Cpne5 belongs to Copine (Cpne) family, all of which are Ca2+‐dependent, phospholipid‐binding proteins that are highly conserved in animals. There are eight homologous Copine genes in mammalians. Cpne5 contains two C2 domains at N‐terminus and an A‐domain at C‐terminus, which were conserved in other Cpne members 1. The C2 domain is responsible for mediating Ca2+‐dependent cellular processes. The A‐domain at the C‐terminus is similar to the extracellular “A‐domain” of integrins, which is responsible for protein–protein interaction usually in Ca2+‐, Mg2+‐, and/or Mn2+‐dependent manner 2. Highly conserved molecular structure indicates the pivotal role of Cpne5 in mammalian. Our previous work demonstrated that Cpne5 was highly expressed in embryonic mouse brain. The distribution of Cpne5 in embryonic rodent brain indicated its essential role in the central nervous system (CNS) 3. However, the exact role of Cpne5 in CNS remains unclear.
To investigate the role of Cpne5 in CNS, we generated Cpne5 knockout mouse model. Our strategy is to replace the first exon of Cpne5 and about 2.6 kb of upstream sequence (containing start code) of Cpne5 genome with a PGK‐neo cassette based on the homologous recombination (as shown in Figure 1A). After constructing the Cpne5 targeting vector, the targeting vector was linearized at 5′‐end by NotI 4, 5. The product was purified and delivered into embryonic stem cells (ES cells, R1, 129/SvJ X 129/Sv‐CP) using electroporation. G418 and ganciclovir were used to primarily screen the positive ES clone. PCR analysis was used to confirm the positive clone. The targeting vector was used as a positive control (PC), and 129svJ genomic DNA was used as a negative control (NC). Southern blot was used to further confirm the positive clone. Next, one of the confirmed positive clones was injected into C57BL/6 blastulas, which were subsequently transferred into pseudopregnant females to generate chimeric offspring. Next, the highly chimeric offsprings were bred with 129/svJ female mice to produce heterozygotes. PCR analysis was used to identify heterozygotes 6. Specific oligonucleotide primers were used for the Cpne5 targeted allele (Forward:5′GCATCGCATTGTCTGAGTAGGTG3′ and Reverse: 5′GCCAGTCCCTCTCAATTCTCCT3′, product length: 519 bp).
Figure 1.
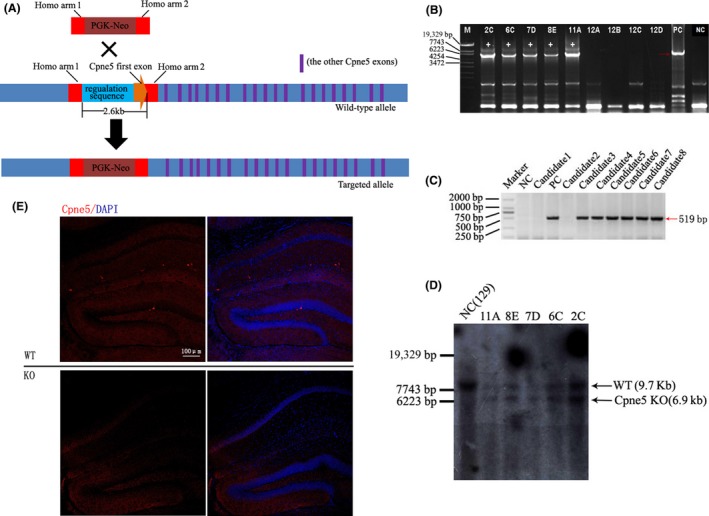
Generation of Cpne5 knockout (KO) mouse model. A: The schematic diagram of strategy for preparing Cpne5 KO mice. Cpne5 will be inactivated by replacing the first exon of Cpne5 and its upstream regulation sequence with a PGK‐Neo‐cassette via homologous recombination. B: Ninety‐six ES clones were picked up, and five positive clones of them were characterized by PCR analysis. Targeting vector was used as positive control (PC), and 129/SvJ genomic DNA was used as negative control (NC). C: The positive clones were confirmed by Southern blot; a 9.7‐kb and a 6.7‐kb band will be observed in the KO‐positive clones. D: PCR analysis of chimeric offspring. Totally six heterozygotes were found in chimeric offsprings (519‐bp band will be observed in heterozygotes). E: Confirmation of Cpne5 homozygotes using immunofluorescence. Cpne5 cannot be observed in the adult hippocampus of homozygotes compared with wild‐type mice. Nucleus was stained with DAPI. Scale bars: 50 μm.
