Summary
Aims
Lipopolysaccharide (LPS) induces oxidative stress and neuroinflammation both in vivo and in vitro. Here, we provided the first detailed description of the mechanism of melatonin neuroprotection against LPS‐induced oxidative stress, acute neuroinflammation, and neurodegeneration in the hippocampal dentate gyrus (DG) region of the postnatal day 7 (PND7) rat brain.
Methods
The neuroprotective effects of melatonin against LPS‐induced neurotoxicity were analyzed using multiple research techniques, including Western blotting, immunofluorescence, and enzyme‐linked immunosorbent assays (ELISAs) in PND7 rat brain homogenates and BV2 cell lysates in vitro. We also used EX527 to inhibit silent information regulator transcript‐1 (SIRT1).
Results
A single intraperitoneal (i.p) injection of LPS to PND7 rats significantly induced glial cell activation, acute neuroinflammation, reactive oxygen species (ROS) production and apoptotic neurodegeneration in hippocampal DG region after 4 h. However, the coadministration of melatonin significantly inhibited both LPS‐induced acute neuroinflammation and apoptotic neurodegeneration and improved synaptic dysfunction in the hippocampal DG region of PND7 rats. Most importantly, melatonin stimulated the SIRT1/Nrf2 (nuclear factor‐erythroid 2‐related factor 2) signaling pathway to reduce LPS‐induced ROS generation. The beneficial effects of melatonin were further confirmed in LPS‐stimulated BV2 microglia cell lines in vitro using EX527 as an inhibitor of SIRT1. LPS‐induced oxidative stress, Nrf2 inhibition, and neuroinflammation are SIRT1‐dependent in BV2 microglia cell lines.
Conclusion
These results demonstrated that melatonin treatment rescued the hippocampal DG region of PND7 rat brains against LPS‐induced oxidative stress damage, acute neuroinflammation, and apoptotic neurodegeneration via SIRT1/Nrf2 signaling pathway activation.
Keywords: Lipopolysaccharide, Melatonin, Neuroinflammation, Nuclear factor‐erythroid 2‐related factor 2, Reactive oxygen species, Silent information regulator transcript‐1
Introduction
Endotoxemia is among the most critical clinical diseases and has a high death rate due to the difficulty of making an accurate prognosis. A component of the outer membrane of Gram‐negative bacteria, lipopolysaccharide (LPS), has been categorized as an endotoxin as a result of its ability to elicit immune cells and produce several proinflammatory cytokines and chemokines, which damages various organs 1. Recent studies have suggested that LPS induces the activation of microglia cells, thereby increasing neurotoxicity via the production of various proinflammatory and cytotoxic factors, including nuclear factor kappa B (NF‐κB) and downstream mediators 2, 3, 4.
The silent information regulator transcript‐1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD)‐dependent nuclear histone deacetylase that is involved in the regulation of several processes, such as controlling calorie restriction by extending the lifespan, metabolism, senescence, apoptosis, inflammation, and deacetylating histones and nonhistone proteins 5, 6, 7, 8. The deacetylation ability of SIRT1 inhibits the transactivational activity of NF‐kB 8. Several studies have demonstrated that SIRT1 reduces the level of oxidative stress and the extent of inflammation 9, 10, 11, 12.
Nuclear factor‐erythroid 2‐related factor 2 (Nrf2) is a pleiotropic protein and an important antioxidant sensor, and the activation of this protein is crucial for cellular defense mechanisms. Once it is activated, Nrf2 translocates from the cytoplasm to the nucleus and interacts with the antioxidant defense system to mediate the transcription of target genes, such as hemeoxygenase 1 (HO‐1). The transcription of these genes increases resistance to oxidative stress and displays protection against inflammation 13.
Melatonin, which is produced in many organs, including the pineal gland, macrophages, monocytes, and lymphocytes, is a multifunctional hormone that exerts various biological activities, such as the reduction of oxidative stress, inflammation, and neurodegeneration 14, 15, 16, 17, 18, 19, 20, 21. Melatonin is a strong antioxidant due to its free radical scavenging abilities and ability to reduce oxidative damage and inhibit neuroinflammation 14, 16, 18 in many models, including LPS 22, 23, 24, 25. The neuroprotective effects of melatonin against LPS damage in adult rodents, neural stem cells, and neonatal rats have previously been reported 23, 26, 27. This study was conducted to explore the detailed mechanism of action of melatonin against LPS‐induced oxidative stress, acute neuroinflammation, and neurodegeneration in the hippocampal DG region of postnatal day 7 (PND7) rats. The results showed that melatonin stimulates the SIRT1/Nrf2 signaling pathway against LPS‐induced oxidative stress in the DG region of the hippocampus of PND7 rats.
Materials and Methods
Animals and Drug Treatment
Postnatal day 7 (PND7) male pups from Sprague–Dawley rats (n = 5 animals/group) with an 18 g average body weight were randomly divided into four groups: (1) Control (C), (2) LPS, (3) LPS+Melatonin (LPS+Mel), and (4) Melatonin (Mel). The control animals received 0.1% DMSO and 0.9% saline solution, whereas the experimental groups received LPS (250 μg/kg) 28 and melatonin (10 mg/kg) 29 treatments, intraperitoneally (i.p.) administered as a single injection. Melatonin (dissolved in 0.1% DMSO and diluted with 0.9% saline solution) was coadministered after 30 min of LPS, and the animals were sacrificed at 4–12 h after injection. The local animal ethics committee of the Division of Applied Life Sciences, Department of Biology, Gyeongsang National University South Korea, approved all experimental procedures.
Chemicals
Melatonin, LPS, DCFDA, and EX527 were purchased from Sigma‐Aldrich.
Cell Culturing and Drug Treatment
Murine BV2 microglia and mouse hippocampal neuronal HT22 cells were maintained on DMEM medium containing 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 incubator. The cells were treated with LPS (1 μg/mL), LPS plus melatonin (1 μg/mL + 100 μM), LPS plus Ex527 (1 μg/mL + 20 μM), and LPS plus Ex527 plus melatonin (1 μg/mL + 20 μM + 100 μM) for 4 h.
Western Blot Analysis
The Western blot analysis was conducted as previously reported 30. Briefly, the animals were sacrificed after four h following LPS administration with or without melatonin treatment. The brains (hippocampus) were carefully collected, and dry ice was used to freeze the tissue. Similarly, after treatment, the BV2 and HT22 cells were collected in phosphate buffer saline (PBS), centrifuged, and the supernatant was removed. The remaining pellet was dissolved in Pro‐Prep protein extraction solution, according to the manufacturer's instructions (iNtRON BIOTECHNOLOGY) to generate cell lysates. The brain homogenates and cell lysates were quantified using Bio‐Rad protein assay solution. The homogenates (20 μg protein) were fractionated using SDS‐PAGE on 4–12% Bolt™ Mini Gels (Novex; Life Technologies, Kiryat Shmona, Israel). After transfer, the membranes were blocked in 5% skim milk (or BSA), incubated overnight at 4°C with primary antibodies, and cross‐reacting proteins were detected using ECL after reaction with horseradish peroxidase‐conjugated secondary antibodies. The primary antibodies, including rabbit‐derived anti‐COX2, anti‐PARP‐1, anti‐Nrf2, anti‐iNOS, anti‐synaptophysin (Syp), and anti‐p‐NF‐kB, mouse‐derived anti‐β‐actin, anti‐PSD95, anti‐GFAP, anti‐Hemeoxygenase‐1 (HO‐1), and goat‐derived anti‐Iba‐1, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). We also used mouse‐derived anti‐SIRT1 from Cell Signaling Technology, Inc. and mouse‐derived anti‐8‐Oxoguanosine (8‐OxoG) from Millipore. After using membrane‐derived secondary antibodies, ECL (Amersham Pharmacia Biotech, Uppsala, Sweden) detection reagent was used for visualization according to the manufacturer's instructions. The densitometry analysis of the bands was performed using Sigma Gel software (SPSS, Chicago, IL, USA). The density values were calculated in arbitrary units (A.U.) relative to the untreated control.
