Summary
Aims
The purpose of this study was to explore the clinical significance of telomerase reverse transcriptase (TERT) promoter mutations in gliomas.
Methods and results
We used DNA sequencing data to analyze 887 gliomas for TERT promoter mutations based on histological and genetic backgrounds. TERT promoter mutations were detected in 39.6% of low‐grade gliomas, 40.3% of anaplastic gliomas, 44.7% of primary glioblastomas, 29.4% of secondary glioblastomas, and in 29.7% of Proneural, 38.6% of Neural, 41.8% of Classical, and 41.6% of Mesenchymal subtypes. Frequency of C250T mutation in recurrent gliomas was approximately half that in newly diagnosed gliomas. TERT exhibited improved prognosis when co‐occurred with isocitrate dehydrogenase 1 (IDH1) and 1p19q alteration, but experienced inverse survival in the Mesenchymal subtype or tumor protein p53 (TP53) and epidermal growth factor receptor (EGFR) alteration. Furthermore, the five subtypes were classified based on the prognostic impact of the TERT mutation with different genetic backgrounds of glioma.
Conclusion
We describe the TERT promoter mutation spectrum according to the histological, genetic, and molecular subtypes of glioma, which may aid in glioma subtype classification and have clinical implications.
Keywords: biomarker, classification, glioma, prognosis, telomerase reverse transcriptase
1. INTRODUCTION
Glioma is the most common and malignant primary brain tumor in adults.1 Telomerase is considered a tumor cell “clock,” maintaining telomere length and immortalizing cells. Recent studies have suggested that telomerase reverse transcriptase (TERT) promoter mutations (C228T, C250T) are common in primary glioblastoma (pGBM), but occur in only a small fraction of astrocytoma and secondary GBM (sGBM), and that patients with glioma and TERT promoter mutation have poorer prognoses than those with wild‐type TERT.2 Notably, TERT promoter mutations are significantly inversely correlated with IDH1/2 mutations.3, 4, 5
The goal of this study was to determine the clinical significance of TERT promoter mutations in biomarker‐based classification of malignant gliomas. For this purpose, we used DNA sequencing data to screen 887 gliomas for TERT promoter mutations using nested PCR and correlated them with the clinical data, molecular subtypes, and other genetic alterations in a Chinese population cohort. Our results reveal that TERT promoter mutations may contribute significantly to biomarker‐based classification of malignant gliomas.
2. MATERIALS AND METHODS
2.1. Tumor samples
A total of 887 samples from the Chinese Glioma Genome Atlas (CGGA) were included in this study, and they comprised 203 astrocytomas, 37 anaplastic astrocytomas, 70 oligodendrogliomas, 19 anaplastic oligodendrogliomas, 225 oligoastrocytomas, 83 anaplastic oligoastrocytomas, 199 pGBMs, and 51 sGBMs. To ensure the accuracy of pathological diagnosis, resected specimens were flash‐frozen in liquid nitrogen and stored at −80°C until DNA extraction, and graded by two neuropathologists according to the 2007 World Health Organization (WHO) Classification of Tumours of the Central Nervous System and Scherer.6, 7 Only samples with >80% tumor cells were selected. All samples were obtained by surgical resection. All patients provided written informed consent, and the ethics committees of the participating hospitals approved the study.
2.2. Assessment of TERT promoter mutation and other genetic alteration statuses
Genomic DNA was extracted from the flash‐frozen tumor tissues using a QIAamp DNA Mini Kit (Qiagen); DNA concentration and purity were measured by an ND 8000 spectrophotometer (NanoDrop Technologies).
