Summary
Introduction
We recently showed that a single high dose of methamphetamine (METH) induces a persistent frontal cortical monoamine depletion that is accompanied by helpless‐like behavior in mice. However, brain metabolic alterations underlying both neurochemical and mood alterations remain unknown.
Aims
Herein, we aimed at characterizing frontal cortical metabolic alterations associated with early negative mood behavior triggered by METH. Adult C57BL/6 mice were injected with METH (30 mg/kg, i.p.), and their frontal cortical metabolic status was characterized after probing their mood and anxiety‐related phenotypes 3 days postinjection.
Results
Methamphetamine induced depressive‐like behavior, as indicated by the decreased grooming time in the splash test and by a transient decrease in sucrose preference. At this time, METH did not alter anxiety‐like behavior or motor functions. Depolarization‐induced glucose uptake was reduced in frontocortical slices from METH‐treated mice compared to controls. Consistently, astrocytic glucose transporter (GluT1) density was lower in the METH group. A proton high rotation magic angle spinning (HRMAS) spectroscopic approach revealed that METH induced a significant decrease in N‐acetyl aspartate (NAA) and glutamate levels, suggesting that METH decreased neuronal glutamatergic function in frontal cortex.
Conclusions
We report, for the first time, that a single METH injection triggers early self‐care and hedonic deficits and impairs frontal cortical energetics in mice.
Keywords: Depressive‐like behavior, Frontal cortex, Glucose metabolism, Methamphetamine
Introduction
Methamphetamine (METH) is a highly addictive amphetamine‐like stimulant that causes positive feelings of euphoria, arousal and reduced fatigue when ingested at lower doses 1. However, METH abusers undergoing abstinence display depression‐related symptoms including inactivity, fatigue, and anhedonia in the first week of METH abstinence as ranked by the Beck Depression Inventory II 2. We recently demonstrated that a single high neurotoxic dose of METH (30 mg/kg i.p.) evoked a long‐lasting despair‐like behavior as gauged by increased immobility time in the tail suspension test up to 7 weeks post‐METH treatment that was accompanied by dopaminergic abnormalities in the frontal cortex 3. Apart from alterations of monoamine metabolism, there is emerging evidence that alterations of energy metabolism might underlie the negative affects in METH withdrawal states 4. Thus, a positive relationship between the severity of depressive symptoms and glucose hypometabolism in the anterior cingulate cortex (ACC) of METH users abstinent for 4–7 days was reported 5. The brain metabolic impact of METH exposure in animal models is poorly characterized. Repeated METH administration (12.5 mg/kg, i.p., four times every 2 h within a day) was devoid of effects at 14 days, but reduced 2‐[14C]deoxyglucose utilization in the AAC after 60 days 6. Also, a daily dose of METH (15 mg/kg) for 5–6 weeks inhibited glucose transport in the frontal and occipital cortex of mice, following [3H]‐glucose infusion through the right common carotid artery 7. However, these studies were focused on glucose usage but did not address negative mood behavior. Hence, we now aim to carry out a detailed characterization of metabolic changes in the frontal cortex at an early timepoint where METH‐induced negative mood behavior is already present. Therefore, we chose to carry out this metabolic profiling 3 days after a single injection of a high dose of METH (30 mg/kg i.p.).
Methods
Animals
Male adult C57BL/6J mice (10 weeks old; 21–26 g; Charles River Laboratories, Barcelona, Spain) were housed four per cage, under controlled environmental conditions (12 h light/dark schedule, at room temperature of 23 ± 1°C, with food and water supplied adlibitum). All experiments were approved by the Institutional Animal Care and Use Committee from Faculty of Medicine, Coimbra University, and were performed following the European Community directive (2010/63/EU) and by the Reporting Guidelines Working Group (2010) 8. The animal procedures were performed in strict accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press 1996). All efforts to minimize animal suffering and the number of used animals were made.
Drugs and Chemicals
We were issued permission by INFARMED (Portuguese national authority of medicines and healths products) to import methamphetamine‐HCl (METH) from Sigma‐Aldrich® (St. Louis, MO, USA). The other chemicals used were from Sigma‐Aldrich and Merck AG (Darmstadt, Germany).
Drug Administration
Animals were injected intraperitoneally with a single dose of METH (30 mg/kg) or with saline solution (0.9% NaCl; SAL) in a volume of 0.1 mL/10 g of body weight. This METH regimen is representative of an acute toxic dosing (ATD). As previously suggested 9, and extensively discussed in a review of METH neurotoxicity 10, this regimen provides the following evident benefits: (1) it has an excellent epidemiological translational value as it recapitulates the high brain levels on first pass extraction occurring after acute high intravenous or smoked METH, (2) this constitutes an attempt to mimic the large doses taken by human METH abusers, which can amount to several grams in a day, (3) it is a good model of potential effects of an overdose in naive nontolerant users, (4) it offers greater experimental control over variables, (5) this model reduces the inherent complexity present in repeated dosage regimens, (6) it reduces the occurrence of seizures and high mortality seen with multiple doses regimen 9. Furthermore, we and others have successfully exploited this METH protocol 3, 11, 12. Finally, all animals survived this dosing regimen and none showed convulsions or weight reductions.
Behavioral Tests
The battery of behavioral tests consisting of elevated plus maze (EPM) test, splash test, sucrose preference test (SPT), open field (OF), and pole test was conducted in three independent cohorts of animals 3 days after i.p. injection of METH (30 mg/kg) or SAL, except for SPT which onsetted the day prior to METH administration. This timepoint was chosen based on our previous findings showing that this METH regimen triggers a helpless‐like behavior, 3 days after METH treatment 3. All tests were carried out between 9:00 and 17:00 h in a sound‐attenuated room under low‐intensity light (12 lx), and the apparatus was cleaned with a solution of ethanol 10% between tests to remove animal odors or clues. Behavior was scored by the same experimenter in an observation room where the mice had been habituated for at least 1 h before beginning the tests and was monitored through a video camera positioned above the apparatuses, and the images were later analyzed with the ANY Maze video tracking (Stoelting Co., Wood Dale, IL, USA) by an experimenter who was unaware of the experimental group of the animals tested. The first set of animals [SAL (n = 8) and METH (n = 8)] performed EPM in the morning and the splash test in the afternoon; the second set of animals [SAL (n = 8) and METH (n = 8)] performed OF in the morning and the pole test in the afternoon; and the third set of animals [SAL (n = 8) and METH (n = 8)] performed SPT during 4 days, starting on the day prior to METH administration.
