences between female MSA and female PD patients (Table 5).
Table 5.
Comparison of Klt, 25(OH)D, and Hcy between MSA and PD patients according to genders
| Variable | MSA (mean±SD) | PD (mean±SD) | MSA vs PD | ||
|---|---|---|---|---|---|
| Value | P | ||||
| Klt | |||||
| Gender | Male | 2.29±1.07 | 1.71±1.05 | 2.05 | 0.044c , a |
| Female | 2.53±1.26 | 2.41±1.98 | 0.29 | 0.774 | |
| 25(OH)D | |||||
| Gender | Male | 34.2±14.3 | 29.1±15.0 | 2.00 | 0.047c , b |
| Female | 40.1±12.9 | 37.2±12.7 | 0.94 | 0.349 | |
| Hcy | |||||
| Gender | Male | 19.2±4.92 | 15.2±5.48 | 3.17 | 0.002d , a |
| Female | 15.1±5.53 | 14.1±5.19 | 0.75 | 0.455 | |
Student's t test.
Mann‐Whitney U test.
P<0.05.
P<0.01.
3.4. Correlations between Klt, 25(OH)D and HCY Levels and clinical assessing scales
As shown in Table 6, we performed Spearman's correlation analysis to identify the relationship between the inflammatory‐related cytokines and clinical parameters in MSA patients. The serum Klt levels were negatively correlated with age, UMSARS (total), UMSARS‐I, Schwab & England, H&Y and Webster and positively correlated with MMSE. In terms of the burdens of NMSS, the serum Klt levels were negatively correlated with NMS burdens of mood, attention, cardiovascular domain, and urinal domain. Meanwhile, plasma 25(OH)D was positively correlated MMSE and reversely correlated with UMSARS (total), UMSARS‐II, H&Y, Schwab & England and Webster in MSA patients. For the domains of NMSS, negative correlations were also observed between 25(OH)D and NMS burden of mood as well as attention. Additionally, the serum Hcy levels were positively correlated with H&Y, NMS burden of cardiovascular domain, and negatively correlated with MMSE. Table 6 also reveals that 19 patients (MSA‐P subtype) were administrated with l‐dopa. Nevertheless, there was no significantly correlation between l‐dopa and serum levels of Klt, 25(OH)D or Hcy. Our results demonstrated that treatment of l‐dopa in MSA patients has no impact on the validation of Klt/25(OH)D/Hcy evaluation for MSA.
Table 6.
Correlations between clinical parameters and H&Y, MMSE assessing scales
| Variable | Klt | 25(OH)D | Hcy | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Age | −0.22b | 0.001 | 0.13 | 0.647 | 0.15 | 0.560 |
| UMSARS (total) | −0.13b | 0.007 | −0.39b | 0.001 | 0.22 | 0.063 |
| UMSARS (I) | −0.21a | 0.048 | −0.20 | 0.355 | 0.27 | 0.256 |
| UMSARS (II) | −0.04 | 0.745 | −0.26a | 0.036 | 0.18 | 0.247 |
| UMSARS (IV) | −0.05 | 0.445 | −0.04 | 0.445 | 0.22 | 0.263 |
| H&Y | −0.32b | 0.002 | −0.40b | 0.001 | 0.35a | 0.019 |
| MMSE | 0.39b | 0.005 | 0.42b | 0.008 | −0.38b | 0.008 |
| PDSS | −0.06 | 0.642 | −0.04 | 0.88 | −0.03 | 0.873 |
| NMSS | −0.05 | 0.531 | −0.05 | 0.462 | 0.04 | 0.978 |
| Cardiovascular | −0.31a | 0.016 | −0.20 | 0.355 | 0.36a | 0.015 |
| Sleep/fatigue | −0.48 | 0.084 | −0.04 | 0.526 | 0.53 | 0.063 |