Nested PCR was used to amplify the TERT core promoter region harboring the C228T and C250T mutations (nucleotide numbers 1 295 228 [C228T] and 1 295 250 [C250T] from the human reference sequence GRCh37 February 2009; http://genome.ucsc.edu/). PCR products (273 bp) were produced from the first nested PCR using the forward and reverse primer sequences 5′‐GTC CTG CCC CTT CAC CTT‐3′ and 5′‐GCA CCT CGC GGT AGT GG‐3′, respectively.2 The PCR products (25 μL) were used as a DNA template for the second nested PCR using the forward and reverse primer sequences 5′‐CCG TCC TGC CCC TTC ACC‐3′ and 5′‐GGG CCG CGG AAA GGA AG‐3′, respectively. The resultant PCR products (128 bp) were used for DNA sequencing following gel extraction and purification (E.Z.N.A. Gel Extraction Kit, Omega Bio‐Tek). IDH1/2 mutation and O‐6‐methylguanine‐DNA methyltransferase (MGMT) promoter methylation were detected by DNA pyrosequencing as described previously.8 FISH analyses for EGFR and 1p19q alterations were performed as we have described previously.9 P53 and phosphatase and tensin homolog (PTEN) mutation hotspots were PCR‐amplified and Sanger‐sequenced as previously described.10, 11
2.3. Whole‐transcriptome sequencing
Whole‐transcriptome sequencing was performed as described in a previous report.12 Briefly, total RNA was isolated from disrupted and homogenized frozen tissues using an RNeasy Mini Kit (Qiagen) according to the instructions. RNA intensity was measured using a 2100 Bioanalyzer (Agilent Technologies) and filtered to construct a sequencing library based on the RNA integrity number ≥7. The subsequent steps included end repair, adapter ligation, size selection, and PCR enrichment. DNA fragment lengths were confirmed using the Agilent 2100 Bioanalyzer with a median insert size of 200 nucleotides. The libraries were sequenced on an Illumina HiSeq 2000 platform using the 101‐bp paired‐end sequencing strategy. Short sequence reads were aligned to the human reference genome (hg19 RefSeq) using the Burrows‐Wheeler Aligner (version 0.6.2‐r126).13
2.4. Statistical analysis
Kaplan‐Meier survival curves were used to describe overall survival (OS), which was defined as the time from surgery to death or last follow‐up. All reported P‐values are two‐sided, and P ≤ 0.05 was considered to indicate statistical significance. All data analysis was performed using GraphPad Prism 5.
3. RESULTS
3.1. Distribution and prognostic value of TERT promoter mutations according to histological and molecular glioma subtypes
The distribution of TERT promoter mutations (C228T, C250T) was screened in 887 gliomas using nested PCR‐based Sanger sequencing. Oligodendrogliomas had the highest mutation frequency: TERT promoter mutations were found in 75.7% of samples (53/70), followed by oligoastrocytomas (54.2%, 122/225), anaplastic oligodendrogliomas (52.63%, 10/19), pGBMs (44.7%, 89/199), anaplastic oligoastrocytomas (41%, 34/83), anaplastic astrocytomas (32.4%, 12/37), and sGBMs (29.4%, 15/51); astrocytomas had the lowest rate of mutations (10.8%, 22/203) (Figure 1A). Only patients with low‐grade (WHO grade II) gliomas and harboring TERT mutation had significantly improved survival compared to patients who did not (P = 0.0004). However, TERT mutation was not associated with better prognosis in anaplastic gliomas (WHO grade III) and glioblastomas (WHO grade IV) (Figure 1B).
Figure 1.
