Summary
Aim
Asenapine is a new atypical antipsychotic prescribed for the treatment of psychosis/bipolar disorders that presents higher affinity for serotonergic than dopaminergic receptors. The objective of this study was to investigate its antidepressant‐like and antimanic‐like properties on relevant animal models of depression and mania and to assess the acute and chronic effect of Asenapine on dorsal raphe nucleus (DRN) 5‐HT cell firing activity.
Methods
We assessed the effects of Asenapine using in vivo electrophysiological and behavioral assays in rats.
Results
Behavioral experiments showed that Asenapine had no significant effect on immobility time in the forced swim test (FST) in control rats. In the ACTH‐treated rats, a model of antidepressant‐resistance, Asenapine failed to alter immobility time in the FST. In contrast in the sleep deprivation (SD) model of mania, acute administration of Asenapine significantly decreased the hyperlocomotion of SD rats. In the DRN, acute administration of Asenapine reduced the suppressant effect of the selective 5‐HT 7 receptor agonist LP‐44 and of the prototypical 5‐HT 1A receptor agonist 8‐OH‐DPAT on 5‐HT neuronal firing activity. In addition, chronic treatment with Asenapine enhanced DRN 5‐HT neuronal firing and this effect was associated with an alteration of the 5‐HT 7 receptor responsiveness.
Conclusion
These results confirm that Asenapine displays robust antimanic property and effective in vivo antagonistic activity at 5‐HT 1A/7 receptors.
Keywords: 5‐HT1A/7, Asenapine, electrophysiology, manic‐like and depressive‐like behaviors, raphe
Abbreviations
- 5‐HT
serotonin
- 8‐OH‐DPAT
8‐Hydroxy‐2‐(di‐n‐propylamino)tetralin Hydrobromide
- ACTH
Adrenocorticotropic hormone
- Asenapine
(3aS,12bS)‐5‐chloro‐2‐methyl‐2,3,3a,12b‐tetrahydro‐1H‐dibenzo[2,3:6,7]oxepino[4,5‐c]pyrrole
- BD
bipolar disorder
- Dexmedetomidine
5‐[(1S)‐1‐(2,3‐dimethylphenyl)ethyl]‐1H‐imidazole
- DRN
dorsal raphe nucleus
- Flesinoxan
p‐fluoro‐N‐(2‐(4‐(2‐(hydroxymethyl)‐1,4‐benzodioxan‐5‐yl)‐1‐piperazinyl)ethyl)benzamide
- FST
forced swim test
- Imipramine
3‐(5,6‐dihydrobenzo[b][1]benzazepin‐11‐yl)‐N,N‐dimethylpropan‐1‐amine,hydrochloride
- LP‐44
6‐[4‐(2‐methylsulfanylphenyl)piperazin‐1‐yl]‐N‐(1,2,3,4‐tetrahydronaphthalen‐1‐yl)hexanamide hydrochloride
- SB 269970
3‐[(2R)‐2‐[2‐(4‐methylpiperidin‐1‐yl)ethyl]pyrrolidin‐1‐yl]sulfonylphenol,hydrochloride
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitors (SSRIs)
- TCA
tricyclic antidepressant
- WAY‐100635
N‐{2‐[4(2‐methoxyphenyl)‐1‐piperazinyl] ethyl} ‐N‐(2‐pyridinyl) cyclohexanecarboxamide trihydrochloride
1. Introduction
Bipolar disorder (BD) is a severe brain disorder characterized by repeated alternation of mania, depression, and euthymia. It affects millions of people worldwide, with a lifetime prevalence of 1% to 5% making BD a major concern for public health. Up to date available treatments, that is, mood stabilizers and antipsychotic drugs, remain unsatisfactory because of their partial therapeutic efficacy and their delayed action. Accordingly, developing new therapies is an urgent need.1 Ideally, animal models of mental disorders should have construct, face, and predictive validity to identify therapeutic interventions. Actually, the forced swimming test (FST) and amphetamine‐induced hyperactivity test are the most popular paradigms to screen new antidepressant and antimanic candidates, respectively.2 In the FST, Kitamura et al.3 were the first to report that chronically administered ACTH (100 μg/d, s.c.), leading to sustained increase in plasmatic corticosterone, counteracts the decrease in the immobility time caused by classical antidepressants including the TCA imipramine, desipramine and the dual serotonin and noradrenaline reuptake inhibitor milnacipran.3, 4 Remarkably, similar results are obtained after repeated electroconvulsive seizures and treatment with the dual norepinephrine and dopamine reuptake inhibitor buproprion.5, 6 Kitamura et al.5 proposed that animals chronically treated with ACTH can be a useful animal model of TCA treatment‐resistant depression.4, 5 Another relevant model of mania is the rapid eye movement (REM) sleep deprivation (SD) model, firstly proposed by Gessa et al.,7 that produces a transient episode of hyperactivity and insomnia, major clinical symptoms of manic episodes. Nonetheless, even if SD is a triggering factor of manic episodes in BD patients,8 SD is less studied in the view of mania modeling. We suggest that SD should be investigated more deeply, particularly for its predictive validity, for the following reasons. Previous studies have shown that both 96 hours and 72 hours of REM SD reduce hippocampal neurogenesis of adult rats 9, 10 and because neuroplasticity deficits can be associated to BD,11 the SD model might be more suitable in understanding neurobiological characteristics of mania and for screening new drugs. Several physiological functions, such as anxiety/affect or circadian rhythms/sleep, are influenced by 5‐HT system.12 The 5‐HT7 receptor, the most recently identified member of the 5‐HT receptor family,13 has been shown to be involved in several functions modulated by the 5‐HT system such as circadian rhythms.14, 15 5‐HT7 receptor is located in the supra‐chiasmatic nucleus of the hypothalamus, where it is intensely involved in circadian rhythm of animal activity.16, 17 As poor circadian synchrony has been linked to maladaptive changes in bipolar depression,18 one may assume that drugs that target key 5‐HT receptor subtypes involved in circadian rhythm regulation may have therapeutic utility. Asenapine (ORG‐5222) is a novel atypical antipsychotic for the treatment of schizophrenia and BD and has a unique human receptor binding profile. It presents high