| Mood | −0.36a | 0.018 | −0.38a | 0.014 | 0.09 | 0.892 |
| Perceptual problem | −0.21 | 0.198 | −0.04 | 0.831 | 0.07 | 0.263 |
| Attention/memory | −0.12a | 0.014 | −0.34a | 0.024 | 0.03 | 0.850 |
| Gastrointestinal | −0.22 | 0.193 | −0.11 | 0.085 | −0.18 | 0.641 |
| Urinary | −0.34a | 0.034 | −0.21 | 0.198 | 0.04 | 0.985 |
| Sexual function | −0.23 | 0.187 | −0.26 | 0.145 | 0.04 | 0.846 |
| Miscellaneous | −0.03 | 0.343 | −0.15 | 0.382 | 0.01 | 0.902 |
| Schwab & England | −0.37a | 0.012 | −0.39b | 0.007 | 0.01 | 0.168 |
| Webster | −0.36a | 0.015 | −0.29a | 0.025 | 0.10 | 0.485 |
| Daily dose of l‐Dopa (mg) | −0.16 | 0.230 | −0.01 | 0.627 | 0.13 | 0.353 |
r s, Spearman's rank correlation coefficient; H&Y, the modified Hoehn and Yahr staging scale; UMSARS, Unified Multiple system atrophy Rating Scale; PDSS, PD Sleep Scale; Webster, Webster Scale; Schwab & England, the Schwab & England Activities of Daily Living Scale; MMSE, mini‐mental state examination; NMSS, nonmotor symptoms scale.
P<0.05.
P<0.01.
3.5. The ROC analysis of Klt, 25(OH)D and Hcy in the diagnosis of MSA
We performed a ROC curve to examine whether Klt, 25(OH)D, and Hcy could discriminate MSA patients from normal subjects. An area under the curve (AUC) value of .79 (***P<.001, Figure 2A) was showed in the ROC of Klt analysis; the cutoff was at 2.68 μmol/L (sensitivity: 57%, specificity: 83%). The AUC of Hcy was .66 (***P<.001, Figure 2C); the cutoff was at 18.4 μmol/L (sensitivity: 64%, specificity: 79%). Due to the AUC of 25(OH)D was .56 (P=.230, Figure 2B), serum 25(OH)D alone could not differentiate the MSA patients from normal subjects. Furthermore, the AUC of the combination of Klt, 25(OH)D, and Hcy was .87 (***P<.001, Figure 2D), indicating that the combination of Klt, 25(OH)D and Hcy had the best performing ROC curve for distinguishing MSA patients from healthy subjects (cut off: .51, sensitivity: 74%, specificity: 65%).
Figure 2.
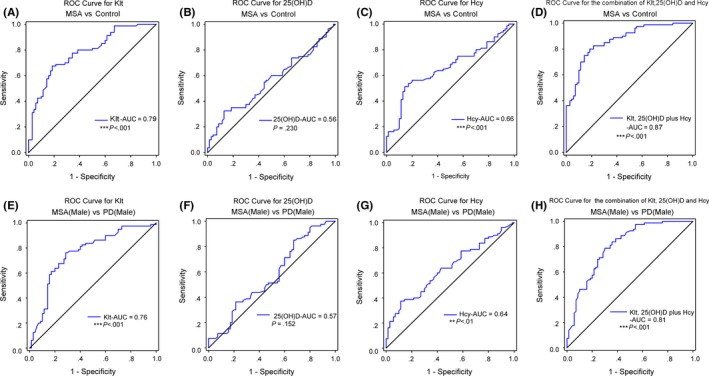
The ROC analysis of Klt, 25(OH)D, and Hcy in diagnosis of MSA and discrimination of male MSA patients from male PD patients. (A–D) The ROC curves of Klt, 25(OH)D, and Hcy in distinguishing MSA patients from normal subjects. The AUC of ROC curves for (A) Klt, (B) 25(OH)D, and (C) Hcy was .79 (***P<0.001), .56 (P=0.230), and .66 (***P<0.001), respectively. The AUC of (D) Klt+25(OH)D+Hcy was .87 (***P<0.001); (E–H) The ROC curves of Klt, 25(OH)D and Hcy in differentiating male MSA patients from male PD patients. The AUC of ROC curves for (E) Klt, (F) 25(OH)D, and (G) Hcy was .76 (***P<0.001), .57 (P=0.152), and .64 (**P<0.01), respectively. The AUC of (H) Klt+25(OH)D+Hcy was .81 (***P<0.001)
3.6. The ROC analysis of Klt, 25(OH)D and Hcy in distinguishing male MSA patients from male PD patients
