Summary
One of the key obstacles to a successful head transplant is the possible onset of central pain, a chronic pain condition that would impair the quality of life of the transplantee. In this review, we provide the reader with a knowledge of this neglected aspect of the head transplant initiative and outline the management should this eventuality occur.
Keywords: Central pain, Cephalosomatic anastomosis, Head transplant, Neuropathic pain
Introduction
Cephalosomatic anastomosis (CSA) 1, 2 requires the severance and reconstruction of the cervical spinal cord 3. This entails damage, however temporary, to the pain conducting pathways coursing in both the white matter (spinothalamic tract) and the gray matter (propriospinal paleospinothalamic system). One dreaded consequence is the possible onset of cord central pain [or central neuropathic pain (CCP)]. Central pain is defined as pain and/or allied symptoms such as dysesthesias and pruritus following damage of whatever kind to the pain conducting pathways in the central nervous system (CNS) 4, 5. In such eventuality, the patient would receive a new body, but go on suffering for the rest of his/her life a most excruciating chronic pain.
Actually, the GEMINI protocol 3 aims at restoring pain conduction too, but the initial damage brought about by the severing blade triggers a so‐called injury discharge, short high‐frequency signals lasting several minutes at most transmitted upstream along nociceptive fibers, rapidly notifying higher brain centers and prompting a pain cascade in predisposed patients [discussed in 4, 5].
For several reasons [reviewed in 4, 5], the exact incidence of CCP remains unknown, with reported figures ranging from a few to the vast majority of patients. In multiple sclerosis, CCP is diagnosed in roughly 18% of the patients. Yet, the best approximation of the risk to develop CCP for a head transplantee comes from older studies of anterolateral cordotomies for the treatment of cancer pain, in which the spinothalamic pain‐conveying tracts were severed surgically. The incidence varied between 1% and 20% [reviewed in 4]. Thus, this may be the expected risk of developing CCP in the context of CSA. At the same time, it may be that, in predisposed subjects, a combination of sensory deficits is responsible 4—the lemniscal fibers too are severed and fused 4, but research up to now has not yet settled the question, thus only approximations as to the true risk for a head transplantee can be offered. In any case, CCP appears to be much more frequent in incomplete cord injuries. Unfortunately, no preemptive way is known to reduce this risk 4, 5, although some experimental strategies have been advanced by the authors (unpublished observations).
Known to medicine since 1891, CP has remained a mystery for over a century until Canavero proposed the Dynamic Reverberation theory in 1992 [see history in 4, 5], which led to a rational treatment. Should the first head anastomoses develop CCP, a cure—however experimental—is available.
Given the poor appreciation of this condition in the medical community at large, in this minireview, we provide the details as relevant to CSA and outline the therapeutic strategy to counter this nefarious syndrome.
General Description
The interested reader is referred to two comprehensive, academic monographs on the topic 4, 5. CP following traumatic spinal cord injury (SCI) is known as cord CP (a.k.a. below‐level pain). CCP is disabling and in many patients may limit their functional ability and daily activities. Injuries that result in severe damage are more prone to produce pain, especially in quadriplegics. CCP can start immediately or even years after injury. Many patients develop it immediately, and practically all within a year (most within 6 months), but 6–8 years have also been reported. Most importantly for CSA, CCP usually appears with some functional recovery.
Central neuropathic pain may involve the entire body region below the level of injury (diffuse pain), but usually is more intense in the sacral dermatomes, buttocks and genitalia, and the feet, never following a dermatomal distribution. Pain is usually diffusely and symmetrically (although not at all times during follow‐up) referred to the parts of the body whose sensation is affected by the cord lesion; however, a quarter complains of localized pain within a much larger area of sensory alteration, some having a pain sharply localized to a small body part, usually the saddle area. While pain generally starts from the level of injury and caudad, there may be a free area from the zone of injury to the area of dysesthesias. The most common locations include the legs, posterior trunk, anterior trunk, and arms (100% in quadriplegics). Pain is described as deep by most patients.