Tissue Collection and Sample Preparation
For the morphological study of brain tissue, the animals were sacrificed after 12 h of drug treatment. An equal number of animals were maintained in each group (n = 5/group), and transcardial perfusion with 4% ice‐cold paraformaldehyde and PBS was performed. After postfixing in 4% paraformaldehyde overnight, the samples were transferred to 20% sucrose solution until the brains sank to the bottom of the tube. Prior to obtaining 16‐μm sections in the coronal planes using a Leica cryostat (CM 3050C; Germany), the brains were frozen in optimum cutting temperature (O.C.T) compound (A.O. USA). The sections were thawed and mounted on probe‐on plus charged slides (Fisher).
Fluoro‐Jade B Staining
Fluoro‐Jade B staining was performed as previously described 31. Brain tissue slides were air‐dried overnight. Initially, the slides were immersed in a solution of 1% sodium hydroxide and 80% ethanol for 5 min, followed by 70% alcohol for 2 min and distilled water for 2 min. The slides were transferred to a 0.06% potassium permanganate solution for 10 min and subsequently rinsed with distilled water. Next, the slides were immersed in a 0.1% acetic acid and 0.01% Fluoro‐jade B solution of for 20 min, rinsed with distilled water, and dried for 10 min. Glass cover slips were mounted onto the glass slides using mounting medium. The images were captured using an FITC filter on a confocal laser scanning microscope (FluoView FV 1000; Olympus, Tokyo, Japan).
Immunofluorescence
Immunofluorescence staining was performed as previously described 32, 33. Briefly, tissue‐containing slides and chamber slides were washed twice for 15 min in 0.01 M PBS, and subsequently, Proteinase K solution was added to the tissue and incubated for 5 min at 37°C. After blocking the brain tissues with normal goat serum, the primary antibodies Iba‐1, Nrf2, 8‐OxoG, and GFAP (1:100 in PBS) were applied, and the slides were incubated at 4°C overnight. The next day, secondary antibodies (FITC, Santa Cruz, 1:50 in PBS) were applied at room temperature for 90 min. The slides were washed twice with PBS for 5 min. DAPI (4′, 6‐diamidino‐2‐phenylindole) was applied to stain the nuclei, and glass cover slips were mounted onto the glass slides using mounting medium. The images were captured using a confocal microscope (FluoView FV 1000).
Oxidative Stress (ROS) Detection In Vivo and In Vitro
To measure the ROS content in the brain homogenates of all experimental groups, we followed a recently published protocol 34. Briefly, the brain homogenates were diluted with ice‐cold Locke's buffer at 1:20 ratio, and after adding DCFH‐DA (5 mM), this mixture was incubated at room temperature for 45 min for the conversion of DCFH‐DA to DCF. Excitation was measured at 484 nm, and emission was measured at 530 nm. The ROS content was expressed as pmol DCF formed/min/mg protein.
The in vitro ROS assay was conducted using BV2 microglia cells as previously described 35. Briefly, the cells were cultured in 96‐well plates containing 200 μL DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin in each well. After incubation for 24 h at 37°C in a humidified 5% CO2 incubator, the cells were treated with LPS (1 μg/mL), LPS plus melatonin (1 μg/mL + 100 μM), LPS plus Ex527 (1 μg/mL + 20 μM), and LPS plus Ex527 plus melatonin (1 μg/mL + 20 μM + 100 μM) for 4 h. Subsequently, 600 μM DCFDA (2′, 7′‐dichloroflourescin diacetate) dissolved in DMSO/PBS was added to each well, followed by incubation for 30 min. The plates were read on an ApoTox‐Glo™ (Promega Corp., Madison, WI, USA) at 488/530 nm.
Enzyme Assays
The hippocampi rat brain homogenates and BV2 cell lysates of the experimental groups were evaluated for different ELISA assays, such as Total NF‐kB p65 ELISA (Invitrogen Corporation, Carlsbad, CA, USA; Catalog #KHO0371) SIRT1 HDAC Fluorimetric Assay kit (Promega Corp., Madison, WI, USA), and COX2 (R&D Systems, Inc., Minneapolis, MN, USA), performed according to the manufacturer's instructions.
Data and Statistical Analysis
The original X‐ray films from the Western blot analyses were scanned and analyzed through densitometry using the computer‐based Sigma Gel System (SPSS Inc., Chicago, IL). The density values were expressed as the means ± SEM. The ImageJ program was used to analyze the integral optical density (IOD). One‐way analysis of variance (ANOVA) was used determine the significant difference, followed by Student's t‐test. P values less than 0.05 (P < 0.05) were considered significant.
Results
Melatonin Ameliorated LPS‐Induced Glial Cells Activation, their Associated Neuroinflammation and Synaptotoxicity in Developing Rat Brain
We first determined whether a single injection of LPS to PND7 rat pups activates glial cells, including microglia, and astrocytes, using an immunofluorescence technique. The results indicated that a single injection of LPS significantly induced the activation of astrocytes (GFAP) and microglia (Iba‐1) in PND7 rat pups brain, as shown in Figure 1A and 1B. Furthermore, LPS also activated proinflammatory markers, including phosphorylated nuclear factor kappa (p‐NF‐κB), cyclooxygenase‐2 (COX‐2), and intrinsic nitric oxide synthetase (iNOS), after 4 h in PND7 rat brains (Figure 2A). Conversely, the coadministration of melatonin not only significantly inhibited LPS‐induced activated astrocytes and microglia but also significantly reversed the increased expression level of different proinflammatory markers, such as p‐NF‐κB, COX 2, and iNOS, as shown in Figure 2A. Additionally, melatonin treatment reduced the LPS‐activated total NF‐kBp65 activity, as measured using an ELISA kit (Figure 2B). Furthermore, the beneficial effects of melatonin against LPS‐induced synaptotoxicity were evaluated after 4 h in the PND7 rat brains. The immunoblot analysis revealed that melatonin markedly increased the expression of LPS‐suppressed pre‐ and postsynapse‐related proteins. The synapse‐related proteins primarily comprised presynapse marker‐like synaptophysin (Syp) and postsynapse density protein 95 (PSD95), as shown in Figure 2C.
Figure 1.