We found serum Klt, 25(OH)D, and Hcy levels in male MSA patients were significantly higher than those in male PD patients (Table 5), which allowed distinguishing male MSA patients from male PD patients by ROC curves. An area under the curve (AUC) value of .76 (***P<.001, Figure 2E) was showed in the ROC of Klt analysis; the cutoff was at 2.01 μmol/L (sensitivity: 65%, specificity: 73%). The AUC of Hcy was .64 (**P<.01, Figure 2G); the cutoff was at 17.3 μmol/L (sensitivity: 55%, specificity: 69%). Due to the AUC of 25(OH)D was .57 (P=.125, Figure 2F), serum 25(OH)D alone could not differentiate male MSA patients from male PD patients. Furthermore, the AUC of the combination of Klt, 25(OH)D and Hcy was .81 (***P<.001, Figure 2H), indicating that the combination of Klt, 25(OH)D and Hcy had the best performing ROC curve for distinguishing male MSA patients from male PD patients (cut off: .45, sensitivity: 71%, specificity: 78%).
4. DISCUSSION
Several interesting results were found in our study. Firstly, we found MSA patients, particularly in male patients, had decreased serum levels of Klt and 25(OH)D and increased serum levels of Hcy compared to healthy subjects. Secondly, significant correlations were observed between serum Klt, 25(OH)D, and Hcy levels and the severity of MSA, including cognition impairment, motor dysfunction, mood/cardiovascular disorder. Thirdly, the ROC curve analysis showed that the combination of Klt, 25(OH)D, and Hcy significantly improves the diagnostic ability to discriminate MSA patients from healthy subjects, as well as distinguishing male MSA patients from male PD patients. As far as we know, this is the first study to explore changes in the serum levels of Klt/25(OH)D/Hcy in MSA patients and to evaluate the potential relationships between serum Klt/25(OH)D/Hcy levels and the severity of MSA. Our findings suggested that serum Klt, 25(OH)D, and Hcy may underlie the pathophysiological mechanisms of MSA and could be used to evaluate the disease severity in MSA.
The pathogenic mechanisms underlying MSA remain unclear. Thus, it is important to investigate potential biomarkers which may be implicated in the pathogenesis of MSA and could be used to assess the outcomes and severity of the disease. Several lines of evidence showed that inflammatory responses occurred during dopaminergic neurons degeneration, while anti‐inflammatory activities are also triggered at the mean time.10, 33, 34, 35 Previous studies indicated that inflammatory mediators including Klt/25(OH)D/Hcy may be used as predictors to evaluate the prevalence of PD and AD.23, 36, 37, 38, 39, 40 Therefore, we conducted this study to investigate whether these inflammatory mediators could also be potential and reliable biomarkers for MSA evaluation. In the present study, we explored the correlation between serum of Klt, 25(OH)D, Hcy, and disease severity. We also identified the predictive value of these inflammatory mediators and their combination for distinguishing patients with MSA from healthy controls.
