Summary
Aims
Estrogens are known to exert a wide spectrum of actions on brain functions including modulation of pain. Besides the circulating estrogens produced mainly by the ovaries, many brain regions are also capable of de novo synthesizing estrogens, which may exert important modulatory effects on neuronal functions. This study was aimed to test the hypothesis that aromatase, the enzyme that catalyzes the conversion of testosterone to estradiols, may be distributed in the rostral ventromedial medulla (RVM), where it may impact on visceral pain.
Methods and results
Adult female rats were treated with cyclophosphamide (CPM, 50 mg/kg, ip, once every 3 days) or saline. At approximately day 10 following the 3rd injection, CPM‐treated rats exhibited colorectal hyperalgesia as they showed significantly greater abdominal withdrawal responses (AWR) to graded colorectal distension (CRD, 0‐100 mm Hg) than the saline group. Immunofluorescent staining and Western blot assay revealed that CPM‐induced colorectal hyperalgesia was associated with significantly increased expression of aromatase and phosphorylated μ‐type opioid receptor (pMOR) and decreased expression of total MOR in the RVM. Intracisternal application of aromatase inhibitors, fadrozole, and letrozole reversed CPM‐induced colorectal hyperalgesia and restored pMOR and MOR expression in the RVM.
Conclusions
Our observations confirmed the expression of aromatase in the RVM, a pivotal brain region in descending modulation of pain and opioid analgesia. The results support the hypothesis that locally produced estrogens in the RVM may be involved in the maintenance of chronic visceral hyperalgesia and the downstream signaling may involve phosphorylation of MOR.
Keywords: μ‐opioid receptors (MOR), aromatase, estrogens, rostral ventromedial medulla (RVM), visceral pain
1. INTRODUCTION
Visceral pain is the most frequent symptom encountered in the clinic, and effective treatment of pain remains a significant clinical challenge. Visceral hyperalgesic diseases such as irritable bowel syndrome and overactive and painful bladder syndromes are more prevalent and often more severe in females.1, 2, 3, 4 Moreover, it has been noted that there is also significant gender differences in the responses to analgesic treatments, with morphine analgesic effect being less potent in females than in males.5
The gender differences in pain and morphine analgesia may be attributable to the actions of estrogens (primarily 17β‐estradiol, E2). Indeed, a large number of preclinical studies have shown that E2 may act at different levels of the pain pathway to impact pain.6 Estrogen receptors (ERs) have been localized on primary afferent neurons including small‐diameter nociceptive neurons and activation of ERα attenuated P2X3‐mediated calcium rise in cultured dorsal root ganglioni (DRG) neurons.7 In the spinal cord, neurons expressing ERα seemed to be confined to the dorsal horn associated with nociceptive transmission8, 9 and spinal administration of E2 was shown to rapidly facilitate nociceptive transmission in the rat.10 At the supraspinal level, ERs are distributed in many brain regions involved in pain processing, including the periaqueductal gray matter (PAG) and the rostral ventromedial medulla (RVM).11 Notably, RVM is an essential component of the endogenous pain modulation pathway, which project to the spinal cord to facilitate or inhibit nociceptive transmission in the dorsal horn.12 In addition, RVM is also one of the main functional areas where endogenous opioid peptides and exogenous morphine exert analgesic effects.13 It has been shown that dysfunction of the descending pain facilitation and inhibition pathways may be associated with the development and maintenance of visceral hyperalgesia.14, 15 Hence, estrogenic effects in RVM may play a role in the regulation of visceral pain.
Circulating estrogens are primarily made in the female ovaries and in smaller amounts in adrenal glands, fat tissues, and male testis. However, it is becoming increasingly clear that many brain regions are also capable of de novo synthesizing estrogens. Aromatase (estrogen synthase), the enzyme that catalyzes the transformation of testosterone to estradiols, has been shown to be distributed widely in the central nervous system (CNS). Locally synthesized estrogens have been implicated in the regulation of neuronal functions such as synaptic plasticity, memory, reproductive behavior, and pain.16, 17, 18, 19, 20 For examples, Ghorbanpoor et al21 reported that pain after spinothalamic tract injury was accompanied by upregulation of aromatase, and ERs in spinal cord dorsal horn and intrathecal application of the aromatase inhibitor letrozole exacerbated pain. More recently, Tran and colleagues employed a transgenic aromatase reporter mouse and provided strong evidence for the expression of aromatase in the laminae I and V of the spinal cord dorsal horn, the caudal spinal trigeminal nucleus and the nucleus of the solitary tract, which are areas critical for the transmission of somatic and visceral nociceptive signals.22 This study has been designed to test the hypothesis that aromatase may be expressed in the RVM, where it might play a role in descending regulation of visceral pain.
2. MATERIAL AND METHODS
2.1. CPM‐induced chronic cystitis in the rat and intracranial application of aromatase inhibitors
Adult female Sprague‐Dawley rats (240‐260 g, 10‐12 weeks old) were housed (4 animals per cage) in a temperature controlled room (22‐25°C) illuminated from 07:00 to 19:00. Food and water were available ad libitum. All animal care and experimental procedures were in compliance with the Guiding Principles in the Care and Use of Animals and the Animal Management Rule of the Ministry of Public Health, People's Republic of China (documentation 545, 2001) and approved by the Ethnic Committee for Experimental Use of Animals of Shanghai Jiaotong University School of Medicine (document #SYXK‐2013‐0050).
Rats were intraperitoneally (i.p.) injected with cyclophosphamide (CPM) (50 mg/kg, Sigma‐Aldrich, Saint Louis, MO, USA) once every 3 days to induce chronic cystitis with referred colorectal hyperalgesia. Control rats received saline injections. From day 7 after the last injection, estrus cycle phase was determined each day through the vaginal smear test as reported previously.23 At around day 10 following the last CPM or saline injection, behavioral test for colorectal sensitivity was carried out in rats at proestrus phase, and tissues samples (urinary bladder, colorectum, brain) were collected for further tests (see below).