For the wild‐type allele, Forward:5′ATGGCGTCGCTCAGCGAGTT 3′ and Reverse: 5′TGGGACCCCTGTTTCCGTGT 3′, product length: 351 bp. Immunostaining was used to characterize the homozygotes. After breeding with C57/BL6J over eight generations, homozygotes were generated and the 129/SvJ background was successfully removed.
To investigate the role of Cpne5, 11 female homozygotes and seven female wild‐type mice from littermate were used to perform behavioral test, including open field (OF), Y‐maze, novelty recognition (NR), light–dark box (LDB), elevated plus maze (EPM), and elevated platform (EP) task 7. The entire behavioral tasks were performed as described previously with minor modifications 8, 9, 10. Briefly, in OF task, mouse was placed at the center of the OF and total travel distance during 5 min was recorded using Any‐maze tracking system (Stoelting, Wood Dale, IL, USA). In LDB task, mice were placed at the center of light box and dark box, and the time spent in each box was recorded. In EPM and EP task, mice were placed at the center zone, and the time spent in each zone was recorded using Any‐maze tracking system. Difference between the two groups was analyzed using Students’ t‐test. Differences were considered statistically significant at P < 0.05. Results are presented as mean ± SEM.
As Figure 1B showed, totally 96 ES clones were G418 and ganciclovir double resistance and five of them contain Cpne5 targeted allele using PCR analysis. Further confirmation using Southern blot showed that all these five positive clones contain Cpne5 targeted allele. As shown in Figure 1C, a 9.7 kb DNA fragment (wild‐type allele) and a 6.7 kb fragment (targeted allele) can be observed in the positive clones. Genomic DNA of 129/SvJ mouse was used as negative control (NC). Next, the confirmed positive clones were injected into C57BL/6 blastulas, which were subsequently transferred into pseudopregnant females to generate chimeric offspring. Subsequently, the highly chimeric offsprings were bred with either 129/SvJ female mice to produce heterozygotes. As shown in Figure 1D, totally six heterozygotes were successfully generated (candidates 3 to candidates 8). To confirm the Cpne5 knockout mice, antibody against mouse Cpne5 (Novus, USA) was used to perform immunostaining. As shown in Figure 1E, Cpne5‐positive cells can be observed in wild‐type mice rather than Cpne5 knockout mice (KO). Thus, the results demonstrated that Cpne5 was successfully deleted in the homozygotes.
Next, to explore the functional clues of Cpne5, we examined Cpne5 knockout mice and found no obvious abnormality in body structure, body weight (Figure S1A,B), and brain structure (Figure S2A–C). Additionally, we examined the Cpne5 knockout mice with various behavioral tasks, including OF, Y‐Maze, NR, LDB, EPM, and EP. As shown in Figure 2A, no difference of total travel distance was found between WT and KO mice, indicating that Cpne5 KO did not affect rodent locomotor activity. Y‐maze and NR tests showed that Cpne5 KO did not obviously affect rodent memory (Figure S3A,B). Meanwhile, LDB task did not find significant difference of time spent in the dark box between Cpne5 KO and WT mice (as shown in Figure 2B). However, in EPM, Cpne5 Knockout mice spent significant more time in the open arms and less time in the closed arms (Figure 2C), indicating the decreased anxiety level in Cpne5 knockout mice. Further examination using EP showed that Cpne5 knockout mice spent significantly more time in the outer zone of EP (Figure 2D, P < 0.05), which indicated that Cpne5‐deficient mice felt less fearful and showed decreased anxiety in EP task. Thus, the present data demonstrated that Cpne5 might be involved in height induced anxiety rather than brightly lit induced anxiety. Expression of Cpne5 in hippocampus and amygdala also supports these results to some extent (data were not shown).
Figure 2.