affinity for serotonin (5‐HT) and noradrenaline receptors, and less for dopamine (DA) receptors. More precisely, Asenapine is pharmacologically characterized by its higher affinity for serotoninergic (including 5‐HT2C: Ki=0.034 nmol L−1, 5‐HT2A: Ki=0.07 nmol L−1, 5‐HT7: Ki=0.11 nmol L−1, 5‐HT2B: Ki=0.18 nmol L−1, and 5‐HT6: Ki=0.25 nmol L−1) and α2‐adrenergic (such as α2B: Ki=0.33 nmol L−1) than dopaminergic (D2: Ki=1.3 nmol L−1 and D3: Ki=0.42 nmol L−1) receptors.19 It has non‐negligible affinity for histamine H1 (Ki=1.0 nmol L−1) and H2 (Ki=6.2 nmol L−1), but much lower affinity for cholinergic receptors (>1 μmol L−1) and therefore presenting less metabolic side effects (weight gain) than, for example, olanzapine.20 Asenapine acts as a potent antagonist on most of 5‐HT receptor subtypes, α2A/B/C adrenoceptors and D2/3 receptors. Therefore, such a potential multitarget action can suggest that it presents anxiolytic/antidepressant properties 21, 22 and might be considered as a new mood stabilizer. Preclinical studies show that Asenapine increases the release of 5‐HT, DA, norepinephrine, and acetylcholine in the prefrontal cortex and hippocampus similarly to other atypical antipsychotics.22, 23, 24 Locally perfused in the prefrontal cortex, Asenapine was shown to antagonize both α2‐adrenergic and 5‐HT2A receptors.23 Furthermore, Asenapine, acutely administered, increases the firing activity of ventral tegmental area dopaminergic and locus coeruleus noradrenergic neurons and prevents the inhibitory effect of DA2/3 receptor and α2‐adrenoceptor agonists.25, 26 In the dorsal raphe nucleus (DRN), acute administration of Asenapine fails to alter the firing rate of 5‐HT neurons25, 26; however, it has been shown to act as a partial 5‐HT1A agonist when applied by microiontophoresis in both the DRN and hippocampus.25 In view of these elements, this study was aimed, first, at investigating the antimanic‐like and antidepressant‐like actions of Asenapine behaviorally in validated manic and depressive models.9, 27 Second, the effects of acute and sustained administration of Asenapine on the firing activity of DRN 5‐HT neurons will be evaluated. Although multiple receptors can be involved in the Asenapine's modulation of the firing activity of DRN 5‐HT neurons, only the 5‐HT1A and 5‐HT7 receptors antagonisms have been tested in this study. Parts of this article have been published previously in abstract form.28
2. Methods
2.1. Animals
Male Sprague‐Dawley rats (Charles River, France), weighing 250‐300 g at the time of the experiments were used. Experiments began at least one week after reception to allow acclimatization. The animals were housed four per cage the first week and then alone for the SD procedure. Animals were kept under standard laboratory conditions (12‐hours light‐dark cycle, lights on at 7:00 AM, room at 22°C, and free access to food and water). Un‐blinded experiments were performed in accordance with the European Communities Council Directives 86/609, OJ L 358,1, December 12 1987, for the care and use of laboratory animals and performed with the approval of the Regional Animal Care Committee (Faculty of Medicine, Claude Bernard University‐Lyon 1).
2.2. Drugs
Asenapine and the selective 5‐HT1A receptor agonist flesinoxan were furnished by H Lundbeck A/S (Denmark). The 5‐HT1A/7 agonist 8‐OH‐DPAT and the tricyclic antidepressant imipramine were purchased from Sigma Aldrich (France). The 5‐HT7 receptor antagonist SB 269970, the 5HT7 agonist LP‐44, and the α2‐adrenergic agonist dexmedetomidine were purchased from Tocris Bioscience (USA). All drugs were dissolved in 0.9% saline, except for LP‐44 which was prepared in 20% 2‐hydroxypropyl‐β‐cyclodextrin (w/v). ACTH (1‐24, human) was purchased from ChinaPeptides (China) and dissolved in saline. The volume of drug's injection for intraperitoneal dosing was of 1 mL/kg. Asenapine has been shown to display potent antagonistic activity according to the regimen used. The active intravenous dose of 1 mg/kg and sustained subcutaneous dose of 0.3 mg/kg/d was chosen on the basis of previous in vivo electrophysiological data from.25, 29 The active and acute subcutaneous dose of 0.01 and 0.03 mg/kg was chosen on the on the basis of previous behavioral data from.21, 30
2.3. Behavioral experiments
2.3.1. REM sleep deprivation model
REM sleep deprivation (SD) was accomplished with the standard “flower pot” procedure.31 Animals were individually located (2:30 PM) in a standard container (0.3×0.3 m, H x Ø), on a platform (0.086×0.066 m, H x Ø) encircled by 0.02 m of water for 72 hours. Because of the muscular atonia associated with the REM sleep onset, the rat fells into the water when the REM sleep starts. Similar previous studies showed that rats displayed a 30%‐35% decrease in slow wave sleep and a 99% decrease in REM sleep.32, 33 Food and water were available ad libitum, and the container was cleaned every day. During cleaning time, animals were located 5 minutes in a dry cage in which they remained awake (grooming and exploratory behaviors). Control rats were kept in their home cages (four rats per cage) during the experiment. Rats received saline or Asenapine (0.01 and 0.03 mg/kg, s.c.) 30 min before the end of REM SD, and just after the 72 hours of REM SD, they were tested for locomotor activity in Plexiglas cages (0.41×0.26×0.20 m) placed in an actimeter (Imetronic, France) endowed with infrared beams positioned 0.04 m and 0.12 m above the floor. The level of illumination of the device was 25 lux. Locomotor activity, that is, the number of beam breaks, was recorded in six bins of 10 minutes for each rat. Positive control using lithium as prototypical on antimanic agent has been previously performed by our group to validate the SD model.