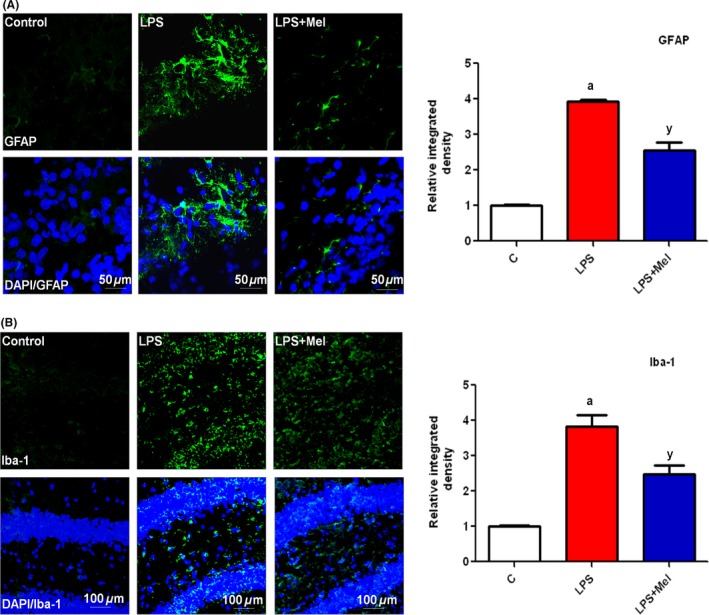
Melatonin attenuated LPS‐induced activated glial cells in the hippocampus of developing rat brain. Representative photomicrographs of the immunofluorescence analysis and the relative integral optical density (IOD) histogram of (A) astrocytes (GFAP)‐positive cells and (B) microglia (Iba‐1)‐positive cells in the DG region of the rats in the experimental groups. The images are representative of immunostaining obtained from sections prepared from at least animal animals per group. The panels represent the hippocampal DG region of the young rat brain immunostained with GFAP (green) and Iba‐1 (green) and counterstained with DAPI (blue), respectively. Significance; a P < 0.01, y P < 0.05.
Figure 2.
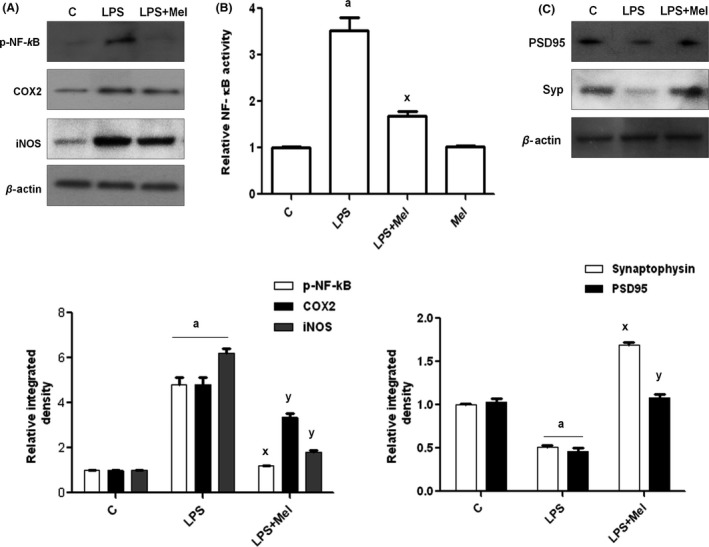
Beneficial effect of melatonin against LPS‐induced neuroinflammation and synaptic deficits in immature rat brain. Immunoblots of pro‐inflammatory markers, including (A) phospho‐NF‐κB, COX2, and iNOS proteins. (B) The ELISA histogram of NF‐κBp65 (total) in the brain homogenates of the experimental groups. The assay was conducted according to the manufacturer's instructions. (C) Immunoblot of synapse‐related proteins, such as synaptophysin (presynapse) and PSD95 (postsynapse), in the hippocampus of the postnatal day 7 rat brain. The bands were quantified using Sigma Gel software and density histograms (expressed in arbitrary units, i.e., A.U) relative to the control using GraphPad Prism software. The values represent the means ± SEM for the indicated proteins (n = 5 animals per group). Significance; aP < 0.01, 0.05 and x,yP < 0.01, 0.05, respectively.
Melatonin Reduced LPS‐Induced Oxidative Stress in the Developing Brain
LPS induces oxidative stress by generating reactive oxygen species (ROS). To investigate whether melatonin inhibits ROS generation in the developing brain, ROS assays and immunohistopathologically 8‐Oxoguanin (8‐OxoG) staining were conducted. The results of the ROS assay indicated that at 4 h after administering LPS to young rats, significantly increased ROS production in the PND7 brain homogenates was observed compared with the saline‐treated control (Figure 3A). However, the brain homogenates of the animals receiving melatonin through either alone or cotreated with LPS displayed reduced ROS production (Figure 3A). Additionally, the immunofluorescence images revealed that compared with the control animals, LPS significantly increased the immunofluorescence reactivity of 8‐OxoG in the DG region of the PND7 rat brain. Similarly, the administration of melatonin to young rats significantly reduced the expression of 8‐OxoG in the DG region of PND7 rat brains (Figure 3B).
Figure 3.
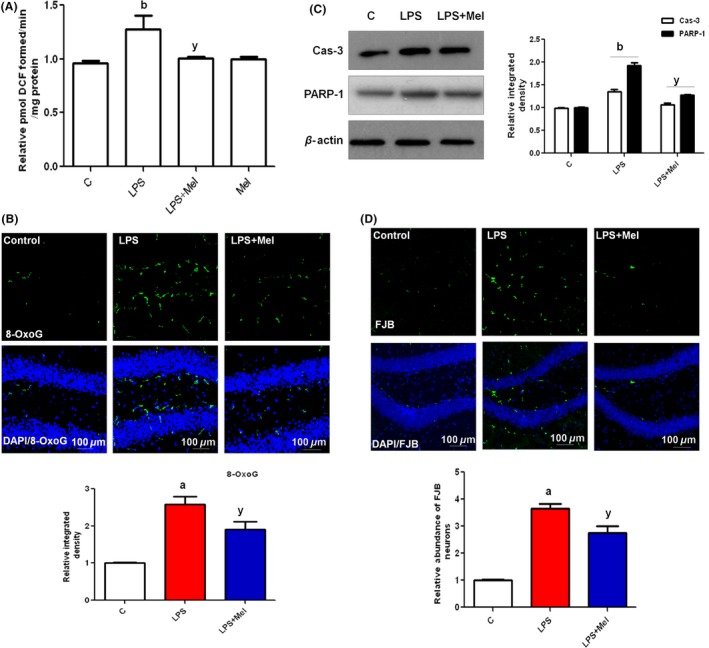
Melatonin abrogated LPS‐induced oxidative stress and apoptotic neurodegeneration in the hippocampus of developing rat brain. (A) The histogram of the ROS assay conducted with brain homogenates from rats in the experimental groups. The assay was repeated three times with the same results. (B) The immunostaining images and respective relative IOD histograms of 8‐OxoG in experimental groups. (C) The Western blot analysis of caspase‐3 and PARP‐1 proteins in the hippocampus of PND7 rat brains, following LPS and melatonin treatment. The relative integrated density for above‐mentioned proteins is depicted in the histograms. The membranes were redeveloped for β‐actin and used as a loading control. (D) The immunofluorescence images and respective relative IOD histograms of FJB‐positive neuronal cells in the DG region of immature rat brains. The density values are expressed in arbitrary units as the means ± SEM for the indicated proteins (n = 5 animals per group). The details are shown in the Methods section. Significance; a,bP < 0.01, 0.05 and yP < 0.05, respectively.