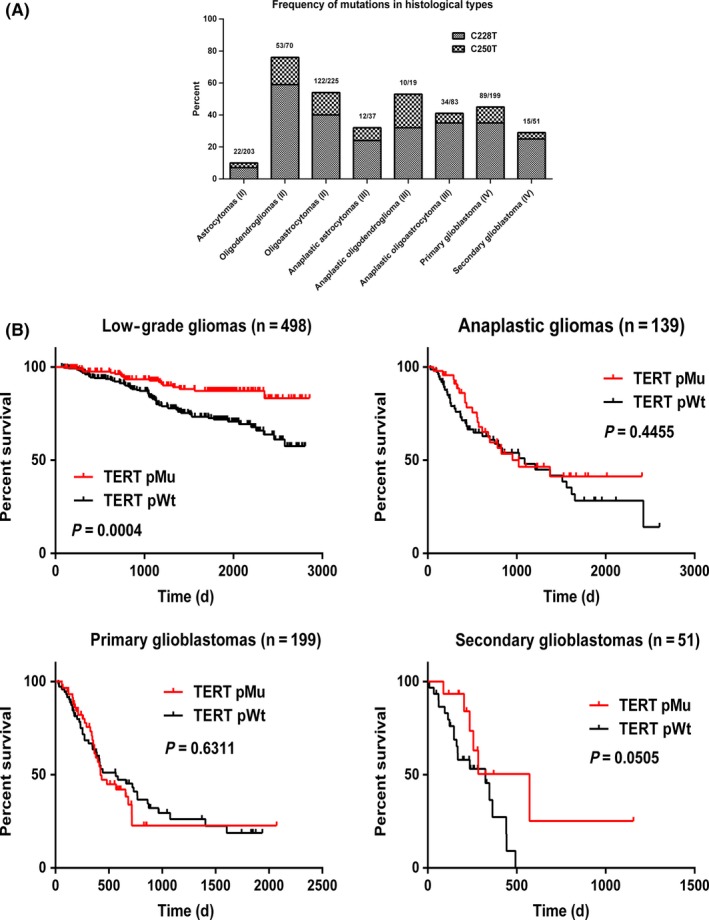
Frequency and prognostic impact of TERT mutation in glioma according to histological type. (A) Frequency of TERT mutation in glioma according to histological type. Values in parentheses indicate WHO grade. (B) OS of patients with low‐grade glioma, anaplastic glioma, pGBM, and sGBM with and without TERT mutation. pMu: patients with TERT mutation; pWt: patients with wild‐type TERT
Notably, the frequency of C250T mutation in recurrent gliomas (7/135, 5.2%) was only half that in newly diagnosed gliomas (77/752, 10.2%), and patients carrying the C250T mutation had longer survival compared to patients with C228T mutation (Figure 2A). Moreover, TERT mutation frequency, both C228T and C250T, increased with age: <19 years, 12% (3/25); 20‐39 years, 34.5% (134/389); 40‐59 years, 45% (181/402); and >60 years, 56.5% (39/69). Compared to patients with wild‐type TERT, younger patients with TERT mutation survived longer than those in their 60 seconds, but patients with TERT mutation and aged >60 years had shorter survival (Figure 2B).
Figure 2.
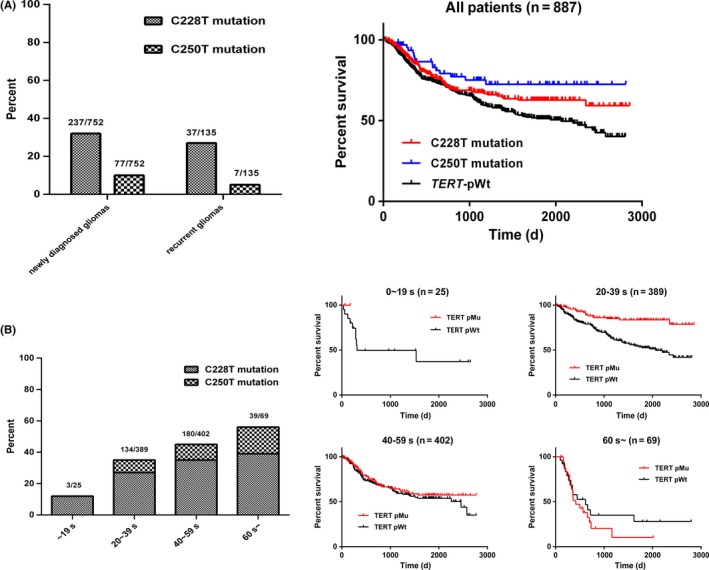
Analysis of TERT mutation in newly diagnosed and recurrent gliomas and different age groups. (A) Frequency of TERT promoter mutation in newly diagnosed and recurrent gliomas; Kaplan‐Meier curves show the survival of patients with C228T and C250T mutation and wild‐type TERT of the whole cohort. (B) Frequency and prognostic impact of TERT mutation in each age group (~19 years, 20‐39 years, 40‐59 years, ≥60 years). pMu: patients with TERT mutation; pWt: patients with wild‐type TERT
We annotated 368 samples based on the TCGA molecular subtypes using single‐sample gene set enrichment analysis (ssGSEA) in the GenePattern server (Figure 3A): 29.7% of Proneural (33/111), 38.6% of Neural (39/101), 41.8% of Classical (28/67), and 41.6% of Mesenchymal (37/89) gliomas carried TERT promoter mutations. Patients with the Classical and Neural, and Proneural subtype in particular, and carrying the TERT mutation had better prognosis than those who did not (P < 0.05), whereas significantly opposite results were obtained for patients with the Mesenchymal subtype of glioma (P < 0.05) (Figure 3B).