To observe the effects of pharmacological inhibition of aromatase in the RVM on CPM‐induced colorectal hyperalgesia, a cohort of CPM‐treated rats (n = 19) were implanted with catheter to the cisterna magna a week before the behavior test. Briefly, the rats were anesthetized with pentobarbital sodium (60 mg/kg, i.p.). Under aseptic condition, a midline incision was made at the dorsal neck and the dura mater between the foramen magnum and C1 lamina was exposed following blunt dissection of muscles. The dura was perforated with a 22‐gauge syringe needle, and a PE‐10 catheter (Clay Adams, Sparks, MD, USA) was advanced for 2 mm into the cisterna magna. The catheter was sealed to the dura with tissue glue and tunneled under the skin and externalized over the head. The incision was then closed with sutures. The catheter was flushed with 10 μL saline and closed with an electrical cauterizer. Rats were allowed to recover for a week. Aromatase inhibitors (fadrozle and letrozole, 10 mmol L−1, 10 μL, n = 6 for fadrozole, n = 7 for letrozole) or vehicle (10 μL, n = 6) were injected into the cisterna magna via the catheter 3 hour before the behavior test.
2.2. Behavior test for colorectal sensitivity
Colorectal sensitivity was measured by recording the response to colorectal distention (CRD) as has been described in detail previously.24, 25 Briefly, all animals were habituated to the test environment for 3 days before measurement. On the day of behavior test, rats were lightly anesthetized with isoflurane. A plastic balloon attached to a tygon tubing was inserted 6 cm into the colorectum via the anus and fixed by taping the tubing to the tail. Rats were placed in small Lucite cubicles (20 × 8 × 8 cm) and allowed to adapt for 30 minute. CRD was performed by rapidly inflating the balloon using a sphygmomanometer. The balloon was inflated to various pressures (10, 20, 40, 60, 80, and 100 mm Hg) for 20 seconds followed by 4‐ minute rest. Abdominal withdrawal reflex (AWR) responses to each CRD were observed by a person blinded to the distension pressure and prior treatment (CPM or saline). AWR was scored as: 0‐normal behavior, 1‐slight head movement without abdominal response, 2‐contraction of abdominal muscles, 3‐lifting of abdominal wall, or 4‐body arching and lifting of pelvis. Each measurement was performed three times, and the repetitive AWR scores for each distension pressure were averaged.
2.3. Collection of tissue samples
Following the behavior test, rats were killed by an overdose of sodium pentobarbital. The urinary bladder and the colorectum were excised and fixed in 10% formaldehyde solution for histological examination. The brainstem was removed and the RVM region was collected under a dissection microscope and frozen immediately for Western blot analysis. To collect brain tissues for immunohistochemistry, rats were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and transcardially perfused with saline followed by 4% of paraformaldehyde and 0.14% of picric acid in phosphate buffer (PB, 0.1 mol L−1, pH 7.4). The brainstem was then removed and postfixed in the same fixative at 4°C.
2.4. Hematoxylin‐eosin staining of the urinary bladder and the colorectum
To examine the histological states or changes of the urinary bladder and the colorectum, the tissues were paraffin embedded and sectioned. The paraffin slides were deparaffinized in xylene I, II, and III for 15, 15, and 10 minute, respectively, and dehydrated in 100%, 95%, 85%, and 75% ethanol for 4 minute, respectively. The sections were stained with hematoxylin‐eosin (HE), dehydrated in 95%, 85%, and 75% ethanol, cleared in xylene, and finally mounted with Permount mounting medium. Morphological changes in the colon and bladder were observed and photographed under a light microscope (Leica DM2500, Leica Microsystems Limited, Wetzlar, Germany).
2.5. Immunofluorescence (IF) staining
To examine the expression of aromatase in the RVM, the postfixed brainstem was cryoprotected with 30% sucrose in 0.1 mol L−1 PB overnight at 4°C. The samples were cut at 20 μm for staining as described previously.26 The sections were first incubated with 0.05 mol L−1 phosphate‐buffered saline (PBS) containing 10% normal goat serum and 0.5% TritonX‐100 at room temperature for 2 hour to block nonspecific binding and this was followed by incubation with primary mouse antiaromatase antibody (1:300, Acris Antibodies GmbH, Herford, Germany) at 4°C overnight. The sections were rinsed with PBS for four times and were then incubated with goat anti‐mouse Alexa fluor 568 secondary antibody (1:1000; Molecular Probes‐Invitrogen, Eugene, OR, USA) at room temperature for 1.5 hour. After washing with PBS, the sections were mounted on glass slides and viewed under the fluorescent microscope (Leica DM2500, Leica Microsystems Limited).
2.6. Western blot
RVM samples were homogenized in lysis buffer containing 20 mmol L−1 Tris‐HCl (pH 8.0), 150 mmol L−1 NaCl, 1 mmol L−1 EDTA, 1% NP‐40, 1 mmol L−1 PMSF, protease inhibitor cocktail (Sigma, St. Louis, MO) and phosphatase inhibitor cocktail (Thermo, Indianapolis, IN) for 1 hour at 4°C. The lysates were centrifuged at 10 000 g for 30 minute at 4°C, and the concentration of protein in each supernatant was determined using a BCA assay (Pierce, Rackford, IL). Twenty‐five‐microgram aliquots were separated on 4%‐20% Tris‐glycine ready gels (Bio‐rad, Hercules, CA), and the separated proteins were transferred from the gel to the surface of nitrocellulose membranes (Bio‐rad). The membranes were blocked with 5% fat‐free dry milk or 5% BSA (only for pMOR) in Tris‐buffered saline (TBS) containing 0.1% Tween‐20 for 2 hour and were then incubated for 18 hour at 4°C with primary antibodies mouse antiaromatase (1:300, abcam, Cambridge, MA, USA), rabbit anti‐pMOR (1:1000, Cell Signaling), rabbit anti‐MOR (1:2000, Immunostar), and mouse anti‐β‐actin (1:2000, abcam). Bound primary antibodies were detected with HRP‐conjugated anti‐rabbit or anti‐mouse secondary antibody (1:3000, Bio‐rad). Immunoreactive bands were visualized using enhanced chemiluminescence (Thermo, Indianapolis, IN), and digital imaging was captured with an Image Quant LAS 4000 mini (GE Healthcare, Life Science). The density of specific bands was measured with NIH ImageJsoftware and was normalized against a loading control (β‐actin).