Most patients experience one or more pain qualities simultaneously (two to four), in the same or different body regions and seemingly identical lesions may cause different combinations of pain qualities in different patients. CCP can have any quality, although some qualities are commoner; bizarre qualities are the exception rather than the rule. Variation in pain qualities is high in CCP. Whereas the majority have pain that can be described, several have no pain at all, but an unpleasant and difficult‐to‐describe sensation that drastically reduces their quality of life; moreover, there may be no sharp transition from nonpainful to painful dysesthesias. Some patients complain of pruritus, singly or in combination with other above‐cited qualities. Paresthesias can also be the main complaint. Numbness is experienced by many; it can occur with both total loss of tactile sensibility, but also normal thresholds to touch, and sometimes describes patients' paresthesias or dysesthesias. CCP is rather bizarre. There are different pains present in different patients and also different pains present in the same patient at different times or simultaneously. Sometimes, characteristics change as they appear or disappear.
Central neuropathic pain consists of three components: a steady, spontaneous pain (almost all), an intermittent, spontaneous pain (about one‐third, singly found in 1% of patients), and evoked pain (about one‐half, singly in 3%). The steady, intermittent and evoked components are often associated with a single patient. A single patient may complain of episodic lightning pains down a leg, superimposed on a continuous background of burning pain. The type of pain has no rapport with the causative lesion.
The steady component of CCP is generally burning or aching: burning is significantly associated with pain in frontal parts of torso and genitals, buttocks, and lower extremities, whereas aching with neck and shoulders and back. Many other descriptors are possible, but no quality seems to be prevailing, although a dysesthetic element, for example, “pins and needles”, stretching or pressure of the skin, and cold may predominate.
Intermittent pain is generally described as shooting or coming in electric shocks; it tends to run around the trunk at the level of the cord lesion in complete cases.
The intensity of the CCP varies from mild, unpleasant tingling to one of the most agonizing torments known to humans, but, generally speaking, CCP tends to be very intense. Pain located in the frontal aspects of torso (including genitals), “burning” or “electric” pain are especially so. When more components of pain are present, the intermittent will be the more severe. The steady component is generally fluctuating during the day and from day to day, also in bursts of activity and cyclically (namely, every other day or even every other week) and is not always so harassing as to induce the patient to ask for medical help. Pain may be more intense in the legs. CCP may or may not be perceived as worse than motor deficits.
The spontaneous discomfort of CP is often (≈70%: 50–90%) accompanied by unpleasant (dysesthesias, paresthesias) or painful sensations induced by somatosensory stimuli applied to areas of complete somatosensory interruption; it is unusual in the complete absence of clinically detectable sensory loss. Infrequently, these can be the only symptoms, that is, in the absence of constant pain. Evoked sensations may be unbearable and evoke violent emotional and defensive reactions, often being referred to as the worst component of CCP. Poorly localized to the hemisoma, patchily or diffusely, they may be elicited either by normally nonpainful stimuli, namely touch (including caresses)—but not, at least initially, deep pressure—vibration, moderate cold and heat (cold ≫ heat) (allodynia) or by mildly to moderately painful stimuli, particularly sharp objects plus noxious cold and heat (hyperesthesia: hyperalgesia and hyperpathia) delivered to an area of nearly (but not) always elevated threshold to stimuli of one or more somatosensory modalities (thermal, mechanical static, and dynamic). These evoked pains are elicited most prominently by a single sensory modality, a little more often than by several. In patients with complete thermanesthesia, extremes of heat and cold may evoke disagreeable nonthermal sensations. Hyperpathia refers to an abnormally painful reaction to a stimulus, especially a repetitive stimulus: the painful sensation develops explosively. There is usually little relation between the strength of the stimulus and the amount of sensation excited: it is nearly all or nothing. Moreover, there is no refractory period for hyperpathic responses. The effective stimulus may include all somatosensory stimuli or only a specific type of input (such as cold or draft, the light touch of clothing or pinprick, even smoke). These grossly unpleasant sensations may demonstrate temporal or spatial spread. In sum, provoked pains are characterized by late onset and poor localization, generally radiate from the stimulated point to the entire half of the body or lesser body areas and may persist for an unusually long time after stimulation has ceased. Evoked pains have a distribution which is less widespread than that of steady or intermittent pain. As a rule, somatic stimuli can cause or aggravate pain only when applied to the affected side, but sometimes even the stimulation of the normal side gives rise to exacerbation of pain (synesthesalgia).
Patients may wear as little clothing as possible over affected areas and seek a narrow window of room temperature, or alternatively wear gloves to avoid contact with the painful hand.
In cord lesions, evoked pain does not depend on the vertebral level nor on the completeness of the spinal lesion and exclusively occurs in areas of incompletely or clinically undetectable sensory loss or as a band at the upper margin of complete sensory loss; it can be elicited throughout the entire area of hypoesthesia or only in part of it, by one or several modalities of sensory stimulation. Trigger points can be identified even distant from areas of sensory deficit. In rare instances, evoked pain affects skin with clinically normal sensation (hyperesthesia).