Elevated Plus Maze
Elevated plus maze (EPM) is often used to evaluate the anxiety‐related behavior in mice 13. It was performed in a black acrylic apparatus (four arms, 18 (length) × 6 (width) cm; LE 848 PANLAB, Barcelona, Spain) placed 55 cm above the floor. Two opposite arms were surrounded by gray opaque walls of 15 cm height (closed arms), while the other two had no walls (open arms). Each animal was placed in the center of the apparatus, facing an open arm, and its exploratory behavior measured during 5 min. The following parameters were evaluated: time spent and the number of entries into the open and closed arms. The anxiogenic‐like behavior was defined by a decreased proportion between the number of entries on the open arms divided by the total number of entries in the four arms and also by a decrease in the time spent on the open arms, compared to the total time spent in both types of arms. The total number of entries in the closed arm was used as a measure of locomotor activity 14.
Splash Test
This test was used to evaluate the impact of exposure to METH on mouse grooming behavior, as an index of self‐care phenotype of experimental groups. For this purpose, 10% sucrose solution was squirted on the dorsal coat of mice in their home cage. Following the dirtying of the mice fur with this viscous solution, the animals initiated grooming behavior. Total grooming time was recorded in an acrylic chamber (40 cm × 40 cm × 40 cm) during 5 min 15.
Sucrose Preference Test
Sucrose consumption is frequently evaluated to assess anhedonia in rodents, by measuring an animal's appetite for a highly palatable, rewarding substance 16. One day before i.p. METH administration, animals were single housed and each mouse was habituated to the presence of two water bottles on the extreme sides of the cage for a 24 h period. To prevent interference of metabolic factors and acute stress, no previous food or water deprivation was applied before the test. On the day of METH administration and for the following 2 days, mice were given a two‐bottle choice of tap water versus 4% sucrose in tap water. To avoid potential side preferences, the position of the bottles was switched halfway through the procedure (36 h). Consumption of water and sucrose in control and experimental groups was measured once daily 17. Food consumption was also measured once daily, following METH administration. The preference for sucrose was calculated as a percentage of consumed sucrose solution over the total amount of liquid (sucrose plus water) consumption.
Open Field Test
To discard the effects of METH on locomotor activity, the animals were individually placed for 5 min in the center of an open field (OF) arena (new environment) made up of a wooden box (40 cm × 40 cm × 50 cm), and the horizontal locomotion (total distance traveled) was recorded 18.
Pole Test
The pole test was used to assess the agility and motor coordination of animals exposed to METH, thus further confirming the absence of motor impairments induced by METH. It consisted of a 55 cm‐high wooden pole, 0.8 cm in diameter, wrapped in gauze to prevent slipping and having the base position in the home cage. Mice were placed head upward on the top of this vertical rough‐surfaced pole, oriented themselves downward and descended into their home cage (descent time). On the test day, animals were exposed to five trials, and the time to descend (descent time) was measured. The best performance over five trials was used. If the mouse was unable to turn completely downward, fell or slipped down, the default value of 120 seconds was taken as maximal severity of impairment 14.
Ex Vivo Glucose Uptake
We evaluated glucose uptake using a previously optimized protocol for acute mouse brain slices 19, 20, which allows simultaneously to compare the effect of various treatments in pairwise arrangement. Briefly, mice from first set of animals were decapitated under halothane anesthesia (no response to tail pinch) 3 days post‐METH injection, their brains were rapidly removed and placed in ice‐cold carboxygenated (95% O2 and 5% CO2) Krebs–HEPES (KH) solution with the following composition (in mM): 133 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 25 NaHCO3, 5.5 glucose, and 10 HEPES (pH 7.4). Transversal coronal frontocortical acute slices of 400 μm thickness were prepared and divided randomly into two pools from each mouse. After 1‐h recovery at 37°C with continuous oxygenation, one pool of slices from each mouse was challenged with 15 mM KCl (depolarized slices) and the other pool with 15 mM NaCl (osmotic control, aka “resting” slices) in batch assay, as before 19. One minute later, 3H‐2‐D‐deoxyglucose [3H]DG (American Radiolabeled Chemicals, ARC, USA; specific activity, 60 Ci/mmol) was added to the bath at the final concentration of 2 nM for 30 min to monitor resting and depolarization‐stimulated uptake rates. After 30 min, the slices were washed twice in ice‐cold assay solution for 5 min and collected in 1 mL NaOH (0.5 M) to dissolve them. An aliquot of 800 μL was then assayed for 3H in a Tricarb β‐counter, while the remaining sample was used to quantify the protein in a bicinchoninic acid assay (BCA; Merck Biosciences, Germany). Tritium uptake was multiplied by a factor of 2.75 × 10 to estimate the total glucose uptake (corresponding to the concentration difference between D‐glucose and [3H]DG in the uptake medium 19, 20). Nonspecific uptake rates (~8 nmol/mg protein) were determined in spare cortical slices on ice, under treatments otherwise similar to the experiments at 37°C. Total D‐glucose uptake was calculated for the 30 min uptake period as before 19, 20 and expressed as nmol/mg protein 19.
Neurochemical Analysis
Mice from the second and third sets of animals were sacrificed by cervical dislocation following the behavioral tests. Frontal cortices were dissected on ice and stored at −80°C until further analyses. This brain region was chosen on account of its relevance to depressive‐like behavior 21. Right cortices were used for the proton‐NMR analysis, while left cortices were used for Western blot analysis.