2.7. Data analysis
The data are expressed as mean ± SEM. Statistical analysis was performed using SPSS software (IBM SPSS Inc., USA). For pressure‐AWR score analysis, Independent‐Samples t‐test was used to assess the difference in AWR scores at various pressures between experimental groups. For area under the curve (AUC) analysis in behavioral test, the AUC of individual pressure‐AWR score curve was determined and was then averaged. One‐Way ANOVA followed by Tukey post hoc test was used to assess the difference in averaged AUC between experimental groups. For aromatase, pMOR or MOR expression analysis, One‐Way ANOVA followed by Tukey post hoc test was used to assess differences between experimental groups. Differences were considered statistically significant when a P value was less than .05.
3. RESULTS
3.1. CPM‐induced colorectal hyperalgesia
The key experimental protocols for animal model preparation and behavior test are illustrated in Figure 1A. Proestrus is characterized by the predominance of nucleated epithelial cells which appear in clusters or individually with occasional cornified cells (Figure 1B). Consistent with previous reports,27, 28 histological examination revealed chronic cystitis characterized by mild edema and bleeding without apparent pathological changes in the colorectum in CPM‐treated rats (Figure 1C). Despite the absence of apparent pathological changes in the colorectum, however, CPM‐treated rats displayed significant colorectal hyperalgesia as compared with the saline‐treated rats. Thus, the CPM‐treated rats (n = 6) had consistently higher CRD‐induced AWR scores than the saline‐treated rats (n = 6) at each distension pressure (P < .05 or P < .01, Independent‐Samples t‐test, Figure 1D). The averaged pressure‐AWR score curve of the CPM group was shifted upward (Figure 1D) with a greater averaged AUC (area under the curve) compared with that of the saline group (P < .001, Independent‐Samples t‐test, Figure 1E). These results indicate that CPM‐treated rats developed chronic colorectal hyperalgesia, which was not associated with apparent pathological changes in the colorectum. A central mechanism likely contributed to the CPM‐induced colorectal hyperalgesia.
Figure 1.
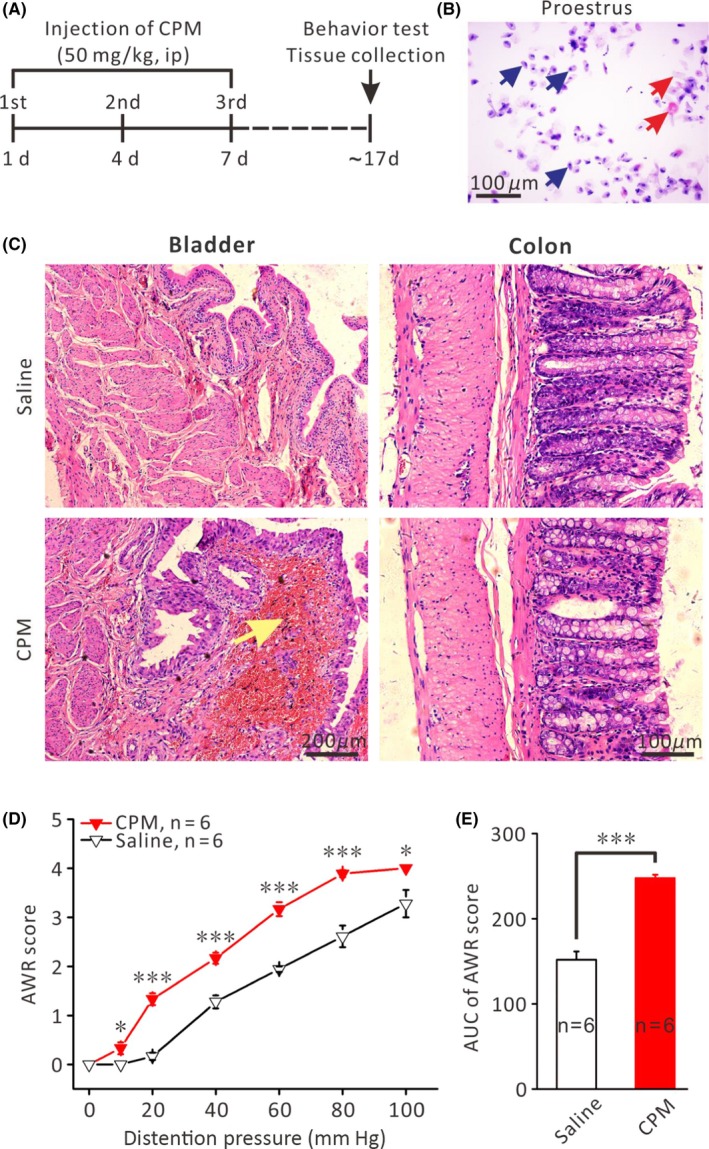
Repetitive application of CPM led to chronic cystitis and colorectal hyperalgesia. A, Protocol of the experiment. B, Representative histological state of the vaginal smear in proestrus female rats. Arrows in blue indicate nucleated epithelial cells; arrows in red indicate cornified cells. C, Representative pictures of HE staining of the urinary bladder and colorectum. CPM‐treated rats exhibited chronic cystitis characterized by edema and hemorrhage (arrows) in the mucosal lamina propria, without apparent histological change in colorectal tissue. D, Plot of the CRD pressure‐AWR score curves for CPM‐ and saline‐treated rats (n = 6 each). Note that the curve for CPM‐treated group was shifted upward compared with the saline group. *P < .05, ***P < .001, comparison of the averaged AWR scores of the CPM‐ and saline‐treated rats at a given CRD pressure using Independent‐Samples t‐test. E, Comparison of the averaged area under the curves (AUC) between CPM‐ and saline‐treated rats (n = 6 each). ***P < .001, Independent‐Samples t‐test
3.2. Increased aromatase expression in the RVM of CPM‐treated rats
Dysfunction of the brainstem descending pain modulation system has been implicated in chronic visceral pain.14 To investigate the possibility that locally synthesized estrogens might play a role in the discordant descending pain modulation system in chronic visceral pain conditions, we set out to detect the expression of aromatase in the RVM of CPM‐ and saline‐treated rats through immunohistochemistry and Western blot. Indeed, aromatase‐immunoreactive neurons were detected in the RVM of saline‐ and CPM‐treated rats (Figure 2A). Moreover, Western blot analysis revealed that the expression of aromatase was markedly upregulated in the CPM‐ than in saline‐treated rats (n = 6 each, P < .05, Independent‐Samples t‐test, Figure 2C). Therefore, CPM‐induced colorectal hyperalgesia is associated with an increased expression of aromatase in the RVM.