Figure 3.

Frequency and prognosis of TERT mutation in gliomas according to molecular subtype. (A) Frequency of TERT mutation in Proneural, Neural, Classical, and Mesenchymal gliomas. (B) OS of patients with or without TERT mutation in the four molecular subtypes of glioma. pMu: patients with TERT mutation; pWt: patients with wild‐type TERT
3.2. Combined analysis of TERT, IDH1/2, EGFR, TP53, PTEN, MGMT, and 1p19q alteration status in glioma
As shown in Figure 4, low‐grade glioma had the highest frequency of co‐occurrence with IDH1/2 mutation (87.3% of 197 TERT mutation tumors), whereas the opposite was true for GBM (11.5% of 104 TERT mutation tumors). Moreover, TERT and TP53 mutations in low‐grade and anaplastic gliomas were mutually exclusive, where only 2% and 3.6% of TERT mutation tumors harbored TP53 mutations, respectively. Additionally, 54.6% of 11 low‐grade gliomas, 71.4% of 21 GBMs, and all seven anaplastic gliomas had TERT and PTEN comutation. Similarly, TERT mutation was clustered in tumors with 1p19q LOH (59/99 low‐grade gliomas, 8/12 anaplastic gliomas) and MGMT promoter hypermethylation (25/49 low‐grade gliomas, 31/71 pGBMs).
Figure 4.
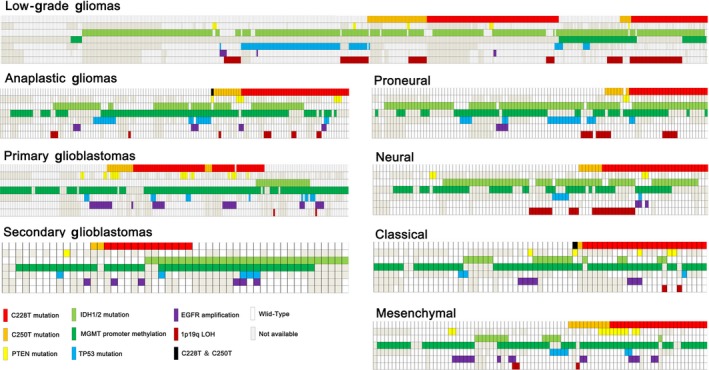
Correlations between TERT promoter mutations (C228T and C250T) and other alterations
In Proneural gliomas, 90.9% of 33 tumors with TERT mutations contained IDH1/2 mutation, whereas in the Classical and Mesenchymal subtypes, only 14.3% and 6.7% did, respectively. TERT mutation showed an inverse correlation with TP53 mutation and EGFR amplification in Proneural glioma, but co‐occurred with 1p19q LOH in the Proneural, Neural, and Classical subtypes. Only one sample in our cohort had C228T and C250T comutation.
