Moyamoya disease (MMD) is a progressive occlusive cerebrovascular disorder of the internal carotid arteries or their branches, with compensatory development of an abnormal basal collateral vascular network 1. Surgical revascularization has been proposed as effective in preventing clinical events, via either a direct or indirect procedure 2, 3. As a commonly used indirect revascularization method, encephaloduroarteriosynangiosis (EDAS) is considered easier and safer than direct bypass 4. However, its effectiveness remains controversial 3, 4. Therefore, it is important to identify whether a patient is suitable for EDAS to determine the best surgical approach.
Increased and dilated cortical microvasculature is considered a MMD‐specific alteration, and it might represent a compensatory mechanism for reduced cerebral blood flow 5, 6. According to our surgical experience in the treatment of MMD, there seems to be a correlation between the cortical microvascular density and the effect of EDAS. Therefore, we sought to verify this hypothesis in this study.
We identified all consecutive patients with MMD treated by EDAS at our department from October 2011 to October 2014. The diagnosis of MMD was made according to the guidelines published in 2012 2.
All EDAS surgeries were performed on the middle cerebral artery (MCA) territory, and the parietal branch of the superficial temporal artery (STA) was used. General anesthesia was maintained using sevoflurane in oxygen and air, combined infusions of propofol and remifentanil. End‐tidal carbon dioxide (ETCO2) was maintained at 38–42 mmHg in all patients during surgery.
For the analysis of cortical vascularization, we took photographs of the cerebral cortex during surgery. Postoperatively, we analyzed two suitable regions of interest (ROIs) per cortex image. ROIs were characterized as an area of 5 × 5 or 10 × 10 mm grids without obvious arteries and veins 5. A microvascular density (MD) was calculated as the percentage of visible microvessel grids and total grids per analyzed ROI, and the final MD scores were represented as the means of the two ROIs in each image. The cases were divided into three groups with the following cutoff points: “low,” MD less than 33%; “medium,” MD between 33% and 66%; and “high,” MD higher than 66% 7 (Figure 1).
Figure 1.
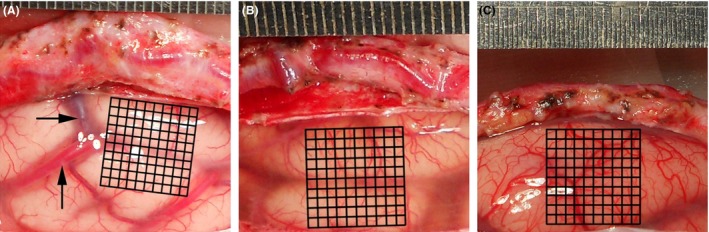
Intraoperative images of cortical surface (territory of MCA) during revascularization surgery in patients with moyamoya disease. For analysis, the millimeter grids were placed and the obvious arteries and veins were avoided. A microvascular density (MD) score was calculated as the percentage of visible microvessels grids (VG) and total grids (TG) as follows: MD = VG/TG. Sample A was classified as “Low” (with a MD score of 31/100); sample B as “Medium” (52/100); and sample C as “High” (82/100).
Postoperative cerebral digital subtraction angiography (DSA) was routinely performed 6–8 months after surgery. The development of collateral circulation through the bypass was graded according to the system described by Matsushima et al. 8: Grade I, revascularization of less than 1/3 of the MCA distribution; Grade II, between 1/3 and 2/3; and Grade III over 2/3. The extent of collateral formation was dichotomized into favorable (Grade II and Grade III) and unfavorable (Grade I).
The long‐term outcomes were ascertained during clinical visits and telephone interviews. The overall clinical outcomes were divided into four grades 9: (1) excellent, preoperative symptoms had totally disappeared without fixed neurological deficits; (2) good, symptoms had markedly decreased, but mild neurological deficits remained; (3) fair, symptoms persisted albeit less frequently; and (4) poor, preoperative symptoms remained unchanged or worsened and with the appearance of new symptoms. The clinical outcome was also dichotomized into favorable (excellent and good) and unfavorable (fair and poor).
In statistical analysis, the categorical variables were analyzed using the chi‐square test. Clinical characteristics were analyzed using the Wilcoxon rank‐sum test. For all tests, P < 0.05 was considered statistically significant. All analyses were performed with the use of SPSS 22.0 (International Business Machines Corp., Almond, NY, USA).
In total, 184 patients (93 men, 27.5 ± 15.9 years) were enrolled in our study. There were 66 children and 118 adults. In these 184 patients, 243 cerebral hemispheres underwent revascularization with EDAS procedure. The MD we assessed ranged from 23/100 to 94/100. The 243 hemispheres were divided into three grades: 81 hemispheres were evaluated as “low,” 91 hemispheres as “medium,” and 71 hemispheres as “high.” In our study population, the proportion of high MD in pediatric patients was markedly higher than that in adult patients (P < 0.001).
The surgical outcomes of EDAS are illustrated in Table 1. In terms of the development of collateral circulation, in both pediatric and adult patients, the rates of favorable revascularization were increased with the grades of MD (50.0%, 78.8%, and 90.9% in children, P < 0.001; 30.4%, 72.4%, and 81.5% in adults, P < 0.001, respectively). Overall, pediatric patients demonstrated significantly better revascularization on the surgical cerebral hemispheres than adult patients (80.9% vs. 55.2%, P < 0.001).
Table 1.