Until now, the role of Klt in the central nervous system remains unclear. It has been documented that the absence of Klt in mice leads to an increase of oxidative stress and inflammatory response in the central nervous system, leading to motor neuron degeneration and impairment of cognitive function.13, 41, 42 Our data indicated serum klt levels significantly differ in patients with PD patients, MSA patients, and healthy controls. The Klt levels exhibited a downward trend in MSA, when compared to the healthy controls. Our study is contrast to the alteration of serum klt levels in multiple sclerosis (MS),43 shown by decreased levels of Klt in MSA/PD and increased levels in MS. Significantly lower plasma Klt levels in MSA/PD patients implied that Klt may attribute to the pathogenesis of the disease.16, 44 Recently, 25(OH)D has gained much attention due to its crucial role in the pathological mechanism of PD.21, 22, 28 Other reports have revealed that 25(OH)D deficiency may lead to a high risk of developing MS.28, 45, 46 Consistent with these reports, we found that in MSA patients exhibited lower serum 25(OH)D levels than healthy controls. Interestingly, MSA and PD patients had no significant difference in serum Klt and 25(OH)D levels. These results were in accordance with the view that MSA share neuropathogeneses similarities with PD. Our data further suggest that Klt and 25(OH)D may act as a neuroprotective role in MSA and PD via attenuating inflammation.14, 15, 20, 21 Therefore, in the assessment and diagnosis of MSA/PD, klt and 25(OH)D were found to be suitable in this study.
Previous studies have showed that serum levels of Hcy have a close correlation with dopaminergic neurodegeneration in AD and PD.47, 48 Similar to these reports, significant higher plasma Hcy levels were observed in MSA and PD patients compared with healthy subjects. The high concentrations of Hcy imply that Hcy may participate in the pathological mechanism of MSA and PD. To our knowledge, Hcy involves in the pathogenesis of MSA via mediating inflammation response.49, 50 Therefore, Hcy may be a possible modifier in MSA and PD.
After dividing the subjects based on gender, we observed several interesting findings in MSA patients (Table 4; Figure 2). The male MSA patients showed lower concentrations of 25(OH)D and higher serum Hcy levels compared to female MSA patients. This finding suggests that 25(OH)D and Hcy may be more valuable in assessing males MSA patients. The physiological associations among 25(OH)D and Hcy and sex‐specific hormones metabolism may be partly contributed to a higher risk of developing MSA in men.38, 51, 52 We also observed the male MSA patients exhibited lower serum klt compared to female MSA patients; however, this downward trend was no statistically significant. We then examined the differences between MSA and PD patients. Despite the three serum biomarkers exhibited no difference between the two groups, serum Klt, 25(OH)D and Hcy levels in male MSA patients were higher than those in male PD patients (Table 5), indicating the difference of Klt, 25(OH)D and Hcy between MSA and PD patients was more significant in men. Subsequently, serum Klt, 25(OH)D and Hcy levels may be potential indicators for differentiating between MSA from PD patients in men.53, 54
In MSA patients, Klt and 25(OH)D levels were inversely correlated with age, UMSARS, H&Y, Schwab & England, Webster and NMSS (mood and attention/memory) and a positive correlation with MMSE. These results suggest that Klt and 25(OH)D influence the motor/nonmotor function and cognitive impairment of MSA patients. Previous studies have indicated that Klt may increase risk of several age‐dependent diseases.13, 55 Semba et al.56 indicated that the decreased serum level of Klt was independently correlated to weaker muscle strength. Kuro‐o et al.13 demonstrated that aging‐associated disorders occurred earlier in Klt‐knockout mice compared with wild‐type mice, for example, dementia, decreased bone mineral density, sterility. Similar to this report, we observed that the serum Klt was inversely correlated to age/H&Y/UMSARS/UMSARS‐I/Schwab & England/Webster in the MSA group, further indicating that low serum level of Klt in MSA patients may attribute to motor dysfunction and aging. Correlations were also previously found between lower serum 25(OH)D and higher total UPDRS scores in PD patients at baseline and during follow‐up.38 A double‐blind trial conducted by Suzuki et al.57 demonstrated that 25(OH)D3 supplementation may participate in protecting PD patients from