Melatonin Abrogated LPS‐Induced Apoptotic Neurodegeneration in the Developing Brain
A single injection of LPS in PND7 rat brains was further investigated for the induction of caspase‐3 and PARP‐1 as apoptosis and neurodegeneration markers. The immunoblot results reveal that LPS‐treated young rats shown a significantly high expression of caspase‐3 and PARP‐1 proteins. However, the cotreatment of melatonin significantly reduced the expression levels of caspase‐3 and PARP‐1 in the PND7 rat brains (Figure 3C). Additionally, to determine the extent of neurodegeneration induced through LPS in the PND7 rat brains, Flouro Jade B (FJB) staining was performed. The images indicated that LPS induced widespread neurodegeneration in the DG region of the hippocampus of PND7 rat brains. In contrast to LPS treatment alone, the rat pups cotreated with melatonin and LPS showed markedly low numbers of FJB‐positive neurons in the hippocampal DG region (Figure 3D).
Melatonin‐Stimulated SIRT1/Nrf2 Signaling Pathway against LPS‐Induced Oxidative Stress in the Developing Brain
To characterize the expression and activity of SIRT1, the brain homogenates of all treated young rats were subjected to Western blotting and SIRT1 activity assays. The results revealed that LPS significantly inhibited SIRT1 protein expression (Figure 4A) and activity (Figure 4B) compared with the saline‐treated control. In contrast, melatonin treatment rescued the developing brain by significantly (P < 0.001) activating SIRT1 protein expression and activity (Figure 4A,B).
Figure 4.
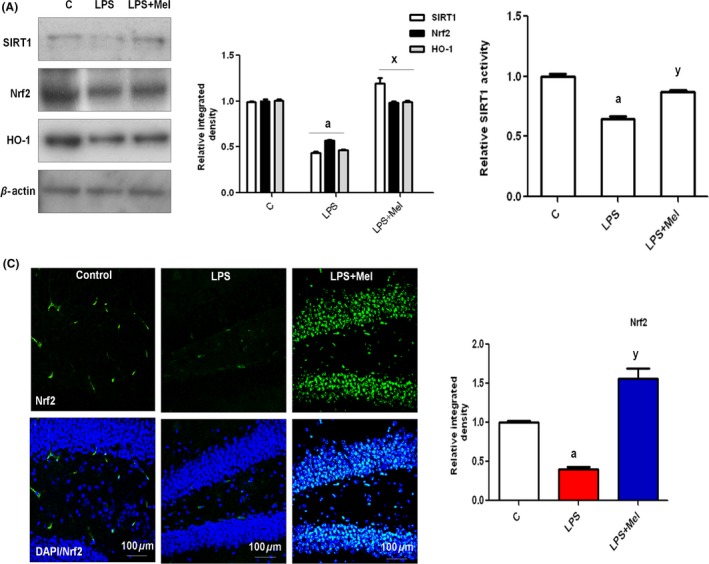
Melatonin stimulated the SIRT1/Nrf2 signaling pathway against LPS in the hippocampus of the developing rat brain. (A) The Western blot analysis of SIRT1, Nrf2, and HO‐1 proteins in the hippocampus of PND7 rat brains following LPS and melatonin treatment. The relative integrated density for the above‐mentioned proteins is depicted in the histograms. The membranes were redeveloped for β‐actin and used as a loading control. (B) The histogram of SIRT1 activity in the hippocampal brain homogenates of young rats subjected to LPS and melatonin treatment. The assay was repeated three times. (C) The immunostained images of Nrf2 (green) protein counterstained with DAPI (blue) in the hippocampal DG region of PND7 rat brains. The results are expressed as the means ± SEM. (n = 5). Significance; a P < 0.01 and x,yP < 0.01, 0.05, respectively.
To analyze the antioxidant effect of melatonin, we measured the protein expression of Nrf2 and its downstream signaling gene hemeoxygenase‐1 (HO‐1). As shown in Figure 4A, LPS administration was significantly suppressed, and melatonin treatment markedly increased the expression of Nrf2 and HO‐1 proteins in the developing brain. The immunohistological investigation of Nrf2 also supported the Western blot results, indicating that melatonin treatment increased the expression of Nrf2 in the hippocampal DG region of the developing rat brain (Figure 4A,C).
The Beneficial Effects of Melatonin against LPS‐Induced Oxidative Stress in BV2 Cells In Vitro
Glial cells, particularly microglia, are well‐known LPS‐responsive cells in the CNS. To elucidate the antiinflammatory and antioxidative role of melatonin against LPS‐induced neuroinflammation and oxidative stress, BV2 microglial cells were used in vitro. The double immunofluorescence results (Figure 5A) revealed that LPS treatment not only stimulated BV2 microglial cells but also inhibited SIRT1 after 4 h compared with untreated BV2 cells in vitro. However, melatonin treatment significantly inhibited BV2 cell stimulation in vitro (Figure 5A). Additionally, melatonin also significantly induced the activation of SIRT1 against BV2 cells treated with LPS alone in vitro (Figure 5A). Similarly, melatonin treatment significantly induced Nrf2 activation and translocation into the nucleus compared with LPS‐treated BV2 cells in vitro (Figure 5B). Additionally, melatonin treatment reduced LPS‐induced oxidative stress by reducing the expression of 8‐OxoG in vitro (Figure 6A).
Figure 5.
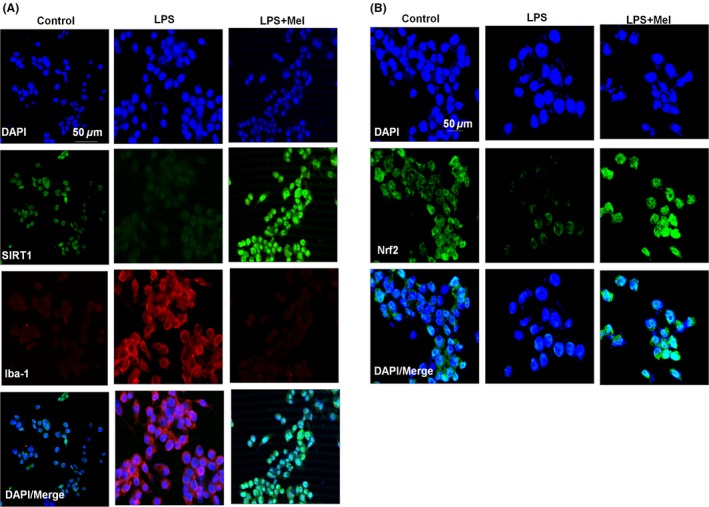
Melatonin inhibited LPS‐stimulated BV2 cell lines and activated SIRT1 in vitro. The immunostaining images of (A) SIRT1 (green) and Iba‐1 (red) and (B) Nrf2 (green) counterstained with DAPI (blue) in LPS‐stimulated BV2 cell lines in vitro. BV2 cells were cultured on chamber slides and treated for 4 h. The results represent three repeated experiments. All relevant details are shown in the Material and Methods section.