Figure 2.
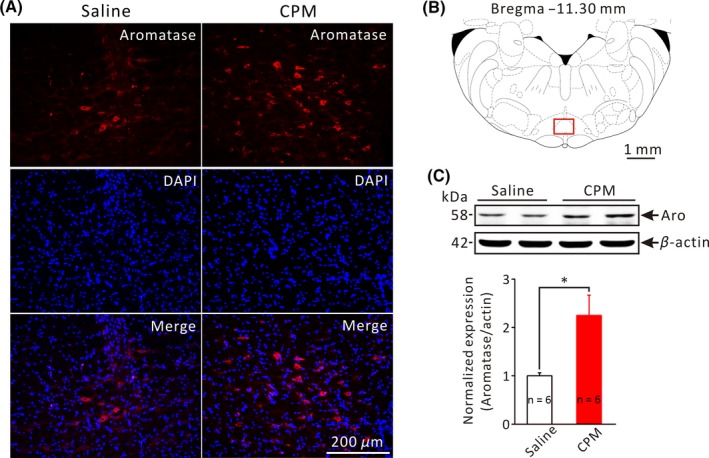
Increased aromatase expression in the RVM of CPM‐treated rats. A, Representative microphotographs of aromatase immunoreactivity (red fluorescence) in the RVM from saline‐ and CPM‐treated rats. B, A schematic coronal section of the brainstem at the level of −11.30 mm from Bregma. Red square indicates area (the nucleus raphe magnus) shown in A. C, Upper panel shows representative Western blot bands of aromatase (MW: 58 kDa) and β‐actin (MW: 42 kDa) in the RVM from saline‐ and CPM‐treated rats. Lower pannel is the bar graph showing the relative density of aromatase in the RVM from saline‐ and CPM‐treated rats. *P < .05, n = 6 for each group, Independent‐Samples t‐test
3.3. Aromatase inhibitors ameliorated CPM‐induced colorectal hyperalgesia
To explore the possible contribution of the upregulated aromatase in the RVM to the CPM‐induced chronic colorectal hyperalgesia, we observed the effects of intracisternal application of aromatase inhibitors, fadrozole (FAD) and letrozole (LET), on visceral pain behavior. Figure 3B compares the averaged AUC of the behavior test (CRD‐induced AWR) among five groups of rats: saline‐treated, CPM‐treated, CPM + vehicle, CPM + FAD, and CPM + LET. As has been described above, repetitive i.p. injections of CPM led to pronounced colorectal hyperalgesia (AUC of AWR score: CPM 248 ± 4.0 vs saline 152 ± 9.6, n = 6 each, P < .001, One‐Way ANOVA, Figure 3B). Intracisternal application of FAD and LET both significantly ameliorated CPM‐induced colorectal hyperalgesia (AUC of AWR score: CPM + FAD 172 ± 11.8 and CPM + LET 174 ± 5.0 vs CPM + vehicle 245 ± 5.4, P < .001 and P < .001, One‐Way ANOVA, Figure 3B). To investigate whether FAD and LET might affect the expression of aromatase, the RVM tissue was collected immediately after the behavior test for Western blot analysis. As shown in Figure 3C,D, aromatase expression was significantly increased in the RVM of CPM‐treated rats compared with saline‐treated rats. Intracisternal application of FAD or LET did not have significant effect on CPM‐induced upregulation of aromatase expression in the RVM (Figure 3C: CPM + FAD 1.8 ± 0.1 vs CPM + Vehicle 1.9 ± 0.2, n = 6 each, P > .05; Figure 3D: CPM + LET 1.9 ± 0.3 vs CPM + Vehicle 2.1 ± 0.3, n = 6 each, P > .05, One‐Way ANOVA). These results suggest that upregulation of aromatase in the RVM contributed to CPM‐induced colorectal hyperalgesia and inhibition of aromatase activity was sufficient to alleviate this form of visceral pain.
Figure 3.