deterioration of the H&Y stage. Similarly, we found that 25(OH)D and total H&Y/UMSARS/UMSARS‐II/ADL/Webster scale scores were inversely correlated, which demonstrated that 25(OH)D may contribute to the deterioration of motor dysfunction and MSA progress. In addition, we observed that Klt and 25(OH)D are statistically significant correlated with NMS burdens of mood in MSA patients (Table 6). Specifically, Klt and 25(OH)D have a negative association with mood and attention/memory, and a positive correlation with MMSE in MSA patients, further indicating that mood and attention/memory disorders may be targets for assessment by serum levels of Klt and 25(OH)D. In agreement with our notions, several studies have also reported that Klt deficiency and a low level of 25(OH)D was associated with mood, memory deterioration, and cognitive dysfunction.57, 58 Consistent with previous reports,59, 60 we also found that plasma levels of Klt have a significant negative correlation with the cardiovascular and urinary domains in MSA patients. Our results suggest that Klt level may be used as a potential predictor for cardiovascular and urinary disease. Interestingly, Hcy was positively correlated with H&Y and cardiovascular domain of NMSS scores and negatively correlated with MMSE score in MSA patients, implying that Hcy plasma levels might be used to evaluate the severity and the cognitive status of MSA patients.26, 61 Our findings demonstrated that Klt, 25(OH)D, and Hcy may attribute to the prevalence and deterioration of MSA.
Our ROC curve data indicated an acceptable sensitivity and specificity for Klt, 25(OH)D and Hcy in distinguishing MSA patients from healthy subjects, as well as distinguishing male MSA patients from male PD patients. Klt displayed more reliable diagnostic ability when compared to 25(OH)D and Hcy (Figure 2). Notably, the combination of Klt, 25(OH)D and Hcy exhibited a better distinguishing ability in distinguishing MSA patients from healthy subjects and differentiating male MSA patients from male PD patients, compared with Klt, 25(OH)D, or Hcy alone. However, serum levels of Klt, 25(OH)D, and Hcy still have a significant overlap between MSA and healthy controls. Our results imply that Klt, 25(OH)D, or Hcy alone is not reliable for detecting MSA or distinguishing MSA patients from PD patients.
Our study has several limitations: (i) Sample size of subjects was small (53 MSA, 65 PD, and 62 healthy controls); (ii) due to the limitation of the cross‐sectional study, longitudinal cohort studies are needed in the future to explore the alterations of the three serum biomarkers (Klt, 25(OH)D, and Hcy) during the disease progression in MSA and PD. In addition, the causal relationships among Klt, 25(OH)D, and Hcy were not established in our study, further investigation is necessary to examine their causality; (iii) the MSA patients in the early stages of H&Y, with sufficient cognitive ability (MMSE score: 25.7±3.27) were recruited; (iv) genetic modifiers, for example, the Klotho genotype and folate or vitamin D medication were not examined in our study; this relatively narrowed the sample size of subjects in our study. Therefore, due to the exploratory nature of our study, comprehensive studies are necessary to conduct in the future.
In conclusion, our study supports the hypothesis that neuroinflammation participate in the pathogenesis of MSA. The inflammatory mediator Klt, 25(OH)D, and Hcy are potential biomarkers. Low serum levels of Klt and 25(OH)D and high serum levels of Hcy may have a risk for motor and nonmotor dysfunctions. Moreover,the combination of serum Klt, 25(OH)D, and Hcy exhibited a better diagnostic ability for distinguishing MSA patients from normal subjects and differentiating male MSA patients from male PD patients. Based on our findings, we propose that serum Klt, 25(OH)D, and Hcy may underlie the pathophysiological mechanisms of MSA and could be used to evaluate the severity of these diseases.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No: 81600206), International Science & Technology Cooperation Program of Guangdong of China (Grant No: 20160903), Social Developing Project of Guangdong Province in China (Grant No: 2014SC107), and the Natural Science Foundations of Guangdong of China (Grant No: 2016A030310140) to XXL.
Guo Y, Zhuang X‐D, Xian W‐B, et al. Serum Klotho, vitamin D, and homocysteine in combination predict the outcomes of Chinese patients with multiple system atrophy. CNS Neurosci Ther. 2017;23:657–666. 10.1111/cns.12711
The first two authors contributed equally to this work.