Proton HRMAS Analysis
Proton (1H) high rotation magic angle spinning (HRMAS) spectroscopy was performed in frontal right cortices samples using a 500‐MHz Brucker NMR spectrometer equipped with a 4 mm HRMAS 500S2 HCND Z‐GRD 4 G probe head, specific for high‐resolution liquid or semi‐solid samples. Each sample consisted of the entire right cortex plus 10 μL of a 100 mM sodium fumarate solution (99.9% D2O), used for lock and quantification purposes (internal reference), put into a 4 mm Zirconium rotor with 50 μL volume capacity. A 1D Carr–Purcell–Meiboom–Gill (CPMG) NMR sequence was used 22. Typical acquisition parameters included a 2.0 seconds acquisition time, defining a 12 kHz sweep width, and a predelay of 4 seconds. This repetition time of 6.0 seconds ensured full relaxation of all protons in the sample as T1s were all below 0.7 seconds. A total of 128 scans were averaged to allow good signal to noise for metabolite quantification. Spectra integration was made using Amix‐viewer (version 3.9.14, BrukerBiospin, Rheinstetten, Germany).
Western Blot Analysis
For measuring glucose transporter (GluT1 and GluT3) levels, total extracts were prepared using left frontal cortices as previously described 3. Total protein concentration was determined by the BCA method. Samples were denatured at 37°C for 60 min in denaturing solution [Tris–HCl, 0.5 M, pH 6.8 with SDS 10% (w/v), glycerol 30% (v/v), DTT 0.6 M, and bromophenol blue 0.01% (w/v)]. Equal amounts of protein (20 μg) were loaded and separated by electrophoresis on sodium dodecyl sulfate polyacrylamide gel (10%), transferred to a polyvinylidene difluoride membrane (Millipore, Madrid, Spain), and blocked with 5% (w/v) nonfat dry milk in phosphate‐buffered saline for 1 h at room temperature. The membranes were probed with rabbit anti‐GluT1 (1:500; Millipore, Danvers, MA, USA) or mouse anti‐GluT3 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. Membranes were then incubated with alkaline phosphatase‐conjugated secondary antibodies (1:1000 anti‐rabbit and 1:5000 anti‐mouse, respectively, GE Healthcare, Buckinghamshire, UK). Finally, membranes were visualized on Fluorescent Image Analyzer Typhoon FLA 900 (GE Healthcare) and analyzed using Image Quant 5.0 software (Molecular Dynamics, Inc., Sunnyvale, CA, USA). Protein densities were normalized against internal controls β‐actin (1:5000; Sigma‐Aldrich) and GAPDH (1:5000; Millipore) and then expressed as percentage of control.
Statistical Analysis
The data are expressed as mean ± SEM. Behavioral and Western blot data were analyzed by unpaired Student's t‐test or one‐way analysis of variance (ANOVA) followed by the Bonferroni post hoc test analysis (sucrose preference test). HRMAS data were analyzed by unpaired Student's t‐test followed by Benjamini–Hochberg 23 correction for multiple comparisons; the false discovery rate (FDR) adopted was 5%. Significant differences were defined at P < 0.05. All analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Effect of METH on Anxiety‐ and Depressive‐Like Behaviors in Mice
The possible anxiogenic‐like and depressive‐like behaviors associated with an early timepoint following METH were addressed in the EPM, and the splash and sucrose preference tests, respectively. The METH group showed a decreased grooming time when compared with SAL group (Figure 1A; P < 0.01). This indicates a disruption of self‐care behavior phenotype associated with METH exposure. Regarding SPT, 1 day after administration, METH‐treated mice demonstrated a significant reduction in sucrose preference when compared to saline group (Figure 1B; P < 0.001), which paralleled a decrease in total liquid consumption (water + sucrose) (data not shown; P < 0.001). However, on days 2 and 3 posttreatment, METH group was not significantly different from saline group in terms of sucrose preference (Figure 1B). Moreover, METH‐treated mice consumed significantly more sucrose solution on day 2 compared to saline group (Figure 1C; P < 0.05). Finally, the parameters related to anxiety in the EPM were not altered by METH administration, with similar percentage of open arms entries (Figure 1D) and percentage of open arms time (Figure 1E) in the METH and SAL groups. Moreover, no significant changes in the number of closed arms entries were observed (Figure 1F) (P > 0.05), indicating the absence of motor deficits.
Figure 1.