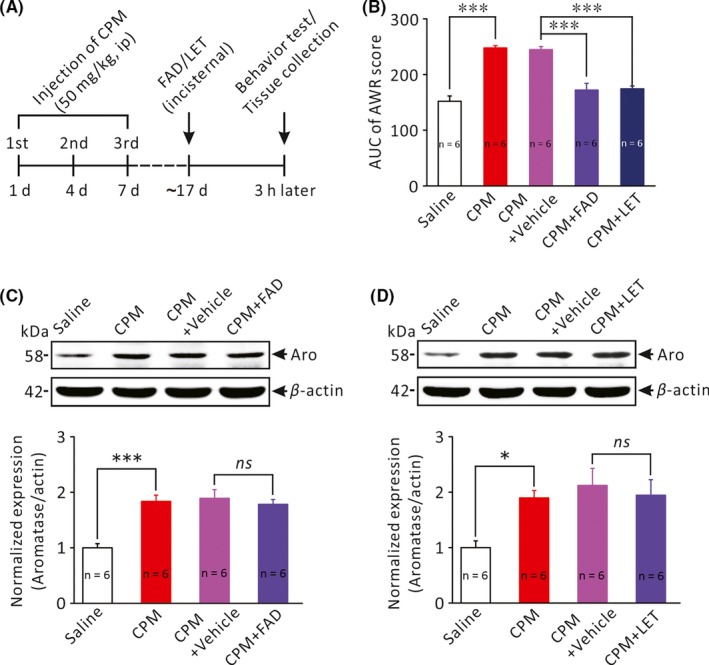
The effects of aromatase inhibitors on colorectal hyperalgesia and aromatase expression in the RVM. A, Protocol of the experiment. B, Bar graph comparing the abdominal withdrawal responses to colorectal distension (averaged AUC of pressure‐AWR score curves) among 5 groups of rats: saline‐treated rats (n = 6), CPM‐treated rats (n = 6), CPM + vehicle (CPM‐treated with intracisternal application of vehicle, n = 6), CPM + FAD (CPM‐treated with intracisternal application of fadrozole, n = 6) and CPM + LET (CPM‐treated with intracisternal application of letrozole, n = 7). ***P < .001, One‐Way ANOVA followed by Tukey post hoc test. C, D, Comparison of the expression level of aromatase (MW: 58 kDa) in the RVM of different groups of rats. *P < .05, ***P < .001, n = 6 for each group, One‐Way ANOVA followed by Tukey post hoc test
3.4. Aromatase inhibitors decreased the phosphorylation of μ‐type opioid receptor (pMOR) in the RVM of CPM‐treated rats
In the RVM, morphine and endogenous opioid peptides produce analgesia primarily via activating the μ‐type opioid receptor (MOR) and phosphorylation of MOR (pMOR) has been implicated in morphine tolerance, that is, decrease in MOR‐mediated analgesia. As previous studies suggested that estrogens may affect MOR signaling,29, 30 we therefore explored whether aromatase inhibitors abrogated CPM‐induced colorectal hyperalgesia via affecting MOR phosphorylation. Figure 4A compares the relative level of pMOR in the RVM in saline‐treated, CPM‐treated, CPM + vehicle and CPM + FAD groups. pMOR expression was significantly elevated in CPM‐treated rats (3.5 ± 0.4, n = 6) compared with the saline‐treated rats (1.0 ± 0.2, n = 6, P < .001) and intracisternal application of FAD restored CPM‐induced upregulation of pMOR (CPM + vehicle 2.8 ± 0.3 vs CPM + FAD 0.9 ± 0.2, n = 6 each, P < .01, Figure 4A). Similarly, CPM‐induced upregulation of pMOR was also restored by intracisternal application of LET (CPM + vehicle 3.0 ± 0.2 vs CPM + LET 1.4 ± 0.1, n = 6 each, P < .01, Figure 4B). Furthermore, there was a decrease in the expression of MOR in the RVM of CPM‐treated rats (CPM‐treated 0.4 ± 0.0 vs saline‐treated 1.0 ± 0.2, n = 6 each, P < .01, Figure 4C). And, CPM‐induced decrease in the expression of MOR in the RVM was also restored by intracisteral injection of FAD (CPM + FAD 0.9 ± 0.1 vs CPM + Vehicle 0.4 ± 0.0, n = 6 each, P < .01, Figure 4C) and LET (CPM + LET 1.1 ± 0.1 vs CPM + Vehicle 0.5 ± 0.0, n = 6 each, P < .001, Figure 4D). These results indicate that CPM‐induced colorectal hyperalgesia is associated with significant upregulation of pMOR and pharmacological inhibition of brainstem aromatase activity was sufficient to restore MOR signaling.
Figure 4.
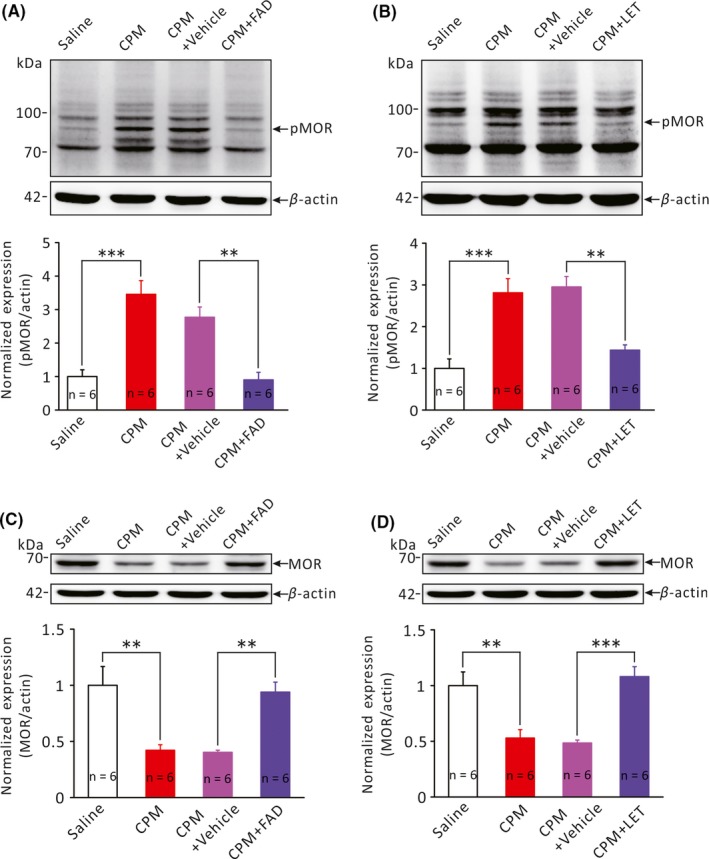
Effects of aromatase inhibitors on the expression of phosphorylated μ‐type opioid receptor (pMOR) in the RVM. A and B, Bar graphs comparing the expression of pMOR (MW: 70~90 kDa) in the RVM of different groups of rats. **P < .01, ***P < .001, n = 6 for each group, One‐Way ANOVA followed by Tukey post hoc test. C and D, Bar graphs comparing the expression of MOR (MW: ~65 kDa) in the RVM of different groups of rats. **P < .01, ***P < .001, n = 6 for each group, One‐Way ANOVA followed by Tukey post hoc test
4. DISCUSSION
It is now becoming increasingly clear that many brain regions are capable of de novo synthesizing neurosteroids including estrogens, which may have important influences on brain functions. In this study, we explored whether aromatase, the enzyme that catalyzes the conversion of androgens to estrogens, is expressed in the RVM, which is an essential component of the brainstem descending pain modulation pathway. A rat model of CPM‐induced chronic cystitis with referred colorectal hyperalgesia was used to assess the possible role of RVM aromatase in the regulation of visceral hyperalgesia. Our data demonstrated that aromatase was indeed detectable in the RVM, and CPM‐induced colorectal hyperalgesia was associated with an upregulated aromatase and pMOR expression in the RVM. Furthermore, pharmacological inhibition of brainstem aromatase activity markedly attenuated CPM‐induced colorectal hyperalgesia and restored pMOR and MOR expression in the RVM. These results strongly suggest that locally synthesized estrogens may negatively impact on opioid signaling in the descending pain modulation pathway, which may contribute to the maintenance of chronic visceral pain.