Contributor Information
Ling Chen, Email: chenl2@mail.sysu.edu.cn.
Xin‐Xue Liao, Email: liaoxinx@mail.sysu.edu.cn.
REFERENCES
- 1. Stefanova N, Bucke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172‐1178. [DOI] [PubMed] [Google Scholar]
- 2. Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94‐98. [DOI] [PubMed] [Google Scholar]
- 3. Kaufman E, Hall S, Surova Y, Widner H, Hansson O, Lindqvist D. Proinflammatory cytokines are elevated in serum of patients with multiple system atrophy. PLoS ONE. 2013;8:e62354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wen XD, Li HF, Wang HX, Ni W, Dong Y, Wu ZY. Mutation analysis of COQ2 in Chinese patients with cerebellar subtype of multiple system atrophy. CNS Neurosci Ther. 2015;21:626‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marques TM, Kuiperij HB, Bruinsma IB, et al. MicroRNAs in cerebrospinal fluid as potential biomarkers for Parkinson's disease and multiple system atrophy. Mol Neurobiol. 2016. 10.1007/s12035-016-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gemma C. Neuroimmunomodulation and aging. Aging Dis. 2010;1:169‐172. [PMC free article] [PubMed] [Google Scholar]
- 7. Dong J, Li S, Mo JL, Cai HB, Le WD. Nurr1‐based therapies for Parkinson's disease. CNS Neurosci Ther. 2016;22:351‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mogi M, Harada M, Kondo T, et al. Interleukin‐1 beta, interleukin‐6, epidermal growth factor and transforming growth factor‐alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147‐150. [DOI] [PubMed] [Google Scholar]
- 9. Song Y, Chen X, Wang LY, Gao W, Zhu MJ. Rho kinase inhibitor fasudil protects against beta‐amyloid‐induced hippocampal neurodegeneration in rats. CNS Neurosci Ther. 2013;19:603‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bachstetter AD, Van Eldik LJ. The p38 MAP kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010;1:199‐211. [PMC free article] [PubMed] [Google Scholar]
- 11. Paredes D, Acosta S, Gemma C, Bickford PC. Role of TNFalpha induced inflammation in delay eyeblink conditioning in young and aged rats. Aging Dis. 2010;1:191‐198. [PMC free article] [PubMed] [Google Scholar]
- 12. Nitta K, Nagano N, Tsuchiya K. Fibroblast growth factor 23/Klotho axis in chronic kidney disease. Nephron Clin Pract. 2014;128:1‐10. [DOI] [PubMed] [Google Scholar]
- 13. Kuro‐o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45‐51. [DOI] [PubMed] [Google Scholar]
- 14. Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG‐I‐mediated senescence‐associated inflammation. Nat Cell Biol. 2011;13:254‐262. [DOI] [PubMed] [Google Scholar]
- 15. Zhao Y, Banerjee S, Dey N, et al. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907‐1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brobey RK, German D, Sonsalla PK, et al. Klotho protects dopaminergic neuron oxidant‐induced degeneration by modulating ASK1 and p38 MAPK signaling pathways. PLoS ONE. 2015;10:e0139914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393‐2403. [DOI] [PubMed] [Google Scholar]
- 18. Kuro‐o M. Klotho and the aging process. Korean J Intern Med. 2011;26:113‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sawires HK, Essam RM, Morgan MF, Mahmoud RA. Serum Klotho: relation to fibroblast growth factor‐23 and other regulators of phosphate metabolism in children with chronic kidney disease. Nephron. 2015;129:293‐299. [DOI] [PubMed] [Google Scholar]
- 20. Ross GW, Petrovitch H, Abbott RD. Serum vitamin D and risk of Parkinson's disease. Mov Disord. 2016;31:933‐935. [DOI] [PubMed] [Google Scholar]