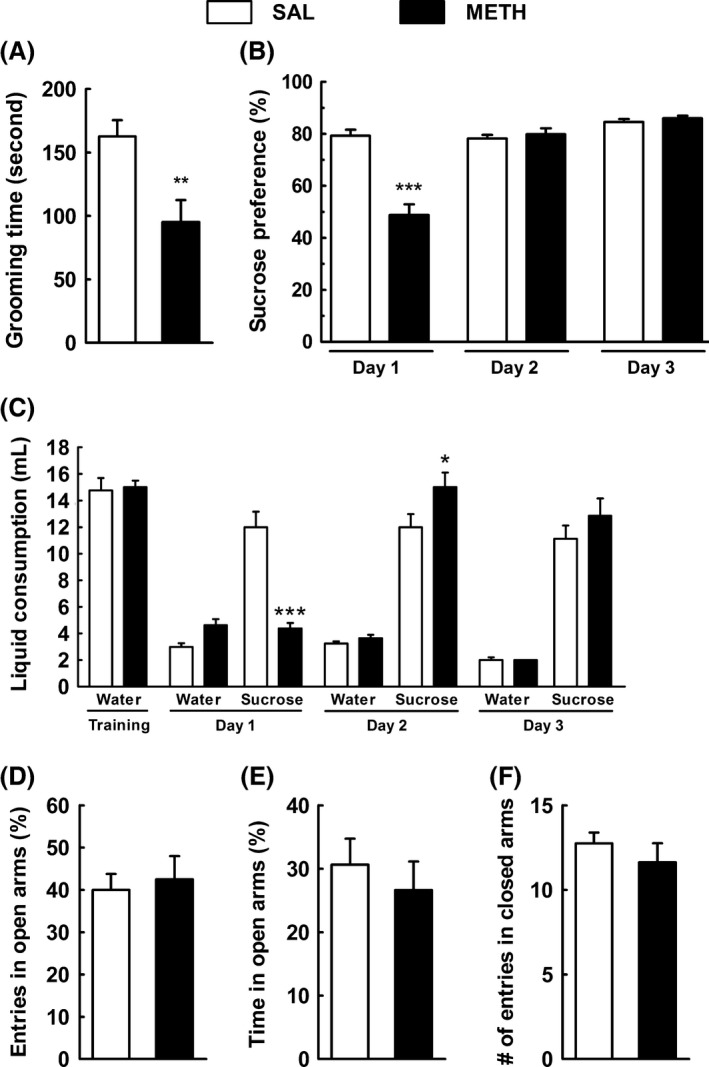
Methamphetamine (METH) decreases grooming time in the splash test and sucrose preference in the sucrose preference test but does not affect anxiety. Effects of a single dose of METH (30 mg/kg, i.p.) on the grooming behavior [splash test (A) and sucrose preference test (B, C)], and on the anxiety‐like behavior [elevated plus maze test (D, E, F)] in mice 3 days after injection. (A) The grooming time, including nose/face grooming (strokes along the snout), head washing (semicircular movements over the top of the head and behind the ears), and body grooming (body fur licking), was significantly decreased in METH mice; (B) sucrose preference was significantly decreased 1 day after METH treatment and returned to basal levels at day 2 post‐METH injection; (C) sucrose consumption was significantly decreased 1 day after METH treatment and significantly increased 2 days after treatment; (D) the percentage of entries in the open arms; (E) the percentage of time spent in open arms; and (F) the number of entries in the closed arms was not statistically different between groups. Animals were monitored during 5 min in the splash test and in the elevated plus maze test. The sucrose preference test was performed during 4 days. Results are mean ± SEM of 7–8 mice in SAL group and eight mice in METH group. *P < 0.05; **P < 0.01; ***P < 0.001 versus saline group using an unpaired Student's t‐test and one‐way ANOVA followed by the Bonferroni post hoc analysis (sucrose preference test) between the indicated experimental groups.
Effect of METH on Motor Function
The putative locomotor impairments associated with METH were further evaluated in the OF and pole tests. Neither the total distance traveled in the OF (Figure 2A) nor the descent time in the pole test (Figure 2B) was significantly changed in METH group when compared to SAL group (P > 0.05). These results are indicative of that the current METH regime did not cause locomotor impairments at the time of behavioral analysis.
Figure 2.
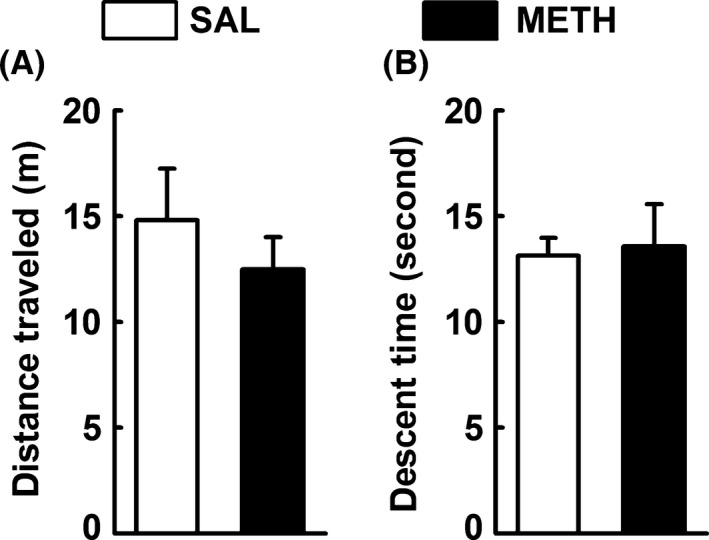
Methamphetamine (METH) does not affect motor function. Effects of a single dose of METH (30 mg/kg, i.p.) on motor function [open field test (A) and pole test (B)] in mice 3 days after injection. The total distance traveled on the open field (A) and the descent time on the pole test (B) were not significantly different between groups. Animals were monitored during 5 min. Results are mean ± SEM of seven mice in SAL group and eight mice in METH group. Statistical analysis was performed using an unpaired Student's t‐test between the indicated experimental groups.
Effect of METH on Glucose Uptake in Depolarized Frontocortical Slices of Mice
Whereas resting glucose uptake in frontocortical slices was not significantly different between the SAL and METH groups (P > 0.05), the lasting depolarization‐stimulated glucose uptake was lower (P < 0.01) in slices prepared from the METH group (Figure 3A). Both astrocytes and neurons are engaged in glucose uptake 20, 24, 25. Hence, we quantitatively analyzed by Western blotting (see Figure 3; inset B’ and C’ for representative images) the density of the major astrocytic glucose transporter isoform, the 45 kDa GluT1 26, which was lower in the frontal cortex of the METH group (P < 0.01; Figure 3B). On the other hand, the density of the 55 kDa GluT1 (Figure 3B; P > 0.05), which is predominantly localized in microvessels 26, as well as the density of the GluT3 (45 kDa) (Figure 3C; P > 0.05), localized exclusively in neurons 27, were not modified.
Figure 3.