Figure 6.
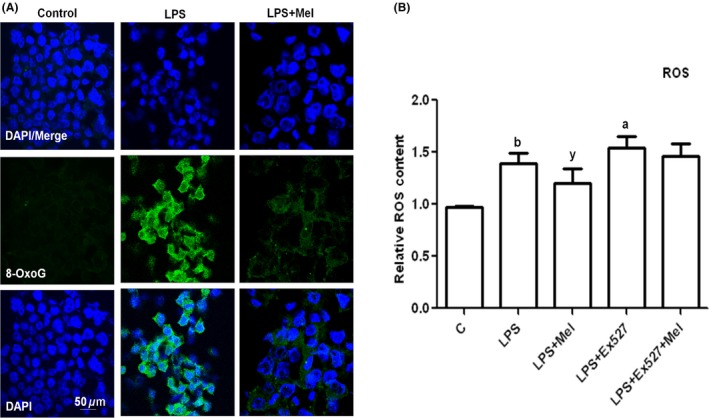
Melatonin reduced LPS‐induced oxidative stress in BV2 cells in vitro. (A) The immunostaining images of 8‐OxoG (green) and DAPI (blue) in BV2 cells treated with LPS and melatonin for 4 h. (B) The histogram of ROS assay conducted in BV2 cell lines cultured in 96‐well plates and treated with LPS, melatonin with or without EX527 (SIRT1 inhibitor) for 4 h. The assay was repeated in triplicate. Significance; a,bP < 0.01, 0.05 and y P < 0.05, respectively.
To determine the mechanism of melatonin against LPS‐induced oxidative stress in vitro, BV2 microglial cells were treated with LPS and melatonin and with or without SIRT1 inhibitor, that is, EX527, for 4 h, and the reactive oxygen species (ROS) production was analyzed using an ROS assay. The results (Figure 6B) showed that melatonin significantly reduced LPS‐induced ROS production in vitro. Interestingly, when SIRT1 was blocked with the inhibitor EX527, the LPS‐induced ROS was further increased, and the ability of melatonin to reduce ROS production was abolished. This finding suggests that the ability of melatonin to reduce LPS‐induced ROS is SIRT1 dependent (Figure 6B).
Melatonin Activation of Nrf2 is SIT1 Dependent in BV2 Cells In Vitro
Melatonin stimulated the SIRT1/Nrf2 pathway to reduce LPS‐induced oxidative stress. Thus, we investigated the SIRT1/Nrf2 signaling pathway through Western blot analysis in BV2 cells after inhibiting SIRT1 using the inhibitor EX527. The results indicated that melatonin significantly activated SIRT1 and Nrf2 protein expression and reduced caspase‐3 expression against LPS in BV2 cells. However, in the presence of SIRT1 inhibitor, LPS not only further suppressed SIRT1 and Nrf2 expressions but also increased the caspase‐3 protein levels in BV2 cells. However, EX527 also slightly affected the ability of melatonin to significantly inhibit caspase‐3 and activate both SIRT1 and Nrf2 proteins as shown in Figure 7A. Similarly, the histogram of the results of the SIRT1 activity assay also support the Western blot results, showing that LPS significantly inhibits SIRT1 and melatonin, thereby increasing SIRT1 activity (Figure 7B).
Figure 7.
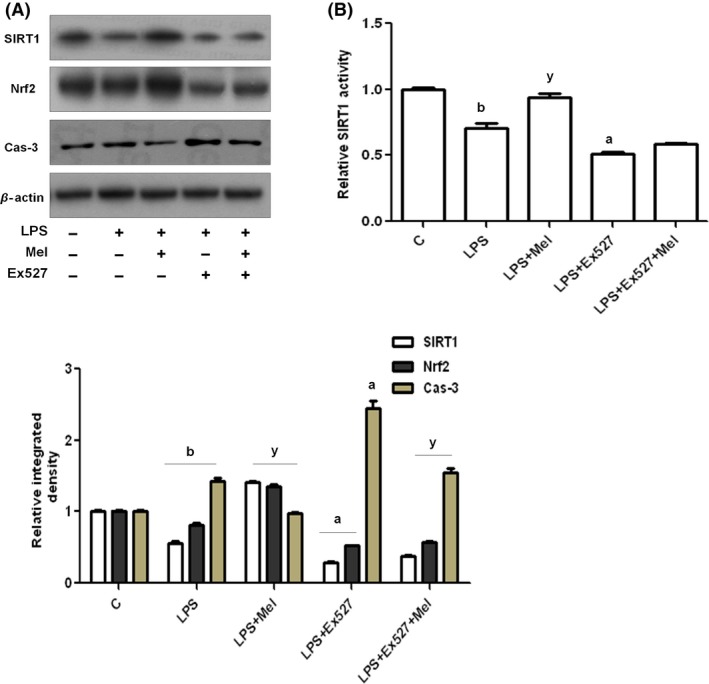
Melatonin stimulated the SIRT1/Nrf2 signaling pathway against LPS in BV2 cells in vitro. (A) The Western blot analysis and relative integrated density histograms of SIRT1, Nrf2, and Caspase‐3 proteins after LPS and melatonin treatment, with or without EX527 treatment for 4 h in BV2 cell lines. β‐Actin was used as a loading control. (B) The histogram of SIRT1 activity in cell lysates subjected to LPS and melatonin, with or without EX527 treatment for 4 h in BV2 cell lines. The assay was repeated for three times, and the values represent the means ± SEM. Significance;a,bP < 0.01, 0.05 and y P < 0.05, respectively.
Melatonin Inhibited LPS‐Upregulated NF‐κB and COX2 Protein in SIT1‐Dependent Manner in BV2 Cells In Vitro
NF‐κB activation is considered the main event in the progression of the proinflammatory signaling pathway. Melatonin administration reduces NF‐κB and the regulation of its downstream signaling molecules in the developing brain. To analyze whether the melatonin‐mediated deactivation of the NF‐κB signaling pathway is SIRT1‐dependent, we used the SIRT1 inhibitor EX527 in BV2 cells, followed by Western blot analysis. The immunoblot results indicated that melatonin treatment reduced LPS‐induced NF‐κB and COX2 expression, whereas SIRT1 inhibition further upregulated NF‐κB and COX2 expression (Figure 8A). In addition, melatonin significantly downregulated the expression of both NF‐κB and COX2 proteins (Figure 8A). Similarly, we also conducted ELISA assays for both NF‐κB and COX2 proteins in BV2 and HT22 cells. The results also showed that the LPS‐induced activation of NF‐κB and COX2 protein expression is SIRT1 dependent and that melatonin significantly decreased the expression of these proteins in both cell lines (Figure 8B,C).
Figure 8.