There has been an increasing interest in the role of estrogens in the regulation of pain, due to the prevalence of chronic pain conditions in females than in males. Circulating estrogens are primarily made in the ovaries and the variation in circulating estrogen level likely accounts for the gender differences in pain and analgesia as well as the variation of pain symptom across the estrous cycle in female subjects.31, 32, 33 In addition to the peripheral sources of estrogens which may impact on neuronal regulation of pain, many areas in the CNS such as the hypothalamus, the amygdala, and the hippocampus are known to be capable of locally synthesizing estrogens from cholesterol.16, 17, 18, 19, 20, 34, 35, 36 Therefore, it is possible that locally synthesized estrogens in discrete regions of the pain pathways may impact on pain and analgesia. Consistent with this notion, there have been reports demonstrating the expression of aromatase, the key enzyme responsible for the conversion of testosterone to estradiols, in areas important for the ascending transmission of pain signals, such as the dorsal horn of the spinal cord and the nucleus of the solitary tract.37, 38
In this study, we focused on the RVM, a pivotal brain region in descending modulation of pain and an important site of morphine analgesia.39, 40, 41, 42, 43 Previous studies suggest that the RVM exerts bidirectional regulatory effects on visceral pain: facilitation or inhibition of visceral pain depending on the type of neurons activated in the RVM.44, 45, 46 Importantly, imbalance of the descending facilitation and descending inhibition may contribute to chronic visceral pain conditions. For example, descending facilitation from the RVM reportedly played a critical role in the maintenance of pancreatic pain.14 Through immunofluorescent staining and Western blot assay, we revealed the existence of aromatase immunoreactivity in the RVM. In addition, we found that aromatase expression was significantly increased in the CPM‐induced chronic cystitis model. These results indicate that aromatase and hence locally synthesized estrogens may impact on the descending modulation of visceral pain.
CPM‐induced cystitis is an established and highly reproducible visceral pain model. Single i.p. injection of a large dose of CPM (typically 150 mg/kg) would induce acute hemorrhagic cystitis characterized by intense inflammatory changes in the urinary bladder accompanied by marked pain symptoms.47 Repetitive application of smaller dose of CPM has been shown to induce chronic but mild to moderate cystitis which was accompanied by referred hyperalgesia and colorectal hypersensitivity.27, 28 As discussed by Brumovsky et al28, both peripheral (ie, sensitization of the primary afferent neurons) and central mechanisms (ie, plasticity of the ascending transmission and central processing of pain) may mediate the colorectal hypersensitivity seen in chronic cystitis. Therefore, CPM‐induced chronic cystitis represents a valid model for us to test the hypothesis that aromatase and locally synthesized estrogens in the RVM might play a role in the regulation of chronic visceral pain. As the severity of visceral pain may vary across the menstrual cycle, we determined the animals' estrous cycle phase through the vaginal smear test and carried out the behavior tests for colorectal sensitivity only at the proestrus phase. Consistent with previous observations,28, 48 we detected moderate cystitis approximately 10 days following the last of three injections of 50 mg/kg CPM. Compared with the saline‐treated rats, CPM‐treated rats displayed significantly enhanced abdominal withdrawal responses (AWR) to graded colorectal distension (CRD) despite the absence of apparent pathology in the colorectum, indicative of central colorectal hyperalgesia.
We found that CPM‐induced colorectal hyperalgesia was accompanied by a significantly increased aromatase expression in the RVM. Furthermore, inhibition of brainstem aromatase activity through intracisternal application of fadrozole (FAD) or letrozole (LET) resulted in rapid reversal of the enhanced nocifensive responses to colorectal distension. Previous reports indicated that intracisternal infusion of drugs was an effective means to pharmacologically manipulate the ventral areas of the brainstem.49, 50 Undoubtedly, however, areas of the dorsal brainstem might also be affected by intracisternally applied agents. Nevertheless, the RVM is the most important region of the brainstem in the regulation of colorectal hyperalgesia, and our results show that aromatase is abdundant in the RVM. The nucleus of the solitary tract (NTS) of the dorsal medulla has also been shown to express aromatase,22 but it relays sensory signals from upper GI tract and is not directly related to colorectal sensitivity. Therefore, it is reasonable to consider the inhibitory effects of letrozole and fadrozole on colorectal hyperalgesia being mediated by inhibition of RVM aromatase activity.