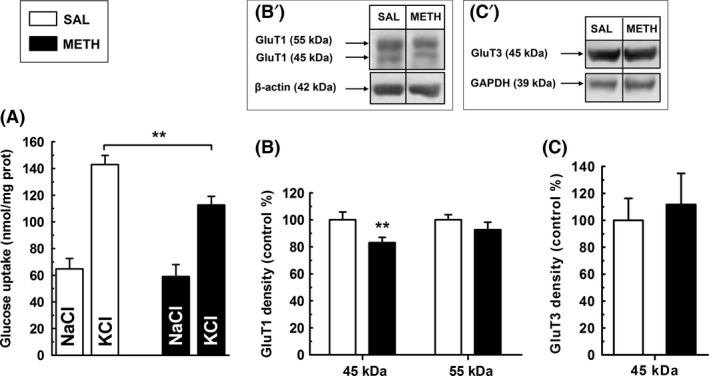
Methamphetamine (METH) impairs ex vivo glucose uptake in depolarized frontocortical slices. (A) Whereas resting glucose uptake by frontocortical slices was not significantly different between groups (n = 8; P > 0.05), glucose uptake in response to lasting depolarization was lower in the METH group; (B) The density of the 45 kDa but not that of the 55 kDa GluT1 isoform was decreased in the frontal cortex of the METH group (n = 8). Inset B’ shows representative Western blot of the 45 and 55 kDa glucose transporter (GluT1) isoforms in frontocortical extracts from SAL and METH groups; (C) The density of GluT3 (45 kDa) in the frontal cortex was not significantly different between groups (n = 7, P > 0.05). Inset C’ shows representative Western blots of GluT3 (45 kDa) in frontocortical extracts from SAL and METH groups. **P < 0.01 versus saline group using an unpaired Student's t‐test between the indicated experimental groups.
Effects of METH on Frontal Cortical Metabolic Parameters
Figure 4A shows representative 1H‐HRMAS spectra obtained from frontal cortices of mice 3 days following SAL or METH injection. METH‐exposed mice showed a decrease in both N‐acetylaspartate/total creatine (NAA/tCr) and glutamate/total creatine (Glu/tCr) ratios, as compared to the SAL group (Figure 4B; P adj < 0.05). The amounts of all other analyzed metabolites including myo‐inositol (a marker of astroglial activation), taurine (a neuroprotective aminoacid), GABA, and glutamine were not significantly different between groups (Figure 4B; P adj > 0.05). METH injection also left unaffected the measured metabolite ratios of Lac/Ala, GABA/Glu, and Glu/Gln (Figure 4C; P adj > 0.05).
Figure 4.
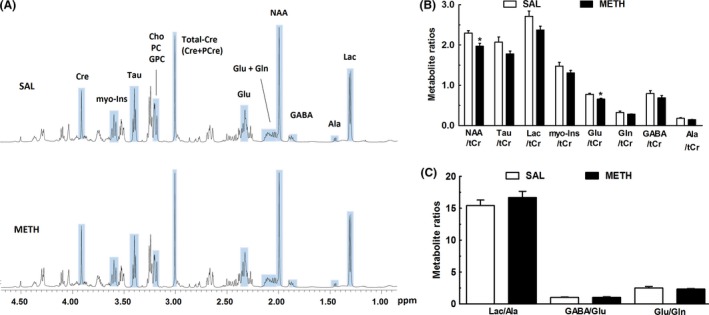
METH changes the metabolic profile of the frontal cortex. A typical 500 MHz CPMG 1H NMR spectrum of the frontal cortex from mice injected with saline (SAL) or a single dose of METH (30 mg/kg, i.p.) (A). The chemical shift range was from 0.80 to 4.70 ppm. Peak assignments: Ala, alanine; Cre, creatine; PCre, phospho‐creatine; Total‐Cre, tCr (total creatine), Cre+PCre; Cho, choline; PC, phosphocholine; GPC, glycerophosphocholine; GABA, γ‐aminobutyric acid; Glu, glutamate; Gln, glutamine; Lac, lactate; myo‐Ins, myo‐inositol; NAA, N‐acetyl‐aspartate; Tau, taurine; (B) 1H HRMAS spectral integration for metabolite quantification showing a significant decrease of the Glu and NAA levels in the METH group. Alanine, lactate, taurine, myo‐inositol, glutamine, and GABA levels were not significantly different between groups (P adj > 0.05). (C) The ratios Lac/Ala, GABA/Glu, and Glu/Gln were also not significantly different between groups (P adj > 0.05). Total creatine was used as internal reference for integration. Results are mean ± SEM of six mice in SAL group and eight mice in METH group. HRMAS data was analyzed by unpaired Student's t‐test followed by Benjamini–Hochberg (Benjamini and Hochberg, 1995) correction for multiple comparisons; the false discovery rate (FDR) adopted was 5%. *P adj < 0.05 versus saline group.
Conclusion
The main finding of the present study was the identification of an impaired frontocortical metabolic profile coexisting with early depressive‐like behavior resulting from a single high METH exposure. This impaired frontocortical energy metabolism was typified by an impaired glucose uptake upon activation of the circuitry and by decreased NAA/tCr and Glu/tCr measured by proton HRMAS. Recently, we used tail suspension test, which is a validated behavioral test to probe negative mood states in a variety of mouse models of depression 3, 28. However, it was mandatory to corroborate this finding using other validated behavioral tests. Therefore, we further characterized the negative emotional state of mice during early post‐METH period (3 days postinjection) by probing the anxiety‐ and depressive‐like behavior. The data obtained in the EPM test showed that the METH group did not exhibit an anxiety‐like trait as inferred from the similar exploration of open arms when compared to SAL. However, the splash test unveiled a significant decrease in the grooming time in the METH group, which is indicative of decreased self‐care behavior, mimicking the apathy observed in depressed patients 29. Moreover, stress‐induced grooming perturbation is associated with reduced hedonic reactivity in the SPT 30. This is the first study evaluating whether METH induced anhedonic‐like behavior in mice, using SPT. Sucrose preference was reduced at 24 h but returned to basal levels at 48 h, where it remained at 72 h post‐METH injection. Sucrose intake was enhanced at 48 h and returned to basal levels at 78 h post‐METH injection. This is suggestive of a rebound effect in sucrose intake and further suggests that METH induces a transient anhedonic‐like behavior. Interestingly, C57Bl6 mice subjected to a stress protocol for 7 weeks seemed to exhibit a rebound effect in sucrose intake following a drop in this measure 31. METH seemingly disrupts self‐care behavior (splash test) that outlasts anhedonia (SPT). This may suggest that splash test is more sensitive than SPT to probe depressive‐like symptoms when using this METH model. Nonetheless, one cannot exclude that using a lower sucrose concentration (e.g., 1%) that would probably impose a lower initial sucrose preference might have disclosed a longer anhedonic behavior. The decreased self‐care behavior seen in the splash test is in agreement with the helpless‐like behavior evaluated by the tail suspension test 3 days after the same METH administration to mice 3. Overall, these results are consistent with a recent study suggesting a depressive‐like state rather than an anxiety‐like state during METH‐early withdrawal using a rat self‐administration model 32. Moreover, Haidar et al. 33 showed that female C57BL6 mice subjected to chronic METH regimen exhibited behavioral despair during the first 48 h of withdrawal without evident anhedonic‐like (SPT) and anxiety‐like behaviors (measured by SPT and EPM, respectively). The lack of alterations during the performance of the open field and pole test indicates that the METH‐exposed animals have normal motor function; thus, ruling out that putative motor deficits may be responsible for the observed depressive‐like behavior.