- 21. Shrestha S, Lutsey PL, Alonso A, Huang X, Mosley TH Jr, Chen H. Serum 25‐hydroxyvitamin D concentrations in Mid‐adulthood and Parkinson's disease risk. Mov Disord. 2016;31:972‐978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J, Yang D, Yu Y, Shao G, Wang Q. Vitamin D and sunlight exposure in newly‐diagnosed Parkinson's disease. Nutrients. 2016;8:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kosakai A, Ito D, Nihei Y, et al. Degeneration of mesencephalic dopaminergic neurons in Klotho mouse related to vitamin D exposure. Brain Res. 2011;1382:109‐117. [DOI] [PubMed] [Google Scholar]
- 24. Pelham CJ, Drews EM, Agrawal DK. Vitamin D controls resistance artery function through regulation of perivascular adipose tissue hypoxia and inflammation. J Mol Cell Cardiol. 2016;98:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez‐Oroz MC, Lage PM, Sanchez‐Mut J, et al. Homocysteine and cognitive impairment in Parkinson's disease: a biochemical, neuroimaging, and genetic study. Mov Disord. 2009;24:1437‐1444. [DOI] [PubMed] [Google Scholar]
- 26. Shimomura T, Anan F, Masaki T, et al. Homocysteine levels are associated with hippocampus volume in type 2 diabetic patients. Eur J Clin Invest. 2011;41:751‐758. [DOI] [PubMed] [Google Scholar]
- 27. Chen D, Wei X, Zou J, et al. Contra‐directional expression of serum homocysteine and uric acid as important biomarkers of multiple system atrophy severity: a cross‐sectional study. Front Cell Neurosci. 2015;9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smolders J, Menheere P, Thewissen M, et al. Regulatory T cell function correlates with serum 25‐hydroxyvitamin D, but not with 1,25‐dihydroxyvitamin D, parathyroid hormone and calcium levels in patients with relapsing remitting multiple sclerosis. J Steroid Biochem Mol Biol. 2010;121:243‐246. [DOI] [PubMed] [Google Scholar]
- 29. Huang RF, Huang SM, Lin BS, Wei JS, Liu TZ. Homocysteine thiolactone induces apoptotic DNA damage mediated by increased intracellular hydrogen peroxide and caspase 3 activation in HL‐60 cells. Life Sci. 2001;68:2799‐2811. [DOI] [PubMed] [Google Scholar]
- 30. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kerley CP, Hutchinson K, Bolger K, McGowan A, Faul J, Cormican L. Serum vitamin D is significantly inversely associated with disease severity in Caucasian adults with obstructive sleep apnea syndrome. Sleep. 2016;39:293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wyss‐Coray T, Mucke L. Inflammation in neurodegenerative disease–a double‐edged sword. Neuron. 2002;35:419‐432. [DOI] [PubMed] [Google Scholar]
- 34. Reese LC, Taglialatela G. Neuroimmunomodulation by calcineurin in aging and Alzheimer's disease. Aging Dis. 2010;1:245‐253. [PMC free article] [PubMed] [Google Scholar]
- 35. Sekiyama K, Sugama S, Fujita M, et al. Neuroinflammation in Parkinson's disease and related disorders: a lesson from genetically manipulated mouse models of alpha‐synucleinopathies. Parkinsons Dis. 2012;2012:271732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dubal DB, Zhu L, Sanchez PE, et al. Life extension factor Klotho prevents mortality and enhances cognition in hAPP transgenic mice. J Neurosci. 2015;35:2358‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fyfe I. Parkinson disease. Reduced level of dietary vitamin D is associated with PD. Nat Rev Neurol. 2015;11:68. [DOI] [PubMed] [Google Scholar]
- 38. Ding H, Dhima K, Lockhart KC, et al. Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology. 2013;81:1531‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non‐motor symptoms in patients with Parkinson's disease ‐ correlations with inflammatory cytokines in serum. PLoS ONE. 2012;7:e47387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shtilbans A, Henchcliffe C. Biomarkers in Parkinson's disease: an update. Curr Opin Neurol. 2012;25:460‐465. [DOI] [PubMed] [Google Scholar]