3.3. Contributions of TERT promoter mutations to biomarker‐based classification of glioma
To assess the value of TERT mutation in the molecular classification in glioma, we separated the samples into five subtypes according to the genetic background of glioma: subtype 1, tumors with TP53 mutation; subtype 2, tumors with 1p19q LOH; subtype 3, tumors with EGFR amplification; subtype 4, tumors with IDH1/2 mutation but without 1p19q and TP53 alteration; and subtype 5, tumors with wild‐type IDH1/2, TP53, 1p19q, and EGFR. As shown in Figure 5, in patients with subtype 2 and 4 tumors, patients carrying TERT mutations had remarkably better prognosis than those who did not, whereas opposite results were obtained for patients with subtypes 1, 3, and 5 (P < 0.05). Furthermore, we merged groups with similar prognoses and found that patients with tumors bearing 1p19q LOH and TERT and IDH1/2 mutations had the best survival, whereas patients carrying TERT mutation and EGFR amplification had the worst clinical outcomes. Patients without these alterations or in whom TERT mutation and other genetic types were mutually exclusive had intermediate survival.
Figure 5.

OS of patients with or without TERT mutation according to the five glioma subtypes. pMu: patients with TERT mutation; pWt: patients with wild‐type TERT
4. DISCUSSION
Gliomas, the most common and aggressive primary brain tumors in adults, are classified as grade II, III, and IV based on histopathological and clinical criteria established by the WHO.6 However, recent studies have shown that there are some limits to this classification for exact prediction of clinical outcomes and formulation of gene target therapy strategies. For example, the prognoses of patients with the same grade gliomas and treated with the same strategy differed, partly due to the subjective opinions of neuropathologists and different tumorigenic mechanisms. Thus, a more objective classification of glioma is needed to guide diagnosis and more meticulous treatment strategies. Advances in molecular biology have found that gene alterations such as IDH1/2 mutation,14, 15, 16 1p19q LOH, EGFR amplification, and MGMT methylation are valuable for glioma diagnosis and prognosis.17, 18, 19, 20 The TERT core promoter mutations (chr5, 1 295 228 C>T and 1 295 250 C>T, C228T and C250T) were first found in melanoma and thought to represent an important tumorigenic mechanism.21, 22
In the present study, a low frequency of TERT promoter mutations (22/203, 10.8%) was detected in astrocytomas, whereas the frequency of such alterations in oligodendrogliomas was high (53/70, 75.7%), which was consistent with previous studies.3, 23, 24, 25
GBM, accounting for 60%‐70% of gliomas, is the most lethal and highest‐grade glioma in adults, with median survival being 15 months.26, 27, 28 Nonoguchi and colleagues surveyed TERT mutation in 358 GBMs and noted that such alteration was significantly more frequent in pGBM (58%) compared to the 28% in sGBM.2 Yan and coworkers also reported the remarkably high prevalence of TERT promoter mutation (83% of 78 tumors) in adult pGBM.24 In our cohort, 44.7% of pGBMs and 29.4% of sGBMs contained TERT mutations. The frequency of TERT mutation in pGBM was not as high as reported. We suspect that pGBM may evolve from relatively low‐grade glioma, which has a significantly higher prevalence rate in China than in Western countries. From the above findings, the high rate of low‐grade gliomas in China, typically astrocytoma, in which TERT mutation is rare, may be the reason for the lower frequency of TERT mutation in the Chinese population with pGBM.29
Following the annotation of the 368 samples according to the TCGA molecular subtypes using ssGSEA on the GenePattern server, we first describe the TERT mutation distribution in the four molecular subtypes. Differing from the remarkable fluctuation of TERT mutation frequency among the pathological types, the molecular subtypes showed moderate frequencies of TERT mutation. As the influence of histological factors had been minimized, the prognostic value of TERT mutation was clearer in the molecular subtypes. In the Mesenchymal subtype, patients with TERT mutation had shorter survival than those with wild‐type TERT (P < 0.005), whereas the results were reversed for Proneural, Neural, and Classical gliomas. In contrast, only patients with WHO grade II tumor and TERT mutation had improved survival; there was no difference between mutant and wild‐type TERT groups in WHO grade III and IV tumors.