There is a growing wealth of neuroimaging studies of depressed patients showing abnormalities of regional cerebral blood flow and glucose metabolism in various brain regions, namely in the prefrontal cortex 34. Thus, we here focused on possible metabolic perturbations occurring in subjects showing METH‐induced negative mood state. Glucose is the main energy source for the brain and is transported from the circulation to the brain through the blood–brain barrier (BBB) via the 55 kDa endothelial glucose transporter protein‐1 (GluT1) and is then delivered to astrocytes via the astrocytic 45 kDa GluT1 isoform 7, 35 and to neurons via the neuronal GLUT3 protein 27. We here report for the first time that depolarization‐induced glucose uptake in frontal cortical slices from METH‐injected mice was diminished as compared to the SAL group. This is paralleled by a decrease in the density of the astrocytic 45 kDa GLUT1 isoform, and by unaltered neuronal GLUT3 levels in the frontal cortex of METH‐injected mice compared to the SAL group. It is tempting to speculate that compromised glucose uptake under stimulation is associated with the observed decrease in the 45 kDa GLUT1 isoform in astrocytes. It has been shown previously that high‐K+ stimulation triggers a decrease in astrocytic Na+ levels, both in cell culture and slices, leading to marked increases in glucose uptake and glycolysis 36, 37. Additionally, neurons have one order of magnitude lower glucose transport capacity than astrocytes 38. Taken this predominant role of astrocytes in glucose uptake in brain slices, it is logical to assume that changes in total K+‐stimulated glucose uptake in our assay reflected predominantly astrocytic impairments. Likely, GluT1 even at its reduced density was capable of subserving basal glucose uptake, and failure was revealed only when the system was tested for its maximal capacity with high‐K+ stimulation.
Notably although the study of Jakoby et al. 38 investigated resting glucose transport in vitro 37, while in vivo, neurons in the stimulated cortex appear to rely upon glucose oxidation as much as glial cells 25. If so, the deficit in glucose uptake under high‐K+ stimulation still can be exclusively astrocytic in our study, but it is equally possible that METH treatment led to reduced neuronal activity, and consequently to smaller rates of glucose uptake under depolarization. In fact, this notion is supported by the observed reduction in NAA and Glu levels and hypoactive neurons requiring less glucose may trigger a consequent downregulation in astrocytic GluT1 levels.
In summary, these findings are in accordance with an hypometabolic phenotype typical of the limbic system in human mood disorders 39. Although in those human imaging studies the frontal cortex was not mapped under external depolarization, it is the mild KCl‐depolarization rather than the resting condition of the ex vivo slice which better resembles the activity of the in situ circuitry in a conscious individual.
Besides glucose uptake, we also measured for the first time the frontocortical 1H‐NMR metabolomics in early post‐METH timepoint. We disclosed significant changes in NAA and glutamate levels. Reductions of the brain levels of NAA are paralleled by the reductions in ATP in several experimental paradigms illustrating brain energy metabolism impairment 40. Therefore, NAA is regarded as a marker of neuronal function indexed to mitochondrial energy metabolism 41 and has been envisioned as a reliable candidate for confirming oxidative injury caused by METH 42. The observed decrease in frontocortical NAA levels in the METH group are consistent with the previous findings described by Bu et al. 43 showing a decrease in frontocortical NAA levels on the seventh day of a twice‐daily 2.5 mg/kg METH injection regimen, using 1H‐NMR spectroscopy. Our findings are also in line with previous studies showing lower anterior cingulate cortex NAA levels in METH users undergoing short‐term abstinence 44, 45. Moreover, 1H spectroscopy studies by Gruber et al. 46 reported diminished NAA levels in the frontal lobes of depressed adults. Therefore, decreased NAA levels seen herein are consistent with compromised frontal cortex energetics in METH‐treated mice and decreased neuronal function. This is also corroborated by the observed reduction in glutamate levels. Being glutamate the major excitatory neurotransmitter, it is predictable that METH treatment causes alterations in the glutamatergic activity. Veeraiah et al. 47 reported dysfunctional glutamatergic and GABAergic activities in the prefrontal cortex of mice in social defeat model of depression. These authors also described that mice showing a depression‐like phenotype exhibited significant reductions in the levels of glutamate, glutamine, NAA, and taurine in the prefrontal cortex. In our studies, we only observed significant changes in glutamate and NAA levels, suggesting that our model causes less severe metabolic alterations. Glutamate homeostasis was also proven dysregulated in cortex in rats following METH self‐administration 48, and in humans during early abstinence from chronic METH abuse 49. Our metabolic data in mice are consistent with previous data reporting energy metabolism dysfunction in frontal cortices from rats using different METH paradigms 50, 51, 52. Myo‐inositol is an astrocytic activation marker 53, and the presently observed lack of changes seen in myo‐inositol levels in METH mice is not suggestive of major alterations of astrocytic phenotype in the frontal cortex. This is consistent with our previous report of normal levels of frontocortical GFAP in METH mice 3 and suggests that the neurotoxic properties of METH are associated with changes in activity and metabolism, rather than phenotype of astrocytes. However, increases in myo‐inositol in ACC in the rhesus monkeys at 1 week following METH withdrawal relative to controls were recently found 54. Different animal models (mice vs. rhesus monkeys; single METH administration vs. METH self‐administration) and brain regions (frontal cortex vs. ACC) might be responsible for this apparent contradiction. Levels of remaining investigated markers did not change.