Central neuropathic pain can be exacerbated by many factors, generally negative mood, prolonged afferent activity (bowel, bladder, somatic), weather, voluntary physical activity (movement/kinesthetic/proprioceptive/muscle (myo) allodynia, which can hinder rehabilitation and virtually paralyze some patients), and transient somatic afferent activity.
Other signs and symptoms are described elsewhere 4, 5. It is worth mentioning that often, following total spinal cord transection, after the phase of spinal shock, the patient complains of phantom sensations referred to the legs, and these are very similar to amputees' sensations, being painful, uncomfortable, and unpleasant, but not disabling. They appear early, almost immediately after SCI and vanish soon after SCI (rarely they linger on for months). Unlike amputees, telescoping or shrinkage of the involved body parts occurs only rarely in paraplegics and the length or posture of the phantom do not change; in addition, they are less vivid. Paraplegics describe sensations projected from the surface, but few postural sensations, with both voluntary and involuntary movements of the phantoms. Phantom sensations must be distinguished from phantom pain. CP appears when phantom sensations fade.
Genesis of Central Pain
The spontaneous component of CP is the result of a localized reverberating loop between the parietal cortex (SI) and the sensory thalamus 4, 5, 6, 7. In subjects with (presumed) dysfunctional GABA A receptors, STT injury is followed by the establishment of an “attractor state” in SI (Locked SI): in this way, information processing decorrelates (“garbling”). Simultaneously, the outflow down the facilitatory cortico (SI)‐thalamic fiber system, no longer held in check, feeds continuously into the thalamus and caudal regions, thereby engaging an out‐of‐balance “pain loop” (Figure 1). Different qualities of pain may be explained by individual degrees of activation of the same cells or activation (frequency discharge/oscillatory changes) of several sets of cells, in different cortical layers and thalamic nuclei, depending on site and extent of damage. The loop would be under the influence of cognitive, emotional, and attentional networks, explaining fluctuations in time of CP. Immediate or delayed onset would hinge on the degree of inhibitory defectiveness in the single patient.
Figure 1.
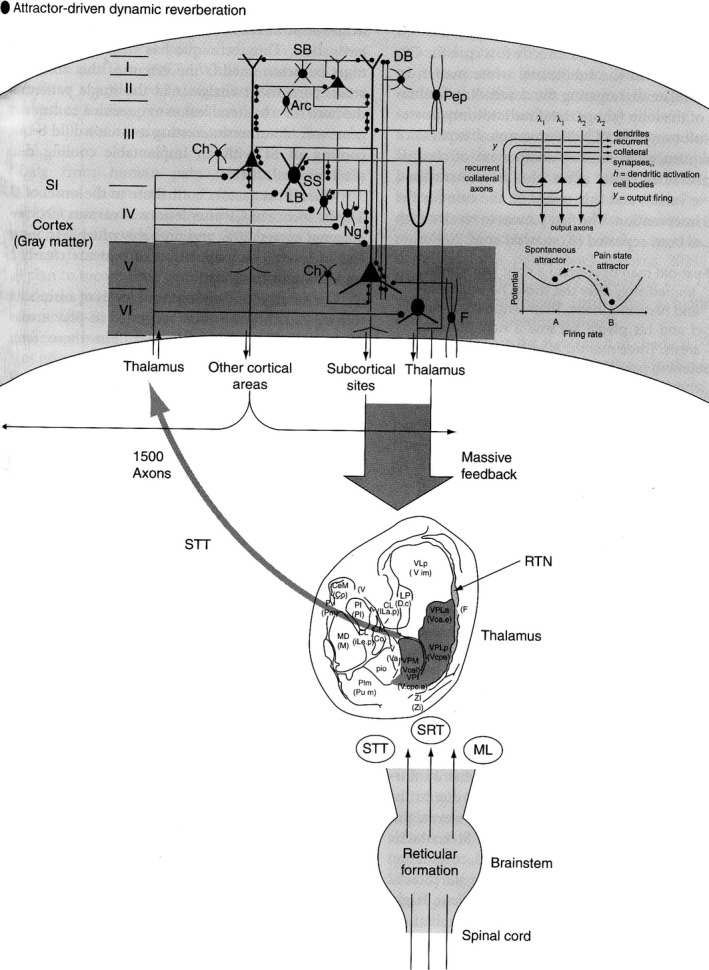
Diagram displaying the genesis of the spontaneous component of central pain. Damage to the spinothalamocortical pathway in subjects whose inhibitory systems at cortical levels are dysfunctional sets off a cascade of effects leading to a basin of attraction of neural activity centered in the somatosensory cortical areas which feeds onto the thalamus via a massive feedback from cortex to sensory thalamus. By interrupting this descending input, the generator is interrupted (from ref. 5, with permission).