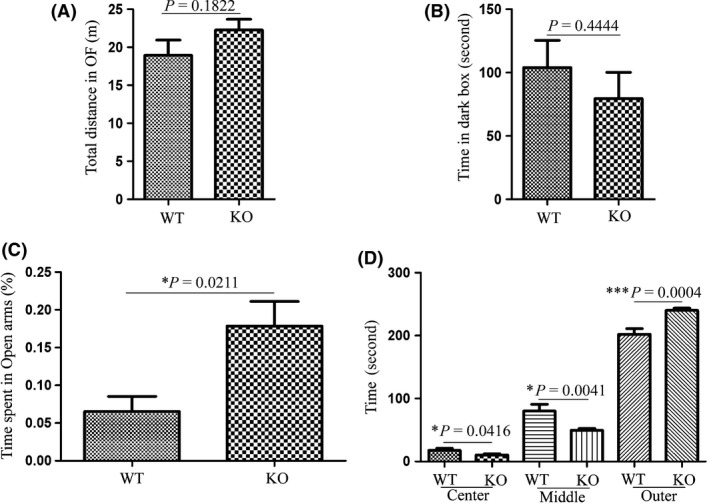
Behavioral characterization of wild‐type (n = 7) and Cpne5 knockout mice (n = 11). A: There is no significant of travel distance in open field task. B:There is no significant difference of time spent in the dark box of light–dark box task (LDB) was found between Cpne5 KO and WT mice. C: Cpne5 KO mice spent significantly more time in the open arm of elevated plus maze (EPM) compared with WT mice, P < 0.05, Percentage = time in open arms/(time in open arms + time in closed arms. D: Cpne5 KO mice spent significantly more time in the outer zone (P < 0.001) and less time in the center and middle zone of elevated platform (EP) compared with WT mice.
In summary, we successfully generated Cpne5 KO mice model and found that Cpne5 deficiency did not affect rodent locomotor activity and memory, but significantly decrease rodent anxiety level in EPM and EP task, which indicates the important role of Cpne5 in regulating anxiety level.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Figure S1. Examination of Cpne5 knockout mice.
Figure S2. Cpne5 deletion does not affect brain structure.
Figure S3. Behavioral characterization of wild‐type and Cpne5 knockout mice.
Figure S4. Cpne5 was expressed in the rodent hippocampus and partly colocalized with NeuN.
Acknowledgments
This work was supported by The Natural Science Foundation of China (31200822 & 30871030), the grants of Beijing Natural Science Foundation (5122033), and Beijing Municipal Science & Technology Commission (Z161100000216142).
References
- 1. Tomsig JL, Creutz CE. Copines: A ubiquitous family of Ca(2+)‐dependent phospholipid‐binding proteins. Cell Mol Life Sci 2002;59:1467–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Creutz CE, Tomsig JL, Snyder SL, et al. The copines, a novel class of C2 domain co ntaining, calcium‐dependent, phospholipid‐binding proteins conserved from Paramecium to humans. J Biol Chem 1998;273:1393–1402. [DOI] [PubMed] [Google Scholar]
- 3. Ding X, Jin Y, Wu Y, et al. Localization and cellular distribution of CPNE5 in embryonic mouse brain. Brain Res 2008;1224:20–28. [DOI] [PubMed] [Google Scholar]
- 4. Simon J, Arthur C, McGuire VA. Gene‐targeting vectors. Methods Mol Biol 2009;561:127–144. [DOI] [PubMed] [Google Scholar]
- 5. Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering‐based method for generating conditional knockout mutations. Genome Res 2003;13:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corbetta S, Gualdoni S, Albertinazzi C, et al. Generation and characterization of Rac3 knockout mice. Mol Cell Biol 2005;25:5763–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolf A, Bauer B, Abner EL, et al. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS One 2016;11:e0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heredia L, Torrente M, Colomina MT, Domingo JL. Assessing anxiety in C57BL/6J mice: A pharmacological characterization of the open‐field and light/dark tests. J Pharmacol Toxicol Methods 2014;69:108–114. [DOI] [PubMed] [Google Scholar]
- 9. Turgeon SM, Kim D, Pritchard M, et al. The effects of phencyclidine (PCP) on anxiety‐like behavior in the elevated plus maze and the light‐dark exploration test are age dependent, sexually dimorphic, and task dependent. Pharmacol Biochem Behav 2011;100:191–198. [DOI] [PubMed] [Google Scholar]
- 10. Ding XF, Zhao YQ, Liu SH, et al. An improved elevated platform for simultaneously assessing rodent locomotor activity and anxiety. CNS Neurosci Ther 2015;21:536–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Examination of Cpne5 knockout mice.
Figure S2. Cpne5 deletion does not affect brain structure.
Figure S3. Behavioral characterization of wild‐type and Cpne5 knockout mice.
Figure S4. Cpne5 was expressed in the rodent hippocampus and partly colocalized with NeuN.