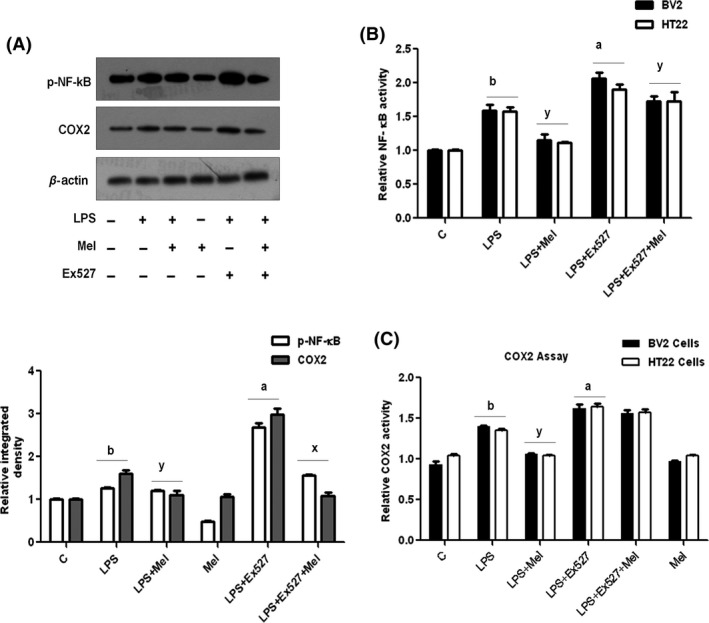
Melatonin reduced LPS‐activated proinflammatory markers in BV2 cell lines in vitro. (A) The Western blot analysis and relative integrated density histograms of p‐NF‐κB and COX2 proteins after LPS, melatonin, and with or without EX527 treatment for 4 h in BV2 cell lines. β‐Actin was used as a loading control. (B) The histogram of NF‐κBp65 activity in cell lysates subjected to LPS, melatonin, and with or without EX527 treatment for 4 h in BV2 and HT22 cell lines, respectively. (C) The histogram of COX2 in BV2 and HT22 cell lines, respectively, cultured in 96‐well plate subjected to LPS and melatonin, with or without EX527 treatment for 4 h. These assays were repeated three times, and the values are expressed as the means ± SEM. Significance; a,bP < 0.01, 0.05 and x,yP < 0.01, 0.05, respectively.
Discussion
The present study contributes to research efforts to identify therapeutic agents to treat neurodegenerative diseases. LPS‐induced neuroinflammation plays a crucial role in the progression of neurological disorders. The present study was designed to evaluate the neuroprotective potential of melatonin against oxidative stress and associated acute neuroinflammation by LPS in the hippocampal DG region of postnatal day 7 rat brains. This mechanistic report provides the first evidence that melatonin abrogates LPS‐induced oxidative stress and acute neuroinflammation in an SIRT1‐/Nrf2‐dependent manner in the developing rat brain (Figure 9).
Figure 9.
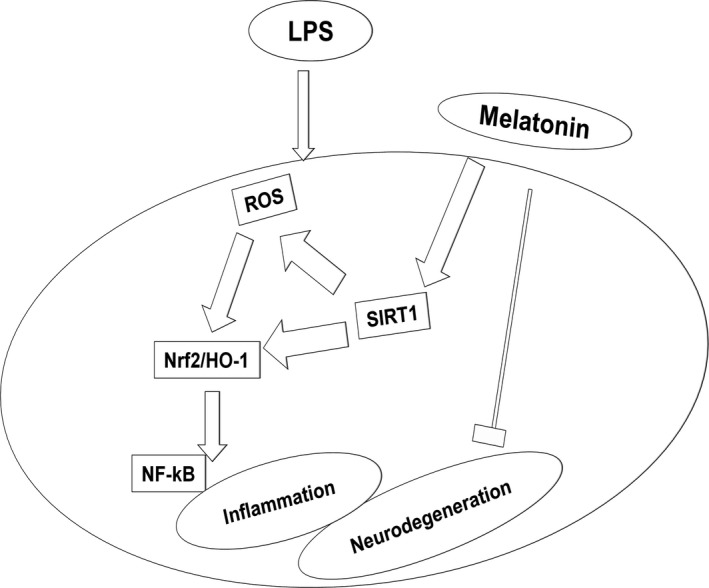
Schematic diagram suggested a neuroprotective mechanism for melatonin against LPS‐induced ROS, acute neuroinflammation, and neurodegeneration. The diagram depicts a potential mechanism by which melatonin prevents LPS‐induced oxidative stress, acute neuroinflammation, and neurodegeneration via a SIRT1/Nrf2 signaling pathway in the hippocampal dentate gyrus region of PND7 rat brains.
LPS is a toxic agent that generates ROS, which subsequently induces inflammation 36, 37, 38. The LPS‐generated ROS then activates NF‐κB and increases iNOS and COX‐2 at both the mRNA and protein level. Previous studies have reported the translocation of NF‐κB to the nucleus in acute neuroinflammation, where this protein binds to DNA and activates the expression of proinflammatory genes, such as iNOS and COX‐2 39. Similar studies have suggested that LPS induces ROS, which activates and translocates NF‐κB and increases the proteins levels of iNOS and COX‐2 40. Thus, we conducted the current study to determine the mechanism of LPS‐induced oxidative stress and acute neuroinflammation in the developing rat brain. The results demonstrated that a single injection of LPS at day 7 into rat pups triggers ROS and acute neuroinflammatory responses in the hippocampal DG region of immature rat brains. In addition, this LPS dose is sufficient to induce the activation of glial cells, including microglia and astrocytes. Most importantly, LPS significantly inhibited the expression of SIRT1 and the endogenous antioxidant response system, that is, Nrf2 and HO‐1 proteins, in rat pup brains. Several previously published studies have shown that melatonin reduces ROS production against different toxins in vitro and in vivo animal models 41, 42, 43, 44, 45, 46, 47, 48. Thus, we also examined the therapeutic efficacy of melatonin in postnatal day 7 rat brains against LPS‐induced oxidative stress and acute neuroinflammation. The results demonstrated that melatonin is potentially effective against LPS shock because melatonin reduced glial cell activation in the developing brain. Specifically, melatonin administration to PND7 rats activated Nrf2/HO‐1 signaling proteins, as an endogenous antioxidant system, in an SIRT1‐dependent manner to rescue the immature brain against LPS‐induced oxidative stress and acute neuroinflammation. In this regard, recent findings have revealed that melatonin treatment could significantly up‐regulate LPS‐suppressed Nrf2 and HO1 protein levels 49. Nrf2 is an important agent in the induction of various antioxidants, which regulates the cellular antioxidant response against ROS. Under normal conditions, Nrf2 exists in the cytoplasm, but in the presence of oxidative stress, Nrf2 translocates to the nucleus, where this protein can activate antioxidant responses by up‐regulating several antioxidant enzymes, particularly HO‐1, and decreasing the sensitivity to oxidative stress damage 50. Additionally, Nrf2 plays an important role in modulating acute inflammatory responses 51.
In the present study, we demonstrated that melatonin is responsible for the antiinflammatory activities in the immature rat brain and in BV2 cells. Most importantly, these results showed that melatonin completely inhibited LPS‐induced glial cell activation, including both microglia and astrocytes, and the associated acute neuroinflammation. It has previously been demonstrated that melatonin, via the melatonin receptor, confers protection against the damage of developing white matter to inhibit microglial activation and oligodendroglial maturation 52. Similarly, numerous studies have reported that melatonin reduces pro‐inflammatory cytokine production and might inhibit the expression of pro‐inflammatory enzymes, such as iNOS and COX‐2, against LPS in vitro and in vivo 53, 54, 55, 56.