Previously, O'Brien et al37 reported that systemic administration of LET by subcutaneous injection reduced tumor‐induced hyperalgesia in fibrosarcoma‐bearing animals. Clinical studies also showed that LET could effectively improve chronic pelvic pain symptoms in patients with refractory endometriosis.51, 52, 53, 54 In contrast, however, Ghorbanpoor et al21 reported that in a model of central pain syndrome (spinothalamic tract injury), While aromatase expression was upregulated in the dorsal horn, inhibition of the spinal cord aromatase through intrathecal application of LET exacerbated (but not relieved) pain. It appears possible that aromatase in different regions of the pain pathway or in different pain conditions may affect pain differently. Nevertheless, our results suggest that upregulation of aromatase in the RVM might be involved in the maintenance of chronic visceral pain and pharmacological inhibition of RVM aromatase might offer effective pain relief.
The RVM is one of the major functional areas where opioids exert analgesic effects primarily through activation of MOR.42, 43, 55, 56 Estrogens have been shown to negatively affect MOR‐mediated signaling in the arcuate nucleus, the medial preoptic nucleus and the dentate gyrus, probably via promoting phosphorylation of MOR.57, 58, 59, 60 Additionally, Ji et al61 demonstrated that E2 attenuated morphine analgesia in a model of visceral pain by acting at the peripheral and supraspinal sites. This study revealed that CPM‐induced colorectal hyperalgesia was accompanied by concomitant increases in aromatase and pMOR expression and that aromatase inhibitors restored pMOR and MOR expression in the RVM. These results suggest that an increased local estradiol synthesis in the RVM contributed to CPM‐induced colorectal hyperalgesia through inhibition of MOR‐mediated signaling.
In summary, this study has provided evidence that aromatase is expressed in the RVM and upregulation of aromatase expression may contribute to chronic visceral hyperalgesia through inhibiting opioid signaling.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by The National Basic Research Program of China (grant #2014CB910303), The National Natural Science Foundation of China (grant #81270464 and 31171066) and The Sino‐German Science Center (grant #GZ919).
Gao P, Ding X‐W, Dong L, Luo P, Zhang G‐H, Rong W‐F. Expression of aromatase in the rostral ventromedial medulla and its role in the regulation of visceral pain. CNS Neurosci Ther. 2017;23:980–989. 10.1111/cns.12769
The first two authors contributed equally to this work.
REFERENCES
- 1. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta‐analysis. Gastroenterology. 2017;152:1042‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol. 2009;194:31‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123‐167. [DOI] [PubMed] [Google Scholar]
- 4. Homma Y, Ueda T, Tomoe H, et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder updated in 2015. Int J Urol. 2016;23:542‐549. [DOI] [PubMed] [Google Scholar]
- 5. Loyd DR, Murphy AZ. The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol. 2014;259:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amandusson Å, Blomqvist A. Estrogenic influences in pain processing. Front Neuroendocrinol. 2013;34:329‐349. [DOI] [PubMed] [Google Scholar]
- 7. Cho T, Chaban VV. Interaction between P2X3 and oestrogen receptor (ER)alpha/ERbeta in ATP‐mediated calcium signalling in mice sensory neurones. J Neuroendocrinol. 2012;24:789‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amandusson A, Hermanson O, Blomqvist A. Estrogen receptor‐like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci Lett. 1995;196:25‐28. [DOI] [PubMed] [Google Scholar]
- 9. Williams SJ, Papka RE. Estrogen receptor‐immunoreactive neurons are present in the female rat lumbosacral spinal cord. J Neurosci Res. 1996;46:492‐501. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Lü N, Zhao ZQ, Zhang YQ. Involvement of estrogen in rapid pain modulation in the rat spinal cord. Neurochem Res. 2012;37:2697‐2705. [DOI] [PubMed] [Google Scholar]
- 11. Loyd DR, Murphy AZ. Androgen and estrogen (alpha) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. J Chem Neuroanat. 2008;36:216‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fields H. State‐dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565‐575. [DOI] [PubMed] [Google Scholar]
- 14. Vera‐Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155‐2164. [DOI] [PubMed] [Google Scholar]
- 15. Olesen SS, Brock C, Krarup AL, et al. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2010;8:724‐730. [DOI] [PubMed] [Google Scholar]
- 16. Hojo Y, Murakami G, Mukai H, et al. Estrogen synthesis in the brain–role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31‐43. [DOI] [PubMed] [Google Scholar]
- 17. Remage‐Healey L, Maidment NT, Schlinger BA. Schlinger, forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain‐derived estrogens. Front Neuroendocrinol. 2012;33:425‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241‐249. [DOI] [PubMed] [Google Scholar]
- 20. Cornil CA, Seredynski AL, de Bournonville C, et al. Rapid control of reproductive behaviour by locally synthesised oestrogens: focus on aromatase. J Neuroendocrinol. 2013;5:1070‐1078. [DOI] [PubMed] [Google Scholar]
- 21. Ghorbanpoor S, Garcia‐Segura LM, Haeri‐Rohani A, Khodagholi F, Jorjani M. Aromatase inhibition exacerbates pain and reactive gliosis in the dorsal horn of the spinal cord of female rats caused by spinothalamic tract injury. Endocrinology. 2014;155:4341‐4355. [DOI] [PubMed] [Google Scholar]
- 22. Tran M, Kuhn JA, Bráz JM, Basbaum AI. Neuronal aromatase expression in pain processing regions of the medullary and spinal cord dorsal horn. J Comp Neurol. 2017;525:3414‐3428. 10.1002/cne.24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ngadjui E, Nkeng‐Efouet PA, Nguelefack TB, Kamanyi A, Watcho P. High fat diet‐induced estrus cycle disruption: effects of Ficus asperifolia. J Complement Integr Med. 2015;12:205‐215. [DOI] [PubMed] [Google Scholar]
- 24. Hu J, Qin X, Song ZY, et al. Alpha‐lipoic acid suppresses P2X receptor activities and visceral hypersensitivity to colorectal distention in diabetic rats. Sci Rep. 2017;7:3928 10.1038/s41598-017-04283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615‐627. [DOI] [PubMed] [Google Scholar]