In conclusion, present results consolidate the negative impact of an acute high dose of METH on mood behavior at an early timepoint. The perturbation in self‐care behavior and the anhedonic‐like phenotype seen herein coincide with a disruption of energy metabolism in frontal cortex. Our data warrant further studies aiming to draw a correlation between metabolic indices and negative mood states in METH context.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We acknowledge Dr. Francisco Caramelo (Laboratory of Biostatistics and Medical Informatics/IBILI, Faculty of Medicine, University of Coimbra, Portugal) for helping with statistical analysis of NMR data. We also acknowledge Fundação para a Ciência e Tecnologia (FCT, Strategic Project PEst‐C/SAU/UI3282/2013, UID/NEU/04539/2013 and COMPETE POCI‐01‐0145‐FEDER‐007440) for funding and CERMAX (Centro de Ressonância Magnética António Xavier) for the use of the NMR spectrometer 800 MHz, part of the National NMR Facility supported by the FCT (RECI/BBB‐BQB/0230/2012). 500 MHz proton HRMAS data was collected at the UC‐NMR facility which is supported in part by FEDER—European Regional Development Fund through the COMPETE Programme (Operational Programme for Competitiveness) and by National Funds through the FCT, grants RECI/QEQ‐QFI/0168/2012, CENTRO‐07‐CT62‐FEDER‐002012, and Rede Nacional de Ressonância Magnética Nuclear (RNRMN). The experiments comply with the current laws of Portugal.
References
- 1. Cruicshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction 2009;104:1085–1099. [DOI] [PubMed] [Google Scholar]
- 2. McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100:1320–1329. [DOI] [PubMed] [Google Scholar]
- 3. Silva CD, Neves AF, Dias AI, et al. A single neurotoxic dose of methamphetamine induces a long‐lasting depressive‐like behavior in mice. Neurotox Res 2014;25:295–304. [DOI] [PubMed] [Google Scholar]
- 4. Berman SM, Voytek B, Mandelkern MA, et al. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry 2008;13:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 2004;61:73–84. [DOI] [PubMed] [Google Scholar]
- 6. Huang Y‐H, Tsai S, Su T, Sim C. Effects of repeated high‐dose methamphetamine on local cerebral glucose utilization in rats. Neuropsychopharmacology 1999;21:427–434. [DOI] [PubMed] [Google Scholar]
- 7. Muneer APM, Alikunju S, Szlachetka AM, Murrin LC, Haorah J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood‐brain barrier dysfunction. Mol Neurodegener 2011;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol 2010;160:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: Necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev 2001;36:1–22. [DOI] [PubMed] [Google Scholar]
- 10. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev 2009;60:379–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pereira FC, Cunha‐Oliveira T, Viana S, et al. Disruption of striatal glutamatergic/GABAergic homeostasis following acute methamphetamine in mice. Neurotoxicol Teratol 2012;34:522–529. [DOI] [PubMed] [Google Scholar]
- 12. Tulloch I, Afanador L, Mexhitaj I, Ghazaryan N, Garzagongora A, Angulo J. A single high dose of methamphetamine induces apoptotic and necrotic striatal cell loss lasting up to 3 months in mice. Neuroscience 2011;193:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lister R. Ethologically‐based animal models of anxiety disorders. Pharmacol Ther 1990;46:321–340. [DOI] [PubMed] [Google Scholar]
- 14. Rial D, Castro AA, Machado N, et al. Behavioral phenotyping of Parkin‐deficient mice: Looking for early preclinical features of Parkinson's disease. PLoS ONE 2014;9:e114216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moretti M, Neis VB, Matheus FC, et al. Effects of agmatine on depressive‐like behavior induced by intracerebroventricular administration of 1‐methyl‐4‐phenylpyridinium (MPP(+)). Neurotox Res 2015;28:222–231. [DOI] [PubMed] [Google Scholar]
- 16. Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress‐induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 2004;29:2007–2017. [DOI] [PubMed] [Google Scholar]
- 17. Strekalova T, Steinbusch HW. Measuring behavior in mice with chronic stress depression paradigm. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:348–361. [DOI] [PubMed] [Google Scholar]
- 18. Dos Santos VV, Santos DB, Lach G, et al. Neuropeptide Y (NPY) prevents depressive‐like behavior, spatial memory deficits and oxidative stress following amyloid‐β (Aβ 1–40) administration in mice. Behav Brain Res 2013;244:107–115. [DOI] [PubMed] [Google Scholar]
- 19. Lemos C, Valério‐Fernandes A, Ghisleni GC, et al. Impaired hippocampal glucoregulation in the cannabinoid CB1 receptor knockout mice as revealed by an optimized in vitro experimental approach. J Neurosci Methods 2012;204:366–373. [DOI] [PubMed] [Google Scholar]
- 20. Lemos C, Pinheiro BS, Beleza RO, et al. Adenosine A2B receptor activation stimulates glucose uptake in the mouse forebrain. Purinergic Signal 2015;11:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krishnan V, Nestler E. Linking molecules to mood: New insight into the biology of depression. Am J Psychiatry 2010;167:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duarte JM, Carvalho RA, Cunha RA, Gruetter R. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin‐induced diabetic rats. J Neurochem 2009;111:368–379. [DOI] [PubMed] [Google Scholar]
- 23. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995;57:289–300. [Google Scholar]
- 24. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci 2013;36:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sonnay S, Duarte JM, Just N, Gruetter R. Compartmentalised energy metabolism supporting glutamatergic neurotransmission in response to increased activity in the rat cerebral cortex: A 13C MRS study in vivo at 14.1 T. J Cereb Blood Flow Metab 2016;36:928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J 1994;8:1003–1011. [DOI] [PubMed] [Google Scholar]