Relationship between microvascular density (MD) and the surgical outcomes of encephaloduroarteriosynangiosis
| MD | Total | Collateral circulation (%) | Improvement of symptoms (%) | ||
|---|---|---|---|---|---|
| Favorable | Unfavorable | Favorable | Unfavorable | ||
| Children | |||||
| Low | 12 | 6 (50.0) | 6 (50.0) | 5 (41.7) | 7 (58.3) |
| Medium | 33 | 26 (78.8) | 7 (21.2) | 25 (75.8) | 8 (24.2) |
| High | 44 | 40 (90.9) | 4 (9.1) | 37 (84.1) | 7 (15.9) |
| Total | 89 | 72 (80.9) | 17 (19.1) | 67 (75.3) | 22 (24.7) |
| Adults | |||||
| Low | 69 | 21 (30.4) | 48 (69.6) | 34 (49.3) | 35 (50.7) |
| Medium | 58 | 42 (72.4) | 16 (27.6) | 42 (72.4) | 16 (27.6) |
| High | 27 | 22 (81.5) | 5 (18.5) | 21 (77.8) | 6 (22.2) |
| Total | 154 | 85 (55.2) | 69 (44.8) | 97 (63.0) | 57 (37.0) |
The mean follow‐up period was 24.1 ± 8.6 months (range: 6–42 months). The rates of favorable outcomes were also increased with the grades of MD in both pediatric and adult patients (41.7%, 75.8%, and 84.1% in children, P = 0.01; 49.3%, 72.4%, and 77.8% in adults, P = 0.006, respectively). Compared with adult patients, pediatric patients were more likely to get favorable outcomes after surgical revascularization (75.3% vs. 63.0%, P = 0.049).
This study is the first to focus on the correlation between cortical microvascularization and outcomes of indirect revascularization in a large population of patients with MMD. In this study, whether in children or in adults, patients with higher MD had more sufficient collateral formation and more favorable clinical improvement following EDAS. The development of collateral circulation through the indirect bypass depends on two main factors: the degree of cerebral ischemia and the capacity for neovascularization 4. First, because cortical microvascularization represents a MMD‐specific compensatory mechanism for impaired cerebral hemodynamic 5, 6, we argue that patients with a higher MD, which reflected a greater degree of cerebral ischemia, consequently demonstrate a stronger demand to stimulate the formation of collateral circulation compared with patients with a lower MD. Second, we assume that increased cortical microvascularization is attributable to an adequate capacity for angiogenesis and arteriogenesis, which also promotes the development of sprouting collateral vessels from the extracranial vascular by indirect revascularization 1. Of course, further studies are required to investigate the specific mechanism.
Indirect revascularization procedures have been considered more effective for pediatric patients than for adult patients in previous studies 4. In our cohort, angiographic and clinical outcomes in pediatric patients were also significantly better than those in adult patients. This phenomenon may be attributable to the above‐mentioned fact that the proportion of high MD in pediatric patients was markedly higher than that in adult patients. Even so, adult patients with medium and high MD could also get a sufficient collateral formation and a favorable clinical improvement following EDAS. Therefore, compared with age, MD is the more decisive factor contributing to the effect of EDAS in our study.
Based on our results, we found a significant predictor of favorable surgical outcome, which might help to identify who is suitable for EDAS. During surgery, if an increased number and diameter of vessels are observed that lead to the “reddish” appearance of the cortex, the patient is considered a good candidate for EDAS. On the contrary, if the cortex appears “white,” the surgical procedures should be direct or combined anastomosis and not EDAS.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This study was supported by grant from National Natural Science Foundation of China (Grant Nos. 81200884, 81171083 and 81571136).
References
- 1. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009;360:1226–1237. [DOI] [PubMed] [Google Scholar]
- 2. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases . Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 2012;52:245–266. [DOI] [PubMed] [Google Scholar]
- 3. Duan L, Bao XY, Yang WZ, et al. Moyamoya disease in China: its clinical features and outcomes. Stroke 2012;43:56–60. [DOI] [PubMed] [Google Scholar]
- 4. Mizoi K, Kayama T, Yoshimoto T, Nagamine Y. Indirect revascularization for moyamoya disease: is there a beneficial effect for adult patients? Surg Neurol 1996;45:541–549. [DOI] [PubMed] [Google Scholar]
- 5. Czabanka M, Peña‐Tapia P, Schubert GA, Woitzik J, Vajkoczy P, Schmiedek P. Characterization of cortical microvascularization in adult moyamoya disease. Stroke 2008;39:1703–1709. [DOI] [PubMed] [Google Scholar]
- 6. Czabanka M, Peña‐Tapia P, Schubert GA, et al. Clinical implications of cortical microvasculature in adult Moyamoya disease. J Cereb Blood Flow Metab 2009;29:1383–1387. [DOI] [PubMed] [Google Scholar]
- 7. Giatromanolaki A, Sivridis E, Brekken R, et al. The angiogenic “vascular endothelial growth factor/flk‐1 (KDR) receptor” pathway in patients with endometrial carcinoma: prognostic and therapeutic implications. Cancer 2001;92:2569–2577. [DOI] [PubMed] [Google Scholar]
- 8. Matsushima T, Inoue T, Suzuki SO, Fujii K, Fukui M, Hasuo K. Surgical treatment of moyamoya disease in pediatric patients‐comparison between the results of indirect and direct revascularization procedures. Neurosurgery 1992;31:401–405. [DOI] [PubMed] [Google Scholar]
- 9. Matsushima T, Fujiwara S, Nagata S, et al. Surgical treatment for paediatric patients with moyamoya disease by indirect revascularization procedures (EDAS, EMS, EMAS). Acta Neurochir (Wien) 1989;98:135–140. [DOI] [PubMed] [Google Scholar]