- 26. Dong L, Liang X, Sun B, et al. Impairments of the primary afferent nerves in a rat model of diabetic visceral hyposensitivity. Mol Pain. 2015;11:74 10.1186/s12990-015-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boudes M, Uvin P, Kerselaers S, Vennekens R, Voets T, De Ridder D. Functional characterization of a chronic cyclophosphamide‐induced overactive bladder model in mice. Neurourol Urodyn. 2011;30:1659‐1665. [DOI] [PubMed] [Google Scholar]
- 28. Brumovsky PR, Feng B, Xu L, McCarthy CJ, Gebhart GF. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol. 2009;297:1250‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson DL Jr, Nag S, Mokha SS. Estrogen facilitates and the kappa and mu opioid receptors mediate antinociception produced by intrathecal (−)‐pentazocine in female rats. Behav Brain Res. 2016;312:163‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Long N, Serey C, Sinchak K. 17beta‐estradiol rapidly facilitates lordosis through G protein‐coupled estrogen receptor 1 (GPER) via deactivation of medial preoptic nucleus mu‐opioid receptors in estradiol primed female rats. Horm Behav. 2014;66:663‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gustafsson JK, Greenwood‐Van MB. Amygdala activation by corticosterone alters visceral and somatic pain in cycling female rats. Am J Physiol Gastrointest Liver Physiol. 2011;300:1080‐1085. [DOI] [PubMed] [Google Scholar]
- 32. Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balthazart J, Foidart A, Harada N. Immunocytochemical localization of aromatase in the brain. Brain Res. 1990;514:327‐333. [DOI] [PubMed] [Google Scholar]
- 35. Voigt C, Gahr M, Leitner S, Lutermann H, Bennett N. Breeding status and social environment differentially affect the expression of sex steroid receptor and aromatase mRNA in the brain of female Damaraland mole‐rats. Front Zool. 2014;11:38 10.1186/1742-9994-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen RE, Wade J. Aromatase mRNA in the brain of adult green anole lizards: effects of sex and season. J Neuroendocrinol. 2011;23:254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Brien EE, Smeester BA, Michlitsch KS, Lee JH, Beitz AJ. Colocalization of aromatase in spinal cord astrocytes: differences in expression and relationship to mechanical and thermal hyperalgesia in murine models of a painful and a non‐painful bone tumor. Neuroscience. 2015;301:235‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evrard HC, Harada N, Balthazart J. Immunocytochemical localization of aromatase in sensory and integrating nuclei of the hindbrain in Japanese quail (Coturnix japonica). J Comp Neurol. 2004;473:194‐212. [DOI] [PubMed] [Google Scholar]
- 39. Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729‐737. [DOI] [PubMed] [Google Scholar]
- 40. Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319‐325. [DOI] [PubMed] [Google Scholar]
- 41. Zhuo M. Descending facilitation. Mol Pain. 2017;13 10.1177/1744806917699212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684‐686. [DOI] [PubMed] [Google Scholar]
- 43. Conroy JL, Nalwalk JW, Phillips JG, Hough LB. CC12, a P450/epoxygenase inhibitor, acts in the rat rostral, ventromedial medulla to attenuate morphine antinociception. Brain Res. 2013;1499:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Urban MO, Coutinho SV, Gebhart GF. Biphasic modulation of visceral nociception by neurotensin in rat rostral ventromedial medulla. J Pharmacol Exp Ther. 1999;290:207‐213. [PubMed] [Google Scholar]
- 45. Zhuo M, Gebhart GF. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002;122:1007‐1019. [DOI] [PubMed] [Google Scholar]
- 46. Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol. 2002;87:2225‐2236. [DOI] [PubMed] [Google Scholar]
- 47. Augé C, Chene G, Dubourdeau M, et al. Relevance of the cyclophosphamide‐induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Eur J Pharmacol. 2013;707:32‐40. [DOI] [PubMed] [Google Scholar]
- 48. Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2006;291:658‐665. [DOI] [PubMed] [Google Scholar]
- 49. Wu KL, Chao YM, Tsay SJ, et al. Role of nitric oxide synthase uncoupling at rostral ventrolateral medulla in redox‐sensitive hypertension associated with metabolic syndrome. Hypertension. 2014;64:815‐824. [DOI] [PubMed] [Google Scholar]
- 50. Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation. 2012;9:212 10.1186/1742-2094-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Remorgida V, Abbamonte HL, Ragni N, Fulcheri E, Ferrero S. Letrozole and norethisterone acetate in rectovaginal endometriosis. Fertil Steril. 2007;88:724‐726. [DOI] [PubMed] [Google Scholar]
- 52. Verma A, Konje JC. Successful treatment of refractory endometriosis‐related chronic pelvic pain with aromatase inhibitors in premenopausal patients. Eur J Obstet Gynecol Reprod Biol. 2009;143:112‐115. [DOI] [PubMed] [Google Scholar]
- 53. Abushahin F, Goldman KN, Barbieri E, Milad M, Rademaker A, Bulun SE. Aromatase inhibition for refractory endometriosis‐related chronic pelvic pain. Fertil Steril. 2011;96:939‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril. 2004;81:290‐296. [DOI] [PubMed] [Google Scholar]
- 55. Hough LB, Nalwalk JW, Yang W, Ding X. Neuronal cytochrome P450 activity and opioid analgesia: relevant sites and mechanisms. Brain Res. 2015;1616:10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol‐17 beta and mu‐opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136:2341‐2344. [DOI] [PubMed] [Google Scholar]
- 58. Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein‐coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605‐612. [DOI] [PubMed] [Google Scholar]
- 59. Mills RH, Sohn RK, Micevych PE. Estrogen‐induced mu‐opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y‐Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Torres‐Reveron A, Williams TJ, Chapleau JD, et al. Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp Neurol. 2009;219:319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ji Y, Murphy AZ, Traub RJ. Traub. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J Pain. 2007;8:494‐502. [DOI] [PubMed] [Google Scholar]