- 27. Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannuci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab 2008;295:E242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 2005;29:571–625. [DOI] [PubMed] [Google Scholar]
- 29. Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural‐neurobiological concordance in the effects of CMS. Neuropsychobiology 2005;52:90–110. [DOI] [PubMed] [Google Scholar]
- 30. Taksande BG, Faldu DS, Dixit MP, et al. Agmatine attenuates chronic unpredictable mild stress induced behavioural alteration in mice. Eur J Pharmacol 2013;720:115–120. [DOI] [PubMed] [Google Scholar]
- 31. Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res 2004;155:135–146. [DOI] [PubMed] [Google Scholar]
- 32. Jang C‐G, Whitfield T, Schulteis G, Koob GF, Wee S. A dysphoric‐like state during early withdrawal from extended access to methamphetamine self‐administration in rats. Psychopharmacology 2013;225:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haidar M, Lam M, Chua BE, Smith CM, Gundlach AL. Sensitivity to Chronic Methamphetamine Administration and Withdrawal in Mice with Relaxin‐3/RXFP3 Deficiency. Neurochem Res 2016;41:481–491. [DOI] [PubMed] [Google Scholar]
- 34. Kalia M. Neurobiological basis of depression: An update. Metabolism 2005;54(5 Suppl 1):24–27. [DOI] [PubMed] [Google Scholar]
- 35. Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J Cereb Blood Flow Metab 2007;27:1766–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rose CR, Ransom BR. Intracellular sodium homeostasis in rat hippocampal astrocytes. J Physiol 1996;491:291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bittner CX, Valdebenito R, Ruminot I, et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci 2011;31:4709–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jakoby P, Schmidt E, Ruminot I, Gutiérrez R, Barros LF, Deitmer JW. Higher transport and metabolism of glucose in astrocytes compared with neurons: A multiphoton study of hippocampal and cerebellar tissue slices. Cereb Cortex 2014;24:222–231. [DOI] [PubMed] [Google Scholar]
- 39. Detka J, Kurek A, Basta‐Kaim A, Kubera M, Lasoń W, Budziszewska B. Neuroendocrine link between stress, depression and diabetes. Pharmacol Rep 2013;65:1591–1600. [DOI] [PubMed] [Google Scholar]
- 40. Moffett JR, Arun P, Ariyannur PS, Namboodiri AM. N‐Acetylaspartate reductions in brain injury: Impact on post‐injury neuroenergetics, lipid synthesis, and protein acetylation. Front Neuroenergetics 2013;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baslow MH. N‐acetylaspartate in the vertebrate brain: Metabolism and function. Neurochem Res 2003;28:941–953. [DOI] [PubMed] [Google Scholar]
- 42. Salo R, Nordahl T, Natsuaki Y, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry 2007;61:1272–1280. [DOI] [PubMed] [Google Scholar]
- 43. Bu Q, Lv L, Yan G, et al. NMR‐based metabonomic in hippocampus, nucleus accumbens and prefrontal cortex of methamphetamine‐sensitized rats. Neurotoxicology 2013;36:17–23. [DOI] [PubMed] [Google Scholar]
- 44. Nordahl TE, Salo R, Natsuaki Y, et al. Methamphetamine users in sustained abstinence: A proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 2005;62:444–452. [DOI] [PubMed] [Google Scholar]
- 45. Salo R, Buonocore MH, Leamon M, et al. Extended findings of brain metabolite normalization in MA‐dependent subjects across sustained abstinence: A proton MRS study. Drug Alcohol Depend 2011;113:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gruber S, Frey R, Mlynarik V, et al. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H‐MRS at 3 Tesla. Invest Radiol 2003;38:403–408. [DOI] [PubMed] [Google Scholar]
- 47. Veeraiah P, Noronha JM, Maitra S, et al. Dysfunctional glutamatergic and γ‐aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol Psychiatry 2014;76:231–238. [DOI] [PubMed] [Google Scholar]
- 48. Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self‐administration and during reinstatement in rats. Neuropsychopharmacology 2014;39:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Neill J, Tobias MC, Hudkins M, London ED. Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. Int J Neuropsychopharmacol 2014;18:pii: pyu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Açikgöz O, Gönenç S, Kayatekin BM, et al. The effects of single dose of methamphetamine on lipid peroxidation levels in the rat striatum and prefrontal cortex. Eur Neuropsychopharmacol 2000;10:415–418. [DOI] [PubMed] [Google Scholar]
- 51. Bachmann RF, Wang Y, Yuan P, et al. Common effects of lithium and valproate on mitochondrial functions: Protection against methamphetamine‐induced mitochondrial damage. Int J Neuropsychopharmacol 2009;12:805–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Feier G, Valvassori SS, Lopes‐Borges J, et al. Behavioral changes and brain energy metabolism dysfunction in rats treated with methamphetamine or dextroamphetamine. Neurosci Lett 2012;530:75–79. [DOI] [PubMed] [Google Scholar]
- 53. Vrenken H, Barkhof F, Uitdehaag BM, Castelijns JA, Polman CH, Pouwels PJ. MR spectroscopic evidence for glial increase but not for neuro‐axonal damage in MS normal‐appearing white matter. Magn Reson Med 2005;53:256–266. [DOI] [PubMed] [Google Scholar]
- 54. Yang S, Belcher AM, Chefer S, et al. Withdrawal from long‐term methamphetamine self‐administration ‘normalizes’ neurometabolites in rhesus monkeys: A (1) H MR spectroscopy study. Addict Biol 2015;20:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]