It is worth noting that the frequency of TERT mutation increased with age. The prognosis of patients with and without TERT mutation became poorer as age increased. Patients aged >60 years who had TERT mutation had worse survival compared to patients without the mutation.
In addition, C250T mutations were significantly inversely correlated with C228T mutations. In the whole cohort, patients with C250T mutations tended to have longer survival compared to patients with C228T mutations. Moreover, the rate of C250T mutations in recurrent gliomas (7/135, 5.2%) was about half that in newly diagnosed gliomas (77/752, 10.2%). These results demonstrate that C250T mutation hotspots may play a role in preventing glioma recurrence.
IDH1/2 mutations are early events in gliomagenesis and result in favorable prognosis,30 followed by gain of TP53 mutations or 1p19q LOH with subsequent astrocytic or oligodendroglial lineage differentiation, respectively.31 Additionally, TP53 is mutated early in the gliomagenesis of sGBM developing from low‐grade astrocytomas.32, 33, 34, 35 EGFR is the most frequently amplified gene in pGBM (40%).36, 37 To estimate the clinical significance of TERT promoter mutation more comprehensively and objectively, we combined the prognostic and diagnostic biomarkers above for analysis. In the present study, TERT mutation was present in a high fraction of low‐grade gliomas and co‐occurred with IDH1/2 mutation and 1p19q LOH, but was mutually exclusive with the TP53 mutation. Similar results were obtained for the Proneural subtype. Additionally, among the GBMs, TERT mutation co‐occurred with PTEN mutation and MGMT methylation but was inversely correlated with IDH1/2 mutation. The prognostic impact of the TERT mutation was further analyzed in the context of the genetic backgrounds above. In tumors with 1p19q LOH (subtype 1) and tumors with IDH mutation but without TP53 and 1p19q alteration (subtype 4), patients with TERT mutations had remarkably better prognosis than patients without TERT mutations, whereas the findings were reversed for the other three subtypes (P < 0.05). Lastly, we derived similar prognoses based on that for TERT mutation in the above subtypes and found that patients in whom there was co‐occurrence of TERT promoter, 1p19q, and IDH1/2 alteration had the best survival, whereas patients carrying TERT mutation and EGFR amplification had the shortest survival.
Huang and colleagues concluded that TERT mutations generate de novo consensus binding motifs for E‐twenty‐six (ETS) transcription factors and increase TERT promoter transcriptional activity by 2‐ to 4‐fold;21 therefore, patients with TERT mutation should theoretically have shorter survival than patients without it. However, our findings in low‐grade anaplastic glioma and sGBM were not consistent with this hypothesis. First, we cannot exclude the combined influence of other biomarkers such as IDH1/2 mutation, 1p19q LOH, and other undetected but important alterations. Second, TERT promoter mutation may represent an important tumorigenic mechanism only, but may not be the catalyst of glioma malignant progression.
Above all, our results describe the TERT promoter mutation spectrum according to the distinct histological, genetic, and molecular subtypes of glioma, dividing malignant gliomas into four molecular classifications based on their genetic backgrounds, which may aid in glioma subtype classification and provide a predictive prognostic tool for glioma.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the Jiangsu Students Platform for innovation and entrepreneurship training program (No. 201413980003Y), National Natural Science Foundation of China (No. 81402068) and National High Technology Research and Development Program of China (863) (No. 2012AA02A508).
You H, Wu Y, Chang K, et al. Paradoxical prognostic impact of TERT promoter mutations in gliomas depends on different histological and genetic backgrounds. CNS Neurosci Ther. 2017;23:790–797. 10.1111/cns.12724
The first two authors contributed equally to this work.