2.3.2. ACTH model
ACTH (1‐24) (0.1 mg/kg/d, s.c.) or saline was delivered through osmotic minipumps (Alzet, USA, purchased from Charles River, France) positioned on the back of the animal under chloral hydrate (400 mg/kg, i.p.) anesthesia. The forced swim test (FST) was accomplished as previously described by.34 Animals were separately placed in a plastic tank (0.50 m high ×0.220 m in Ø) filled with tap water (25 ± 0.5°C), at a depth of 0.35 m. Two swim sessions were conducted in a dimly light room (12 lux): a 15 minutes pretest session on day‐13 of ACTH treatment, followed by a 5‐minutes test session 24 hours later. Rats were injected with saline or Asenapine (0.01 and 0.03 mg/kg, s.c.) 30 minutes before the test session, or with imipramine (30 mg/kg, i.p.) or 8‐OHDPAT (0.3 mg/kg, i.p.) 60 minutes before the test session. After both sessions, rats were removed from the tanks, dried with hand towels, and kept warm for 30 minutes. The last 4 minutes of the test session was recorded and analyzed with a video‐tracking system (Viewpoint, France), allowing to assess immobility time, defined as the rat floating without struggling and making movements only necessary to maintain its head above water and used as an indicator of depression‐like behavior.
2.4. Electrophysiological experiments
Extracellular recordings were performed with single‐barreled glass micropipettes preloaded with fiberglass filaments. The tip was broken back to 2 to 4 μm and filled with a 2 mol L−1 NaCl solution saturated with Blue Chicago dye. Animals were anesthetized with chloral hydrate (400 mg/kg, i.p.), placed in a stereotaxic frame, and a burr hole was drilled on the midline 1 mm anterior to lambda.35 A lateral tail vein was cannulated with a 24‐gauge catheter for the intravenous (i.v.) administration of drugs. Dorsal raphe (DR) 5‐HT neurons were encountered over a distance of 1 mm starting just below the ventral border of the Sylvius aqueduct. These neurons were identified according to the criteria of36: a slow (0.5‐2.5 Hz) and regular firing rate and long‐duration (0.8‐1.2 ms) positive action potentials.35
2.4.1. Acute administrations
Once a putative 5‐HT neuron was identified, a baseline firing rate was established over 2‐3 minutes. Drugs were then administered with a delay of about 80 seconds between injections. First, saline (0.9% NaCl) was administered. Single dose of 8‐OH‐DPAT (5 μg/kg, i.v.) and successive administrations of LP‐44 (1 mg/kg, i.v.) were used to fully suppress neuronal firing activity. Administration of Asenapine (1 mg/kg, i.v.), was performed prior to the administrations of LP‐44 and 8‐OH‐DPAT. We also evaluated the effect of the administration of SB 269970 (1 mg/kg, i.v.) prior to the administrations of LP‐44 and 8‐OH‐DPAT. Drug‐induced changes (after reaching a plateau) in neuronal activities were recorded over 1‐2 minutes and were plotted as percent changes from the pre‐injection baseline rate period defined as 100%.
2.4.2. Chronic treatments
Rats were treated with saline or Asenapine (0.3 mg/kg/d, s.c.) using osmotic minipumps for 3, 14, or 21 days. Osmotic minipumps (model 2ML1, 2ML2, or 2ML4, Alzet®, purchased through Charles River, France; release rate 10, 5 or 2.5 μL per hour, respectively) were implanted s.c. in the back of the rats under chloral hydrate (400 mg/kg, i.p.) anesthesia. The electrophysiological recordings were undertaken at the end of the treatment with the minipump on board. To estimate possible changes in the spontaneous neuronal firing activity of 5‐HT cells, four to five successive descents were carried out in the DRN in sham and Asenapine‐treated rats. In another group of rats treated for 14 d with saline or Asenapine (0.3 mg/kg/d, s.c.), the responsiveness of 5‐HT7, 5‐HT1A receptors, and α2‐adrenoceptors was evaluated using LP‐44 (1‐4 mg/kg, i.v.), flesinoxan (100‐400 μg/kg, i.v.), 37 and dexmedetomidine (10‐20 μg/kg, i.v.).38
2.5. Statistical analysis
Statistical analysis was performed using StatView (Abacus Concepts Inc., USA). We used two‐way repeated‐measures and one‐way ANOVA followed by PLSD Fisher's post hoc test for electrophysiological experiments, two‐way ANOVA followed by PLSD Fisher's post hoc test for behavioral studies. Statistical significance was set at P<.05.
3. Results
3.1. Effects of Asenapine in the sleep deprivation (SD) model of mania
To assess the potential antimanic‐like efficacy of Asenapine, we measured the impact of an acute Asenapine injection (0.01 and 0.03 mg/kg, s.c.) on the hyperlocomotion induced by a 72 hours REM SD. Results are presented in Figure 1.
Figure 1.
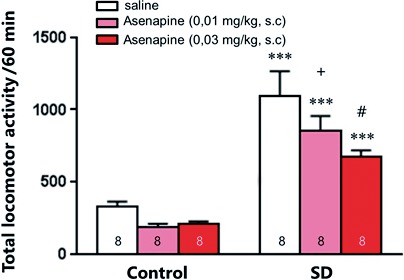
Effect of acute Asenapine on manic‐like behaviors. Effect of acute Asenapine (0.01 or 0.03 mg/kg, s.c.) administration on the locomotor activity of control and REM sleep deprived (SD) animals. Values plotted are mean ± SEM. ***P<.001 vs saline; + P=.052, #P=.001 vs SD‐saline, using two‐way ANOVA followed by Fisher PLSD post hoc test
Two‐way ANOVA indicated a significant effect of SD (F1,42=85.21, P<.001), Asenapine treatments (F2,42 =5.41, P<.001), but no interaction. PLSD post hoc analysis showed that Asenapine, at both doses tested, significantly decreased the hyperlocomotion of SD rats (P<.001). Despite a trend toward a decreased locomotor activity, Asenapine did not significantly reduce the locomotion of control animals.
3.2. Effects of Asenapine in the ACTH model of resistant depression
To evaluate the potential antidepressant‐like activity of Asenapine, we used the ACTH model of resistant depression. Animals were treated chronically with a regimen of ACTH (0.1 mg/kg/d, s.c., for 14 days) and tested behaviorally in the forced swim test (FST). Results are shown in Figure 2. As previously observed by,27 animals that received chronic ACTH‐(1‐24) treatment (100 μg/kg/d) over 14 days did not significantly differ in immobility time compared to control saline animals (P=.0727).
Figure 2.