In the present study, we hypothesized that melatonin exerts antioxidative and antiinflammatory activities via the activation of the SIRT1‐dependent transcription factor Nrf2 in the immature rat brain and in BV2 cells. The protein expression, as measured by Western blot and ELISA, demonstrated that SIRT1 plays an important role in melatonin antioxidative and antiinflammatory effects. Consistent with the results from protein expression, when SIRT1 was inhibited with its inhibitor, the protein expression of SIRT1 and Nrf2 was significantly decreased in BV2 cells, suggesting that the LPS‐induced inhibition of Nrf2 is SIRT1‐dependent in vitro and in the developing rat brain. Similarly, these results showed that as a result of SIRT1 inhibition, the expression of proinflammatory proteins, such as p‐NF‐κB and COX‐2, was further increased, suggesting that SIRT1 plays a significant role in LPS‐induced neuroinflammation.
There are several explanations for the efficacy of melatonin as anti‐ROS agent, including its potential inhibitory effects on lipid peroxidation; melatonin also exhibits stabilizer activity and acts as a protector of cell and organelle membranes 57. Additionally, melatonin regulates various enzymes that play crucial roles in ROS production 58. Similarly, melatonin reduces nitrite/nitrate production by reducing iNOS expression via NF‐κB inhibition, which is activated by LPS. Moreover, melatonin can cross morphophysiological barriers and enter subcellular compartments because of its solubility in lipid and aqueous media, thus making melatonin a good antioxidant agent.
Taken together, these findings highlight melatonin as a potential therapeutic candidate for the treatment of oxidative stress, acute neuroinflammation, and associated neurodegeneration against LPS shock during development. Melatonin is an agent with low or even no side effects and is safe when used under multiple physiological conditions. Studies concerning melatonin protection against various toxins and the availability of this compound as a medicine under different physiological conditions suggest it poses no threat to human health. This study suggests a new strategy to target SIRT1/Nrf2 signaling pathway for the inhibition of LPS‐induced neurodegenerative disorders. However, further studies will be needed to evaluate the mechanism of the role of melatonin in neurodegenerative diseases.
Conflict of Interest
The author declares no conflict of interest.
Acknowledgment
This research was supported by the Brain Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1904391).
References
- 1. Rossignol DP, Lynn M. TLR4 antagonists for endotoxemia and beyond. Curr Opin Investig Drugs 2005;6:496–502. [PubMed] [Google Scholar]
- 2. Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 2010;4:242–247. [DOI] [PubMed] [Google Scholar]
- 3. Gasparini C, Felmann M. NF‐kappa B as a target for modulating inflammatory responses. Curr Pharm Des 2012;18:5735–5745. [DOI] [PubMed] [Google Scholar]
- 4. Rakonczay ZJ, Hegvi P, Taka T, Mccarroll J, Saluja AK. The role of NF‐kappa B activation in the pathogenesis of acute pancreatitis. Gut 2008;57:259–267. [DOI] [PubMed] [Google Scholar]
- 5. Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2a promotes cell survival under stress. Cell 2001;107:137–148. [DOI] [PubMed] [Google Scholar]
- 6. Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Nat Acad Sci USA 2004;101:15998–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2008;2:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF‐kB‐dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004;23:2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 2007;100:1512–1521. [DOI] [PubMed] [Google Scholar]
- 10. Salminen A, Kauppinen A, Suuronen T, Kaarniranta K. SIRT1 longevity factor suppresses NF‐κB‐driven immune responses: regulation of aging via NF‐κB acetylation? BioEssays 2008;30:939–942. [DOI] [PubMed] [Google Scholar]
- 11. Rajendran R, Garva R, Krstic‐Demonacos M, Demonacos C. Sirtuins: Molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J Biomed Biotechnol 2011;2011:368276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salminen A, Hyttinen JMT, Kaarniranta K. AMP‐activated protein kinase inhibits NF‐κB signaling and inflammation: impact on health span and lifespan. J Mol Med 2011;89:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang K, Huang J, Xie X, et al. Sirt1 resists advanced glycation end products‐induced expressions of fibro‐nectin and TGF‐β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radi Biol Med 2013;65:528–540. [DOI] [PubMed] [Google Scholar]
- 14. Boccalandro HE, Gonzalez CV, Wunderlin DA, Silva MF. Melatonin levels, determined by LC‐ESI‐MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: evidence of its antioxidant role in fruits. J Pineal Res 2011;51:226–232. [DOI] [PubMed] [Google Scholar]
- 15. Acuña‐Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential Functions. Cell Mol Life Sci 2014;71:2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 2011;51:1–16. [DOI] [PubMed] [Google Scholar]
- 17. Mukherjee D, Roy SG, Bandyopadhyay A, et al. Melatonin protects against isoproterenol‐induced myocardial injury in the rat: antioxidative mechanisms. J Pineal Res 2010;48:251–262. [DOI] [PubMed] [Google Scholar]
- 18. Sewerynek E, Abe M, Reiter RJ, et al. Melatonin Administration Prevents Lipopolysaccharide‐Induced Oxidative Damage in Phenobarbital‐Treated Animals. J of Cell Bioche 1995;58:436–444. [DOI] [PubMed] [Google Scholar]
- 19. Ochoa JJ, Az‐Castro J, Kajarabille N, et al. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J Pineal Res 2011;51:373–380. [DOI] [PubMed] [Google Scholar]
- 20. Tahan G, Gramignoli R, Marongiu F, et al. Melatonin expresses powerful anti‐inflammatory and antioxidant activities resulting in complete improvement of acetic‐acid‐induced colitis in rats. Dig Dis Sci 2011;56:715–720. [DOI] [PubMed] [Google Scholar]
- 21. Veneroso C, Tunon MJ, Gonzalez‐Gallego J, Collado PS. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J Pineal Res 2009;47:184–191. [DOI] [PubMed] [Google Scholar]
- 22. Lissoni P, Barni S, Tancini G, et al. Role of the pineal gland in the control of macrophage functions and its possible implication in cancer: a study of interactions between tumor necrosis factor‐alpha and the pineal hormone melatonin. J Biol Regul Homeost Agents 1994;8:126–129. [PubMed] [Google Scholar]
- 23. Barjavel MJ, Mamdouh Z, Raghbate N, Bakouche O. Differential expression of the melatonin receptor in human monocytes. J Immunol 1998;160:1191–1197. [PubMed] [Google Scholar]
- 24. Lardone PJ, Guerrero JM, Fernandez‐Santos JM, Rubio A, Martín‐Lacave I, Carrillo‐Vico A. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid‐related orphan receptor. J Pineal Res 2011;51:454–462. [DOI] [PubMed] [Google Scholar]