- 41. Wang YA. Klotho, the long sought‐after elixir and a novel tumor suppressor? Cancer Biol Ther. 2006;5:20‐21. [DOI] [PubMed] [Google Scholar]
- 42. Kuro‐o M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ellidag HY, Yilmaz N, Kurtulus F, et al. The Three Sisters of fate in multiple sclerosis: Klotho (Clotho), fibroblast growth factor‐23 (Lachesis), and vitamin D (Atropos). Ann Neurosci. 2016;23:155‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeldich E, Chen CD, Colvin TA, et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014;289:24700‐24715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harandi AA, Harandi AA, Pakdaman H, Sahraian MA. Vitamin D and multiple sclerosis. Iran J Neurol. 2014;13:1‐6. [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang HL, Wu J. Role of vitamin D in immune responses and autoimmune diseases, with emphasis on its role in multiple sclerosis. Neurosci Bull. 2010;26:445‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zoccolella S, dell'Aquila C, Specchio LM, Logroscino G, Lamberti P. Elevated homocysteine levels in Parkinson's Disease: is there anything besides L‐dopa treatment? Curr Med Chem. 2010;17:213‐221. [DOI] [PubMed] [Google Scholar]
- 48. Ray L, Khemka VK, Behera P, et al. Serum homocysteine, dehydroepiandrosterone sulphate and lipoprotein (a) in Alzheimer's disease and vascular dementia. Aging Dis. 2013;4:57‐64. [PMC free article] [PubMed] [Google Scholar]
- 49. Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476‐483. [DOI] [PubMed] [Google Scholar]
- 50. Xie Y, Feng H, Peng S, Xiao J, Zhang J. Association of plasma homocysteine, vitamin B12 and folate levels with cognitive function in Parkinson's disease: a meta‐analysis. Neurosci Lett. 2017;636:190‐195. [DOI] [PubMed] [Google Scholar]
- 51. Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Saaksjarvi K, Heliovaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee JE, Jacques PF, Dougherty L, et al. Are dietary choline and betaine intakes determinants of total homocysteine concentration? Am J Clin Nutr. 2010;91:1303‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patel MS, Donaldson AV, Lewis A, et al. Klotho and smoking–an interplay influencing the skeletal muscle function deficits that occur in COPD. Respir Med. 2016;113:50‐56. [DOI] [PubMed] [Google Scholar]
- 54. Ramsey JM, Cooper JD, Bot M, et al. Sex differences in serum markers of major depressive disorder in the Netherlands study of depression and anxiety (NESDA). PLoS ONE. 2016;11:e0156624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thal DR, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. 2004;2004:pe26. [DOI] [PubMed] [Google Scholar]
- 56. Semba RD, Cappola AR, Sun K, et al. Relationship of low plasma Klotho with poor grip strength in older community‐dwelling adults: the InCHIANTI study. Eur J Appl Physiol. 2012;112:1215‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suzuki M, Yoshioka M, Hashimoto M, et al. Randomized, double‐blind, placebo‐controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr. 2013;97:1004‐1013. [DOI] [PubMed] [Google Scholar]
- 58. Paroni G, Seripa D, Fontana A, et al. Klotho gene and selective serotonin reuptake inhibitors: response to treatment in late‐life major depressive disorder. Mol Neurobiol. 2017;54:1340. [DOI] [PubMed] [Google Scholar]
- 59. Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol. 2014;10:268‐278. [DOI] [PubMed] [Google Scholar]
- 60. Lim K, Lu TS, Molostvov G, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243‐2255. [DOI] [PubMed] [Google Scholar]
- 61. Bjelland I, Tell GS, Vollset SE, Refsum H, Ueland PM. Folate, vitamin B12, homocysteine, and the MTHFR 677C‐>T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60:618‐626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