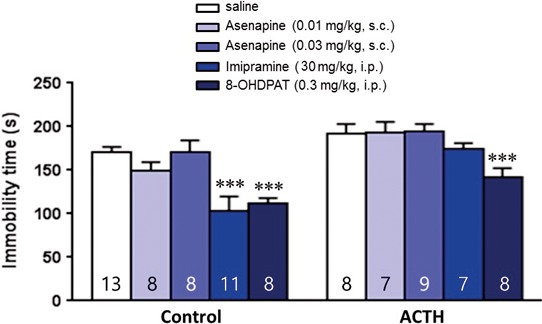
Effect of acute Asenapine on depression‐like behaviors. Effect of acute Asenapine (0.01 or 0.03 mg/kg, s.c.), imipramine (30 mg/kg, i.p.), and 8‐OHDPAT (0.3 mg/kg, i.p.) administrations on the immobility time in the forced swim test in control or ACTH‐treated rats (0.1 mg/kg/d, s.c., for 14 d). Values plotted are mean ± SEM. ***P<.001 vs saline, using two‐way ANOVA followed by Fisher PLSD post hoc test
Two‐way ANOVA indicated a significant effect of ACTH (F1,77 = 29.42, P<.001), the different treatments tested (F4,77=11.20, P<.001), but no interaction. PLSD post hoc analysis showed that imipramine (30 mg/kg, i.p.) and the 5‐HT1A/7 agonist 8‐OH‐DPAT (0.3 mg/kg, i.p.) significantly decreased the immobility duration of control animals (both P<.001), whereas only 8‐OH‐DPAT had an significant anti‐immobility effect in ACTH‐treated rats (P<.01). On the other hand, acute injection of Asenapine (0.01 and 0.03 mg/kg, s.c.) did not alter the immobility time in control nor ACTH‐treated rats.
3.3. Effects of Asenapine and SB 269970 on 5‐HT7 receptor responsiveness of dorsal raphe (DR) serotoninergic (5‐HT) neurons
In the present experiments, DR 5‐HT neurons were identified on the basis of their firing discharge patterns, following previous criteria.35, 36 As expected, administration of cumulative doses of the 5‐HT7 agonist LP‐44 (1‐4 mg/kg, i.v.) in control rats induced a dose‐dependent decrease in the firing activity of DR 5‐HT neurons with ED50=1.45 mg/kg (n=7; Figure 3A,B). Prior administration of the 5‐HT7 receptor antagonist SB 269970 (1 mg/kg, i.v.) partially prevented this suppressant response, with ED50=2.59 mg/kg (n=5; Figure 3B). Two‐way repeated‐measures ANOVA revealed a significant effect of SB 269970 pretreatment (F1,40=6.98, P<.05) on the potency of LP‐44 (F4,40=54.13, P<.001), but no SB 269970 x LP‐44 interaction. Similarly, an injection of Asenapine (1 mg/kg, i.v.) reduced the suppressant effect of LP‐44 on the firing activity of DR 5‐HT neurons, with ED50=3.54 mg/kg (n=7, Figure 3C,D). Two‐way repeated‐measures ANOVA indicated a significant effect of Asenapine (F1,55= 0.17, P<.01) on the potency of LP‐44 (F5,55=61.90, P<.001), and a significant interaction effect (F5,55=5.38, P<.001). However, because LP‐44 has a nanomolar affinity (Ki 52 nmol L−1) for 5‐HT1A receptors 39 and was reported to induce 5‐HT1A receptor‐mediated effects on synaptic transmission,40 it was necessary to assess the 5‐HT1A receptor antagonistic activity of Asenapine in the DRN. Hence, it has been repeatedly shown that 5 μg/kg (i.v.) of the prototypical 5‐HT1A/7 receptors agonist 8‐OH‐DPAT fully suppresses the firing activity of DR 5‐HT neurons.41 Expectedly, a prior administration of SB 269970 (1 mg/kg, i.v.) did not alter the suppressant effect of 8‐OH‐DPAT (5 μg/kg, i.v.): % of inhibition of 5‐HT neuronal firing induced by 8‐OH‐DPAT after saline (n=6) or SB‐269970 (n=4) administration=100±0%. On the other hand, an injection of Asenapine (1 mg/kg, i.v.) reduced significantly the suppressant effect of 8‐OH‐DPAT (5 μg/kg, i.v.) on the firing activity of DR 5‐HT neurons: % of inhibition of 5‐HT neuronal firing induced by 8‐OH‐DPAT after Asenapine (n=4) administration =48±7%, (F2,11=69.70, P<.001).
Figure 3.
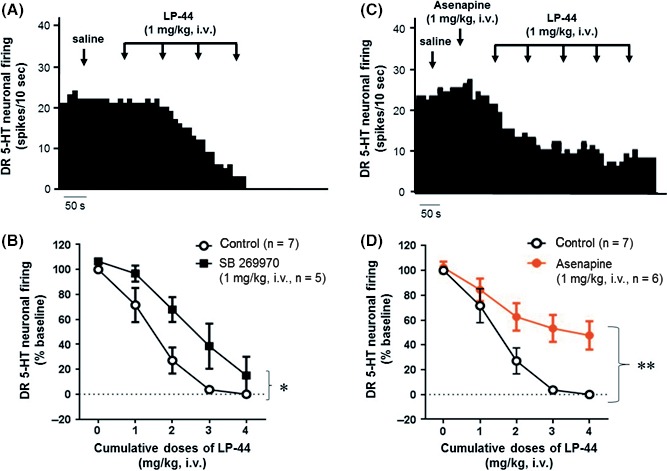
Effect of acute administration of LP‐44 alone or after administration of Asenapine on the neuronal firing activity of dorsal raphe (DR) 5‐HT cells. (A) Integrated firing rate histogram of a presumed DR 5‐HT cell showing its response to successive doses of LP‐44 (1‐4 mg/kg, i.v.). (B) Dose‐response curve of the inhibitory effect of LP‐44 made with cumulative doses (1‐4 mg/kg, i.v.) alone or with a prior administration of SB 269970 (1 mg/kg, i.v.). (C) Integrated firing rate histogram of a presumed DR 5‐HT neuron showing its response to cumulative doses of LP‐44 (1‐5 mg/kg i.v.) after a prior administration of Asenapine (1 mg/kg i.v.). (D) Dose‐response curve of the inhibitory effect of LP‐44 made with cumulative doses (1‐4 mg/kg, i.v.) alone or after administration of Asenapine (1 mg/kg, i.v.). Each symbol represents the mean compare to control of DR 5‐HT cell firing (in percentage ± S.E.M.). *P<.05, and **P<.01, as compared to control using two‐way repeated‐measures ANOVA
3.4. Effects of sustained Asenapine administration on the spontaneous firing activity of DR 5‐HT neurons
The mean spontaneous firing activity of DR 5‐HT neurons in control rats was 0.99 ± 0.10 Hz (n=55 cells; Figure 4A). Sustained administrations of Asenapine (0.3 mg/kg/d, s.c.), with osmotic minipumps for 3 days, 14 days or 21 days, resulted in a significant effect on the spontaneous firing activity of DR 5‐HT neurons (F3,201=3.93, P<.01; one‐way ANOVA followed by Fisher's PLSD test). The spontaneous firing activity of DR 5‐HT neurons of rats treated for 14 d and 21 days with Asenapine was increased by 32% (n=46 cells; 1.31 ± 0.10 Hz, P<.05 compared to control), and by 46% (n=45 cells; 1.44 ± 0.15 Hz, P<.01), respectively. No significant difference was observed for the 3‐d Asenapine regimen (n=59 cells).