The reticular formation (RF) and the propriospinal system become hyperactive after CNS injuries and provide bilateral bottom‐up facilitation to the loop. In particular, evoked pains are due to local generators in the damaged gray matter of the spinal cord, that is hyperactive sensory‐coded propriospinal interneurons, found cranially to the level of cord injury. Happily, the damage to these neurons in CSA is minimal and immediately controlled by fusogens 3. We stress how hyperactivity alone after SCI is not sufficient to trigger and sustain the spontaneous components of CCP, or all patients with SCI would develop CCP, which is not the case 5. Hyperexcitability is a universal phenomenon that follows neural injury: cord foci of hyperactivity play a boosting role only. This diffuse, bilateral, network of multisynaptic reticular propriospinal systems in and around the lesioned gray matter feeds the thalamocorticothalamic loop and spreads upward toward the brainstem RF by way of intersegmenatal cross‐talking interneurons.
Management of Central Pain in CSA
Although several drugs 5 and neuromodulatory (e.g., extradural cortical stimulation) strategies 4, 8, 9 are available to control CP, yet, our aim in the context of CSA is to stop the pain in its tracks should it arise at some point. Happily, the dynamic reverberation theory outlined above leads directly to a cure for CP: a selective lesion in the subparietal white matter, in some cases bilateral, targeting the descending facilitatory arm of the loop (subparietal radiatotomy/posterior capsulotomy, SRPC). Neurosurgical experience shows that, once the sensory component of chronic pain is abolished, pain affect also is renormalized (never vice versa) 4, 5, and this would be the case for the proposed intervention.
SRPC in the HEAVEN/AHBR 1, 2 context would be carried out by high‐intensity focused ultrasound (Hi‐FUs), a technique which entails no radiation, minimizes the risk of bleeding (with no risk of infection) with real time monitoring, and avoids collateral damage 5. The final result is a thermal lesion up to 4 × 5 mm. Targeting of the corticothalamic fibers would be achieved in both cases by DTI‐guided neuronavigation. This technique has clear promise. What must be determined is the extent of this ablation to attain permanent analgesia in the single patient and the need for a bilateral lesion to quench a contralateral generator. An alternative would be focal cooling of SI with an implantable cooling device 5.
Conclusion
All researchers that over the past 100 years proposed a full head/body transplant, and ran experiments toward this goal have not considered the possibility of the transplantation setting off a heinous pain syndrome that would have negated the final result. It was the discovery of the mechanism underlying CP in 1992 that truly opened the way to HEAVEN/AHBR 1, 2.
References
- 1. Canavero S. HEAVEN: The head anastomosis venture Project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int 2013;4(Suppl 1):S335–S342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ren XP, Luther K, Haar L, et al. Concepts, challenges, and opportunities in allo‐head and body reconstruction (AHBR). CNS Neurosci Ther 2014;20:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Canavero S. The “Gemini” spinal cord fusion protocol: Reloaded. Surg Neurol Int 2015;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canavero S, Bonicalzi V. Central Pain Syndrome. New York: Cambridge University Press, 2007. [Google Scholar]
- 5. Canavero S, Bonicalzi V. Central Pain Syndrome, 2nd edn Cambridge: Cambridge University Press, 2011. [Google Scholar]
- 6. Canavero S, Bonicalzi V. Role of primary somatosensory cortex in the coding of pain. Pain 2013;154:1156–1158. [DOI] [PubMed] [Google Scholar]
- 7. Canavero S, Bonicalzi V. Pain myths and the genesis of central pain. Pain Med 2015;16:240–248. [DOI] [PubMed] [Google Scholar]
- 8. Canavero S, editor. Textbook of Therapeutic Cortical Stimulation. New York: Nova Science, 2009. [Google Scholar]
- 9. Canavero S, editor. Textbook of Cortical Brain Stimulation. Berlin: DeGruyter Open, 2014. [Google Scholar]