REFERENCES
- 1. Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005‐2009. Neuro Oncol. 2012;14(Suppl 5):v1‐v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nonoguchi N, Ohta T, Oh JE, et al. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013;126:931‐937. [DOI] [PubMed] [Google Scholar]
- 3. Killela PJ, Pirozzi CJ, Healy P, et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5:1515‐1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nobusawa S, Watanabe T, Kleihues P, et al. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002‐6007. [DOI] [PubMed] [Google Scholar]
- 5. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764‐772. [DOI] [PubMed] [Google Scholar]
- 6. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scherer HJ. Cerebral astrocytomas and their derivatives. Am J Cancer. 1940;40:159‐198. [Google Scholar]
- 8. Yan W, Zhang W, You G, et al. Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China. PLoS ONE. 2012;7:e30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horbinski C, Hobbs J, Cieply K, et al. EGFR expression stratifies oligodendroglioma behavior. Am J Pathol. 2011;179:1638‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cancer Genome Atlas Research . N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arango D, Wilson AJ, Shi Q, et al. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br J Cancer. 2004;91:1931‐1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bao ZS, Chen HM, Yang MY, et al. RNA‐seq of 272 gliomas revealed a novel, recurrent PTPRZ1‐MET fusion transcript in secondary glioblastomas. Genome Res. 2014;24:1765‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H, Durbin R. Fast and accurate short read alignment with Burrows‐Wheeler transform. Bioinformatics. 2009;25:1754‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Appin CL, Brat DJ. Molecular genetics of gliomas. Cancer J. 2014;20:66‐72. [DOI] [PubMed] [Google Scholar]
- 15. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high‐grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157‐173. [DOI] [PubMed] [Google Scholar]
- 16. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabharwal A, Middleton MR. Exploiting the role of O6‐methylguanine‐DNA‐methyltransferase (MGMT) in cancer therapy. Curr Opin Pharmacol. 2006;6:355‐363. [DOI] [PubMed] [Google Scholar]
- 18. Rivera AL, Pelloski CE, Gilbert MR, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12:116‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lam N, Chambers CR. Temozolomide plus radiotherapy for glioblastoma in a Canadian province: efficacy versus effectiveness and the impact of O6‐methylguanine‐DNA‐methyltransferase promoter methylation. J Oncol Pharm Pract. 2012;18:229‐238. [DOI] [PubMed] [Google Scholar]
- 20. Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192‐2197. [DOI] [PubMed] [Google Scholar]
- 21. Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959‐961. [DOI] [PubMed] [Google Scholar]
- 23. Arita H, Narita Y, Fukushima S, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126:267‐276. [DOI] [PubMed] [Google Scholar]
- 24. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self‐renewal. Proc Natl Acad Sci U S A. 2013;110:6021‐6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koelsche C, Sahm F, Capper D, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126:907‐915. [DOI] [PubMed] [Google Scholar]
- 26. Westermark B. Glioblastoma–a moving target. Ups J Med Sci. 2012;117:251‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492‐507. [DOI] [PubMed] [Google Scholar]
- 28. Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang P, Wang Y, Peng X, et al. Management and survival rates in patients with glioma in China (2004‐2010): a retrospective study from a single‐institution. J Neurooncol. 2013;113:259‐266. [DOI] [PubMed] [Google Scholar]
- 30. Ostrom Q, Cohen ML, Ondracek A, et al. Gene markers in brain tumors: what the epileptologist should know. Epilepsia. 2013;54(Suppl 9):25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohgaki H, Kleihues P. Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol. 2011;28:177‐183. [DOI] [PubMed] [Google Scholar]
- 33. Kim YH, Nobusawa S, Mittelbronn M, et al. Molecular classification of low‐grade diffuse gliomas. Am J Pathol. 2010;177:2708‐2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mendrysa SM, Ghassemifar S, Malek R. p53 in the CNS: perspectives on Development, Stem Cells, and Cancer. Genes Cancer. 2011;2:431‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stander M, Peraud A, Leroch B, et al. Prognostic impact of TP53 mutation status for adult patients with supratentorial World Health Organization Grade II astrocytoma or oligoastrocytoma: a long‐term analysis. Cancer. 2004;101:1028‐1035. [DOI] [PubMed] [Google Scholar]
- 36. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population‐based study. Cancer Res. 2004;64:6892‐6899. [DOI] [PubMed] [Google Scholar]