Figure 4.
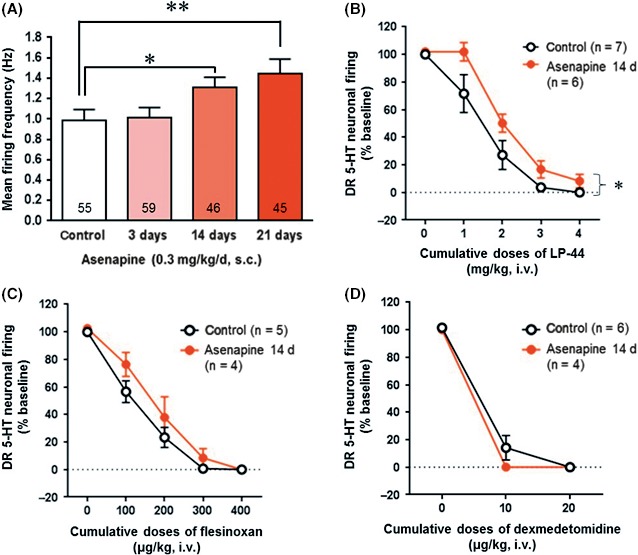
Effect of chronic treatment with Asenapine on the firing activity of DR 5‐HT neurons. (A) Mean ± SEM of the spontaneous firing rate of DR 5‐HT neurons in control and in rats treated with Asenapine (0.3 mg/kg/d, s.c.) for 3, 14 or 21 d. The numbers indicate the total number of recorded neurons in each group of treated rats. *P<.05 and **P<.01, as compared to control using one‐way ANOVA followed by Fisher PLSD post hoc test. Dose‐response curve of the suppressant effect of cumulative doses of (B) LP‐44 (1‐4 mg/kg, i.v.), (C) flesinoxan (100‐400 μg/kg, i.v.), and (D) dexmedetomidine (10‐20 μg/kg, i.v.) in controls and in rats treated for 14 d with Asenapine (0.3 mg/kg/d, s.c.). Each symbol represents the mean compare to control of DR 5‐HT cell firing (in percentage ± SEM). *P<.05, as compared to control using two‐way repeated‐measures ANOVA
3.5. Effects of sustained Asenapine administration on the 5‐HT7, 5‐HT1A receptor, and α2‐adrenoreceptor responsiveness of DR 5‐HT neurons
To evaluate the responsiveness of 5‐HT7 receptors in Asenapine‐treated animals, rats were chronically treated for 14 days with Asenapine (0.3 mg/kg/d, s.c.), and then acutely administered with the 5‐HT7 agonist LP‐44 in order to compare the dose‐response effects in treated and control animals. In control rats, systemic administration of LP‐44 (1‐4 mg/kg, i.v.) produced a dose‐dependent reduction in the 5‐HT neuronal firing rate with ED50=1.45 mg/kg (n=7; Figure 4B). In rats treated with Asenapine, the ED50 increased to 2.08 mg/kg (n=6). Two‐way repeated‐measures ANOVA showed a significant effect of 14‐d treatment with Asenapine (F1,44 =6.60, P<.05) on the potency of LP‐44 (F4,44 =99.87, P<.001), but no significant interaction.
To assess 5‐HT1A autoreceptor responsiveness, rats were treated for 14 days with Asenapine (0.3 mg/kg/d, s.c) and acutely administered the 5‐HT1A agonist flesinoxan. In control rats, flesinoxan (0‐400 μg/kg, i.v.) dose‐dependently reduced the 5‐HT neuronal firing activity with ED50=117.19 μg/kg (n=5; Figure 4C). In Asenapine‐treated animals, the ED50 was 165.63 μg/kg (n=4). Two‐way repeated‐measures ANOVA showed a significant effect of flesinoxan treatment (F1,28 =111.46, P<.001), but no significant effect of Asenapine nor interaction.
Moreover, we tested the α2‐adrenoceptor responsiveness of the firing activity of DR 5‐HT neurons of rats treated with Asenapine (0.3 mg/kg/d, s.c) for 14 days, by injecting the α2‐adrenoceptor agonist dexmedetomidine (10‐20 μg/kg, i.v.; Figure 4D). Again, two‐way repeated‐measures ANOVA detected a significant effect of dexmedetomidine (F1,16=287.36, P<.001), but no Asenapine treatment nor interaction effects.