- 25. Wong CS, Jow GM, Kaizaki A, Fan LW, Tien LT. Melatonin ameliorates brain injury induced by systemic lipopolysaccharide in neonatal rats. Neuroscience 2014;267:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Juhyun S, Mang KS, Kyoung ML, Jong EL. The Protective Effect of Melatonin on Neural Stem Cell against LPS‐Induced Inflammation. BioMed Res Inter 2015;doi: 10.1155/2015/854359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Escames G, Cuna‐Castroviejo D, Lopez LC, et al. Pharmacological utility of melatonin in the treatment of septic shock: experimental and clinical evidence. J Pharm Pharmacol 2006;58:1153–1165. [DOI] [PubMed] [Google Scholar]
- 28. Chen YH, Xu DX, Wang JP, et al. Melatonin protects against lipopolysaccharide‐induced intrauterine fetal death and growth retardation in mice. J Pineal Res 2006;40:40–47. [DOI] [PubMed] [Google Scholar]
- 29. Lee YJ, Choi DY, Choi IS, et al. Inhibitory effect of 4‐O‐methylhonokiol on Lipopolysaccharide‐induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor‐kappa B in vitro and in vivo models. J Neuroinflammation 2012;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah SA, Ullah I, Lee HY, Kim MO. Anthocyanins protect against ethanol‐induced neuronal apoptosis via GABAB1 receptors intracellular signaling in prenatal rat hippocampal neurons. Mol Neuro 2013;48:257–269. [DOI] [PubMed] [Google Scholar]
- 31. Shah SA, Yoon GH, Kim H, Kim MO. Vitamin C Neuroprotection Against Dose‐Dependent Glutamate‐Induced Neurodegeneration in the Postnatal Brain. Neurochem Res 2015;40:875–884. [DOI] [PubMed] [Google Scholar]
- 32. Shahid AS, Hae YL, Ray AB, Dae JY, Kim MO. Novel osmotin attenuates glutamate‐induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death Dis 2014;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah SA, Yoon GH, Chung SS, et al. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer's disease neuropathological deficits. Mole Psychiatry 2016;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tahir A, Haroon B, Tae HK, Kim MO. Melatonin attenuates D‐galactose‐induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF‐KB/JNK signaling pathway in aging mouse model. J Pineal Res 2015;58:71–85. [DOI] [PubMed] [Google Scholar]
- 35. Shah SA, Yoon GH, Kim MO. Protection of the Developing Brain with Anthocyanins against Ethanol‐Induced Oxidative Stress and Neurodegeneration. Mol Neurobiol 2015;51:1278–1291. [DOI] [PubMed] [Google Scholar]
- 36. Zhao L, Chen YH, Wang H, et al. Reactive oxygen species partially contribute to lipopolysaccharide (LPS)‐induced teratogenesis in mice. Toxicol Sci 2008;103:149–157. [DOI] [PubMed] [Google Scholar]
- 37. Xu DX, Chen YH, Wang H, Zhao L, Wang JP, Wei W. Effect of N‐acetylcysteine on lipopolysaccharide‐induced intrauterine fetal death and intra‐uterine growth retardation in mice. Toxicol Sci 2005;88:525–533. [DOI] [PubMed] [Google Scholar]
- 38. Xu DX, Chen YH, Zhao L, Wang H, Wei W. Reactive oxygen species are involved in lipopolysaccharide‐induced intrauterine growth restriction and skeletal development retardation in mice. Am J Obstet Gynecol 2006;195:1707–1714. [DOI] [PubMed] [Google Scholar]
- 39. Baeuerle PA. Pro‐inflammatory signaling: last pieces in the NF kappa B puzzle. Curr Biol 1998;8:19–22. [DOI] [PubMed] [Google Scholar]
- 40. Wang H, Khor TO. Saw CL, et al. Role of Nrf2 in Suppressing LPS‐Induced Inflammation in Mouse Peritoneal Macrophages by Polyunsaturated Fatty Acids Docosahexaenoic Acid and Eicosapentaenoic. Acid. Mole Pharma 2010;7:2185–2193. [DOI] [PubMed] [Google Scholar]
- 41. Hardeland R, Tan DX, Reiter RJ. Kynuramines metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res 2009;47:109–126. [DOI] [PubMed] [Google Scholar]
- 42. Schaefer M, Hardeland R. The melatonin metabolite N1‐acetyl‐5‐methoxykynuramine is a potent singlet oxygen scavenger. J Pineal Res 2009;46:49–52. [DOI] [PubMed] [Google Scholar]
- 43. Pablos MI, Agapito MT, Gutierrez R, et al. Melatonin stimulates the activity of the detoxifying enzyme glutathione peroxidase in several tissues of chicks. J Pineal Res 1995;19:111–115. [DOI] [PubMed] [Google Scholar]
- 44. Rodriguez C, Mayo JC, Sainz RM, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004;36:1–9. [DOI] [PubMed] [Google Scholar]
- 45. Tomas‐Zapico C, Coto‐Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res 2005;39:99–104. [DOI] [PubMed] [Google Scholar]
- 46. Blanchard B, Pompon D, Ducrocq C. Nitrosation of melatonin by nitric oxide and peroxynitrite. J Pineal Res 2000;29:184–192. [DOI] [PubMed] [Google Scholar]
- 47. Nagai R, Watanabe K, Wakatsuki A, et al. Melatonin preserves fetal growth in rats by protecting against ischemia/reperfusion‐induced oxidative/nitrosative mitochondrial damage in the placenta. J Pineal Res 2008;45:271–276. [DOI] [PubMed] [Google Scholar]
- 48. Richter HG, Hansell JA, Raut S, Giussani DA. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J Pineal Res 2009;46:357–364. [DOI] [PubMed] [Google Scholar]
- 49. Aparicio‐soto M, Alarcon C, Cardeno A, Sanchez‐Fidalgo S, Sanchez‐Hidalg M. Melatonin modulates microsomal PGE synthase 1 and NF‐E2‐related factor‐2‐regulated antioxidant enzyme expression in LPS‐induced murine peritoneal macrophages. Br J Pharmacol 2014;171:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Owuor ED, Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol 2002;64:765–770. [DOI] [PubMed] [Google Scholar]
- 51. Jung KH, Hong SW, Zheng HM, et al. Melatonin ameliorates cerulein‐induced pancreatitis by the modulation of nuclear erythroid 2‐related factor 2 and nuclear factor‐kappa B in rats. J Pineal Res 2010b;48:239–250. [DOI] [PubMed] [Google Scholar]
- 52. Olivier P, Fontaine RH, Loron G, et al. Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats. PLoS One 2009;4:e7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Crespo E, Macias M, Pozo D, et al. Acuna‐ Castroviejo D. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide‐induced multiple organ dysfunction syndrome in rats. FASEB J 1999;13:1537–1546. [PubMed] [Google Scholar]
- 54. Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol 2010;8:228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez‐Gallego J. A review of the molecular aspects of melatonin's anti‐inflammatory actions: recent insights and new perspectives. J Pineal Res 2013;54:1–14. [DOI] [PubMed] [Google Scholar]
- 56. Lin GJ, Huang SH, Chen SJ, Wang CH, Chang DM, Sytwu HK. Modulation by melatonin of the pathogenesis of inflammatory autoimmune diseases. Int J Mol Sci 2013;14:11742–11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reitter RJ, Acuna‐Castroviejo D, Tan DX, Burhardt S. Free radical‐mediated molecular damage: mechanisms for the protective actions of melatonin in the central nervous system. Ann NY Acad Sci 2001;93:200–215. [PubMed] [Google Scholar]
- 58. Vane JR, Botting RM. Anti‐inflammatory drugs and their mechanism of action. Inflamm Res 1998;47:S78–S87. [DOI] [PubMed] [Google Scholar]