4. Discussion
The results of the present study show that Asenapine behaved as a potent antimanic‐like agent in the 72 h‐SD model of mania.7, 9 Moreover, neither Asenapine nor the TCA imipramine presented antidepressant properties in the ACTH model of antidepressant‐resistance.3, 4 At last, the electrophysiological results showed, for the first time in vivo, that Asenapine acted as a potent 5‐HT7 receptor antagonist, a receptor known to control DRN 5‐HT neuronal firing activity.42, 43
Current antimanic pharmacotherapy, using frequently a mood stabilizer such as lithium, remains unsatisfactory due to partial therapeutic efficacy.44 Accordingly, the development of novel antimanic agents has been hindered by the deficiency of appropriate animal models. There is an urgent need for animal models that reproduce the oscillating characteristics of BD, and most of preclinical researches use distinct models to evaluate such a disorder, that is, either model of mania or depression.45 Actually to test the efficacy of potential antimanic agents, the “gold‐standard” rodent model of mania is the amphetamine‐induced hyperactivity model.46 Another relevant model of mania is the SD model that produces a transient episode of hyperactivity and insomnia. Therefore, we recently found that, firstly, SD animals display behavioral alterations corresponding to manic symptoms of BD. Secondly, the antimanic drugs (lithium and aripiprazole), but not a classical antidepressant (fluoxetine), yield rapid antimanic‐like effects in SD rats.9 Accordingly, the present study showed that Asenapine decreased potently the hyperlocomotion of SD rats. In agreement, a recent study in Black Swiss mice, evaluating the effects of Asenapine in a validated battery test for assessing manic‐like behaviors, shows that Asenapine reduces locomotor activity in a large open field, increases the time spent in the open‐field center, and reduces amphetamine‐induced hyperactivity, further supporting the antimanic properties of Asenapine.21 Clinically, the potential of 5‐HT7 antagonism of Asenapine in the treatment of bipolar disorders cannot be ruled out 47 and thus far, only one selective 5‐HT7 receptor antagonist JNJ‐18038683 is currently assessed in a clinical trial for bipolar depression (ClinicalTrials.gov: NCT02466685). Conversely, there is only one double‐blind, active, and placebo‐controlled clinical trial in patients suffering from major depressive disorder, showing that neither treatment with pharmacologically active doses of JNJ‐18038683 or the positive control escitalopram separated from placebo, indicating a failed study lacking assay sensitivity.48
Actually, several groups have proposed that chronic treatment with ACTH can be a useful animal model of TCA treatment‐resistant depression.4, 5 More recently, the antidepressant‐like effects of ketamine and nucleus accumbens deep‐brain stimulation have been successfully evaluated in this rodent model of TCA resistance.27, 49 Conversely, the present data showed that neither Asenapine (at both 0.01 and 0.03 mg/kg, s.c.) nor imipramine decreased the immobility time in the FST in ACTH‐treated rats, revealing the lack of antidepressant‐like property of Asenapine in this treatment‐resistant depression model. These latter results fit well with the study in Black Swiss mice 21 showing that Asenapine fails to affect sweet solution preference or to decrease immobility time in the FST.
As mentioned above, Asenapine is pharmacologically characterized by its high affinity for serotoninergic and α2‐adrenergic receptors,19 also it was essential to determine whether and how sustained administrations of Asenapine affected 5‐HT system. In the DRN, 5‐HT neurons are capable of autonomously regulating their own basal firing rate and feedback activation by 5‐HT1A auto‐receptors reduces the excitability of 5‐HT neurons decreasing 5‐HT release both locally within the DRN and in projection regions (see 50). On the other hand, DRN 5‐HT7 receptors are localized on GABAergic interneurons and their activation is shown to reduce firing of 5‐HT cells.42, 43 As previously revealed,25, 26 a single dose of Asenapine (1 mg/kg, i.v.) did not significantly alter the spontaneous firing rate of DRN 5‐HT neurons (Figure 3). Moreover, such a dose of Asenapine fails to modify the suppressant effect of the 5‐HT1A/7 receptors agonist 8‐OH‐DPAT at a dose of 10 μg/kg.25 Importantly, we have previously demonstrated that acute activation of 5‐HT7 receptors with the selective 5‐HT7 receptors agonist AS19 suppresses the firing activity of DRN 5‐HT neurons and that the selective 5‐HT7 receptor antagonist SB‐269970 prevents the latter effect of AS19, suggesting that 5‐HT neuronal firing activity is under negative 5‐HT7 receptor control.42 Accordingly, the present data showed that LP‐44 suppressed, dose‐dependently, the firing activity of DRN 5‐HT neurons and that both SB‐269970 and Asenapine prevented the effect of LP‐44, thus revealing the 5‐HT7 receptor mediation of Asenapine in the DRN. However, Asenapine also has a high affinity for 5‐HT1A receptors (pKi of 8.6, 19). Importantly, our results showed that acute administration of such a dose of Asenapine modified the suppressant effect of 8‐OH‐DPAT at a dose of 5 μg/kg, suggesting that acute administration of Asenapine (1 mg/kg, i.v.) was able to prevent both 5‐HT1A and 5‐HT7 receptors activation in the DRN. Recently, Oosterhof et al. 29 have shown that treatment with Asenapine (0.1 mg/kg/day, s.c.) increases the firing rate of DRN 5‐HT neurons after two days, but not 3 weeks of administration, while the responsiveness of 5‐HT1A receptors is unaltered. On the other, the current study showed, using a higher regimen (0.3 mg/kg/day, s.c.), that only long‐term treatments (2 or 3 weeks) with Asenapine enhanced the spontaneous the neuronal firing activity of DRN 5‐HT cells. Interestingly, the latter effect of chronic treatment with Asenapine was associated with an alteration of the 5‐HT7 receptor responsiveness, but not that of DRN 5‐HT1A receptors nor α2‐adrenoceptors, both receptors also able to control DRN 5‐HT neuronal firing, further highlighting 5‐HT7 receptors as an key pharmacological target for the treatment of mood disorders.42, 51
5. Conclusion
Preclinically, both acute Asenapine and SB‐269970 have been shown to reduce immobility time in the FST in rodents.42, 52 Similarly, both acute Asenapine and SB‐269970 have been shown to reduce amphetamine‐induced hyperactivity in rodents, the mostly used as animal model of mania.21, 53 One may assume that 5‐HT7 receptors antagonists may be good antidepressant and antimanic candidates. Using complementary behavioral and in vivo electrophysiological paradigms, the present study shows that Asenapine is a potent antimanic‐like agent in the 72h‐SD model of mania and is able to regulate DRN 5‐HT neuronal firing activity via its 5‐HT1A/7 receptors antagonism. These results provide further insights in the neural mechanisms by which Asenapine may act in the treatment of acute manic and mixed episodes of BD.54
Conflict of Interest
Dr. Haddjeri has received research grants from H.Lundbeck A/S. Drs. Didriksen and Arnt are full‐time employees of Lundbeck and Sunred Pharma Consulting Aps, respectively. The others authors declare no conflict of interest. This research was supported at Claude Bernard University‐Lyon 1, France and INSERM.
Acknowledgments
The authors thank Helene Scarna and Laura Lambas‐Senas for their assistance in preparing this manuscript, and their technical support was also greatly appreciated.
Delcourte S, Abrial E, Etiévant A, et al. Asenapine modulates mood‐related behaviors and 5‐HT1A/7 receptors‐mediated neurotransmission. CNS Neurosci Ther. 2017;23:518–525. 10.1111/cns.12698
The first two authors contribute equally to this work.
References
- 1. Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476‐486. [DOI] [PubMed] [Google Scholar]
- 2. Kato T, Kasahara T, Kubota‐Sakashita M, Kato TM, Nakajima K. Animal models of recurrent or bipolar depression. Neuroscience. 2016;321:189‐196. [DOI] [PubMed] [Google Scholar]
- 3. Kitamura Y, Araki H, Suemaru K, Gomita Y. Effects of imipramine and lithium on wet‐dog shakes mediated by the 5‐HT2A receptor in ACTH‐treated rats. Pharmacol Biochem Behav. 2002;72:397‐402. [DOI] [PubMed] [Google Scholar]
- 4. Kitamura Y, Akiyama K, Kitagawa K, et al. Chronic coadministration of carbamazepine together with imipramine produces antidepressant‐like effects in an ACTH‐induced animal model of treatment‐resistant depression: involvement of 5‐HT2A receptors? Pharmacol Biochem Behav. 2008;89:235‐240. [DOI] [PubMed] [Google Scholar]
- 5. Kitamura Y, Yagi T, Kitagawa K, et al. Effects of bupropion on the forced swim test and release of dopamine in the nucleus accumbens in ACTH‐treated rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:151‐158. [DOI] [PubMed] [Google Scholar]
- 6. Li B, Suemaru K, Cui R, Kitamura Y, Gomita Y, Araki H. Repeated electroconvulsive stimuli increase brain‐derived neurotrophic factor in ACTH‐treated rats. Eur J Pharmacol. 2006;529:114‐121. [DOI] [PubMed] [Google Scholar]
- 7. Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol. 1995;5:89‐93. [DOI] [PubMed] [Google Scholar]
- 8. Barbini B, Bertelli S, Colombo C, Smeraldi E. Sleep loss, a possible factor in augmenting manic episode. Psychiatry Res. 1996;65:121‐125. [DOI] [PubMed] [Google Scholar]
- 9. Abrial E, Bétourné A, Etiévant A, et al. Protein kinase C inhibition rescues manic‐like behaviors and hippocampal cell proliferation deficits in the sleep deprivation model of mania. Int J Neuropsychopharmacol. 2014;18:1‐11. pii: pyu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guzman‐Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111‐2116. [DOI] [PubMed] [Google Scholar]
- 11. Machado‐Vieira R, Soeiro‐De‐Souza MG, Richards EM, Teixeira AL, Zarate CA Jr. Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: developing treatments using an integrated translational approach. World J Biol Psychiatry. 2014;15:84‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy DL, Andrews AM, Wichems CH, Li Q, Tohda M, Greenberg B. Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry. 1998;59:4‐12. [PubMed] [Google Scholar]
- 13. Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5‐HT7 receptor research. Trends Pharmacol Sci. 2004;2004:481‐486. [DOI] [PubMed] [Google Scholar]
- 14. Thomas DR, Melotto S, Massagrande M. , et al. SB‐656104‐A, a novel selective 5‐HT7 receptor antagonist, modulates REM sleep in rats. Br J Pharmacol. 2003;139:705‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hedlund PB, Huitron‐Resendiz S, Henriksen SJ, Sutcliffe JG. 5‐HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831‐837. [DOI] [PubMed] [Google Scholar]
- 16. Duncan MJ, Congleton MR. Neural mechanisms mediating circadian phase resetting by activation of 5‐HT(7) receptors in the dorsal raphe: roles of GABAergic and glutamatergic neurotransmission. Brain Res. 2010;1366:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas DR, Hagan JJ. 5‐HT7 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:81‐90. [DOI] [PubMed] [Google Scholar]
- 18. Wirz‐Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21:S11‐S15. [DOI] [PubMed] [Google Scholar]
- 19. Shahid M, Walker GB, Zorn SH, Wong EH. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009;23:65‐73. [DOI] [PubMed] [Google Scholar]
- 20. Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta‐analysis. PLoS One. 2014;9:e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ene HM, Kara NZ, Einat H. The effects of the atypical antipsychotic asenapine in a strain‐specific battery of tests for mania‐like behaviors. Behav Pharmacol. 2015;26:331‐337. [DOI] [PubMed] [Google Scholar]
- 22. Ohyama M, Kondo M, Yamauchi M, Imanishi T, Koyama T. Asenapine reduces anxiety‐related behaviours in rat conditioned fear stress model. Acta Neuropsychiatr. 2016;28:327‐336. [DOI] [PubMed] [Google Scholar]
- 23. Huang M, Li Z, Dai J, Shahid M, Wong EH, Meltzer HY. Asenapine increases dopamine, norepinephrine, and acetylcholine efflux in the rat medial prefrontal cortex and hippocampus. Neuropsychopharmacology. 2008;33:2934‐2945. [DOI] [PubMed] [Google Scholar]
- 24. Frånberg O, Marcus MM, Svensson TH. Involvement of 5‐HT2A receptor and α2‐adrenoceptor blockade in the asenapine‐induced elevation of prefrontal cortical monoamine outflow. Synapse. 2012;66:650‐660. [DOI] [PubMed] [Google Scholar]
- 25. Ghanbari R, El Mansari M, Shahid M, Blier P. Electrophysiological characterization of the effects of asenapine at 5‐HT(1A), 5‐HT(2A), alpha(2)‐adrenergic and D(2) receptors in the rat brain. Eur Neuropsychopharmacol. 2009;19:177‐187. [DOI] [PubMed] [Google Scholar]
- 26. Frånberg O, Marcus MM, Ivanov V, Schilström B, Shahid M, Svensson TH. Asenapine elevates cortical dopamine, noradrenaline and serotonin release. Evidence for activation of cortical and subcortical dopamine systems by different mechanisms. Psychopharmacology. 2009;204:251‐264. [DOI] [PubMed] [Google Scholar]
- 27. Walker AJ, Foley BM, Sutor SL, McGillivray JA, Frye MA, Tye SJ. Peripheral proinflammatory markers associated with ketamine response in a preclinical model of antidepressant‐resistance. Behav Brain Res. 2015;293:198‐202. [DOI] [PubMed] [Google Scholar]
- 28. Haddjeri N, Abrial A, Arnt J, Didriksen M, Lambás‐Señas L. Asenapine effects on dorsal raphe 5‐HT7 receptor responsiveness and in animal models of mania and resistant depression”. Eur Neuropsychopharmacol. 2014;24:S408‐S409. [Google Scholar]
- 29. Oosterhof CA, El Mansari M, Blier P. Asenapine alters the activity of monoaminergic systems following its subacute and long‐term administration: an in vivo electrophysiological characterization. Eur Neuropsychopharmacol. 2015;25:531‐543. [DOI] [PubMed] [Google Scholar]
- 30. Snigdha S, Idris N, Grayson B, Shahid M, Neill JC. Asenapine improves phencyclidine‐induced object recognition deficits in the rat: evidence for engagement of a dopamine D1 receptor mechanism. Psychopharmacology. 2011;214:843‐853. [DOI] [PubMed] [Google Scholar]
- 31. Jouvet D, Vimont P, Delorme F. Study of selective deprivation of the paradoxal phase of sleep in the cat. J Physiol (Paris). 1964;56:381. [PubMed] [Google Scholar]
- 32. Verret L, Léger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci. 2005;21:2488‐2504. [DOI] [PubMed] [Google Scholar]
- 33. Sapin E, Lapray D, Bérod A, et al. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009;4:e4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379‐391. [DOI] [PubMed] [Google Scholar]
- 35. Etiévant A, Oosterhof CA, Bétry C, et al. Astroglial control of the antidepressant‐like effects of prefrontal cortex deep brain stimulation. EBiomedicine. 2015;2:896‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aghajanian GK. Feedback regulation of central monoaminergic neurons: evidence from single cell recording studies. Essays Neurochem Neuropharmacol. 1978;3:1‐32. [PubMed] [Google Scholar]
- 37. Haddjeri N, Ortemann C, de Montigny C, Blier P. Effect of sustained administration of the 5‐HT1A receptor agonist flesinoxan on rat 5‐HT neurotransmission. Eur Neuropsychopharmacol. 1999;9:427‐440. [DOI] [PubMed] [Google Scholar]
- 38. Millan MJ, Dekeyne A, Newman‐Tancredi A, et al. S18616, a highly potent, spiroimidazoline agonist at alpha(2)‐adrenoceptors: I. Receptor profile, antinociceptive and hypothermic actions in comparison with dexmedetomidine and clonidine. J Pharmacol Exp Ther. 2000;295:1192‐1205. [PubMed] [Google Scholar]
- 39. Di Pilato P, Niso M, Adriani W, et al. Selective agonists for serotonin 7 (5‐HT7) receptor and their applications in preclinical models: an overview. Rev Neurosci. 2014;25:401‐415. [DOI] [PubMed] [Google Scholar]
- 40. Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L. 5‐HT(1A) and 5‐HT(7) receptors differently modulate AMPA receptor‐mediated hippocampal synaptic transmission. Hippocampus. 2012;22:790‐801. [DOI] [PubMed] [Google Scholar]
- 41. Haddjeri N, Lucas G, Blier P. Role of cholinergic and GABAergic systems in the feedback inhibition of dorsal raphe 5‐HT neurons. NeuroReport. 2000;11:3397‐3401. [DOI] [PubMed] [Google Scholar]
- 42. Mnie‐Filali O, Faure C, Lambás‐Señas L, et al. Pharmacological blockade of 5‐HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology. 2011;36:1275‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kusek M, Sowa J, Kamińska K, Gołembiowska K, Tokarski K, Hess G. 5‐HT7 receptor modulates GABAergic transmission in the rat dorsal raphe nucleus and controls cortical release of serotonin. Front Cell Neurosci. 2015;9:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goodwin GM, Geddes JR. Latest maintenance data on lithium in bipolar disorder. Eur Neuropsychopharmacol. 2003;13:S51‐S55. [DOI] [PubMed] [Google Scholar]
- 45. Einat H, Manji HK. Cellular plasticity cascades: genes‐to‐behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59:1160‐1171. [DOI] [PubMed] [Google Scholar]
- 46. Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164:1263‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marazziti D, Piccinni A, Baroni S, et al. Current trends on antipsychotics: focus on asenapine. Curr Med Chem. 2016;23:2204‐2216. [DOI] [PubMed] [Google Scholar]
- 48. Bonaventure P, Dugovic C, Kramer M, et al. Translational evaluation of JNJ‐18038683, a 5‐hydroxytryptamine type 7 receptor antagonist, on rapid eye movement sleep and in major depressive disorder. J Pharmacol Exp Ther. 2012;342:429‐440. [DOI] [PubMed] [Google Scholar]
- 49. Kim Y, McGee S, Czeczor JK, et al. Nucleus accumbens deep‐brain stimulation efficacy in ACTH‐pretreated rats: alterations in mitochondrial function relate to antidepressant‐like effects. Transl Psychiatry. 2016;6:e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andrade R, Huereca D, Lyons JG, Andrade EM, McGregor KM. 5‐HT1A receptor‐mediated autoinhibition and the control of serotonergic cell firing. ACS Chem Neurosci. 2015;6:1110‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nikiforuk A. Targeting the serotonin 5‐HT7 receptor in the search for treatments for CNS disorders: rationale and progress to date. CNS Drugs. 2015;2015:265‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kołaczkowski M, Mierzejewski P, Bieńkowski P, et al. Antipsychotic, antidepressant, and cognitive‐impairment properties of antipsychotics: rat profile and implications for behavioral and psychological symptoms of dementia. Naunyn Schmiedeberg's Arch Pharmacol. 2014;387:545‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Waters KA, Stean TO, Hammond B, et al. Effects of the selective 5‐HT(7) receptor antagonist SB‐269970 in animal models of psychosis and cognition. Behav Brain Res. 2012;228:211‐218. [DOI] [PubMed] [Google Scholar]
- 54. Scheidemantel T, Korobkova I, Rej S, Sajatovic M. Asenapine for bipolar disorder. Neuropsychiatr Dis Treat. 2015;11:3007‐3017. [DOI] [PMC free article] [PubMed] [Google Scholar]


