Summary
Aims
Multiple sclerosis (MS) is an immune‐mediated demyelinating disorder of the central nervous system (CNS). We described that Candida albicans (Ca) aggravates experimental autoimmune encephalomyelitis (EAE) that is a model to study MS. We also observed that vaccination with a myelin peptide (MOG) in the presence of vitamin D (VitD) protected mice against EAE. In this work, we investigated whether Ca infection interferes with the efficacy of this vaccine.
Methods
EAE was induced in C57BL/6 female mice previously vaccinated with MOG+VitD and then infected 3 days before encephalomyelitis induction.
Results
Vaccination was able to control EAE development in infected mice. These animals gained weight, and only a few progressed to very low clinical scores. Protection was confirmed by a lower inflammatory infiltration in the CNS and was also associated with a reduced production of encephalitogenic cytokines by spleen and CNS cell cultures. The elevated percentage of CD25+FoxP3+ cells suggests that regulatory T cells are involved in the protection. Adoptive transfer of splenocytes from mice vaccinated with MOG+VitD supports the view that protection is mediated by immunoregulatory cells.
Conclusion
Together, these experiments provide evidence demonstrating that EAE can be prevented by the inverse vaccination with MOG+VitD even in the presence of a disease‐aggravating infectious agent.
Keywords: Active vitamin D, Disseminated candidiasis, Multiple sclerosis, Myelin oligodendrocyte glycoprotein, Tolerogenic vaccination
Introduction
Multiple sclerosis (MS) is a chronic inflammatory condition of the central nervous system (CNS) determined by a presumed autoimmune process mainly directed to myelin components 1. More than 2.5 million people have MS worldwide and the incidence continues to increase and leads to substantial disability in most patients 2. Acute demyelination shows as clinical relapses that may fully or partially resolve, while chronic demyelination and neuroaxonal injury lead to continuous and permanent neurological symptoms that usually progress over time 3. Experimental autoimmune encephalomyelitis (EAE) is an animal model that is very useful to understand MS pathophysiology and treatment 4. This model is usually induced by immunization of susceptible mice strains with CNS antigens associated with strong adjuvants 5. Inoculation of C57BL/6 mice with myelin oligodendrocyte glycoprotein‐35‐55 peptide (MOG35‐55) triggers a chronic form of the disease that does not remit 6. This MOG35‐55 EAE model has supplied a growing number of evidences on the role of the immune system the pathogenesis of MS, elucidating the contribution of B, T, and other cell subsets in the pathogenesis of this disease 7, 8, 9.
MS is a multifaceted condition, with a range of environmental, behavioral, and genetic factors implicated in its etiology and clinical course 10. Considering that most of the systemic fungal infections have been associated with dissemination to the CNS 11, these pathogens could contribute to local tissue destruction. Alternatively, the local immune response against them could also participate in the immune process that damages the CNS. A possible association between MS and Candida spp. has been suggested by serological evidences. A significantly higher level of Candida‐specific antibodies was detected in patients with MS than in normal control individuals 12. In addition, Candida spp. antigens were also demonstrated in the cerebrospinal fluid of some patients with MS13. We recently used MOG35‐55‐induced EAE to demonstrate that Candida albicans infection before encephalomyelitis induction aggravated EAE clinical parameters. This higher severity was concomitant to fungus dissemination to the CNS and also to a much higher production of encephalitogenic cytokines by peripheral and local cell cultures 14.
Even though clinical studies are required to test new therapies, this EAE model is considered a first‐line model in the development of novel therapeutic approaches for MS, especially for shedding light on specific mechanistic questions 5. Myelin‐specific induction of tolerance represents a promising strategy to modify the course of autoimmune inflammatory demyelinating diseases such as MS 15. Inverse vaccination, for example, which refers to an antigen‐specific tolerogenic immunization procedure, is able to silence immunity to myelin in MS 16. Different vaccine preparations have given encouraging results. Cytokine–neuroantigen association is a new class of tolerogenic/therapeutic vaccines for treatment of EAE 15, 17, 18. This approach is based on the association between recognition of self‐antigens and the activity of tolerogenic adjuvants. Overall, these fusion proteins are able to induce tolerance and inhibit the effector phase of autoimmune response in EAE 15. Similarly, polymeric biodegradable lactic–glycolic acid nano/microparticles loaded with MOG35‐55 autoantigen and recombinant IL‐10 significantly ameliorated the course of EAE 18.
We recently demonstrated that MOG35‐55 injection in the presence of active vitamin D is highly tolerogenic being able to work as a therapy 19 and also as a prophylaxis 20. In this context, we evaluated whether this kind of tolerogenic vaccination was also efficient in the presence of an aggravating EAE agent as is C. albicans.
Materials and Methods
Experimental Design
Mice were allocated into three groups: EAE, submitted to EAE induction; Ca/EAE, infected with C. albicans 3 days before EAE induction; and, MOG+VitD/Ca/EAE, vaccinated with MOG+VitD, infected with C. albicans and then submitted to EAE induction. Evaluations were performed during the early clinical and clinical disease phases, 10th and 17th days after EAE induction, respectively. The chronology of the experimental design is clarified in Figure 1A.
Figure 1.
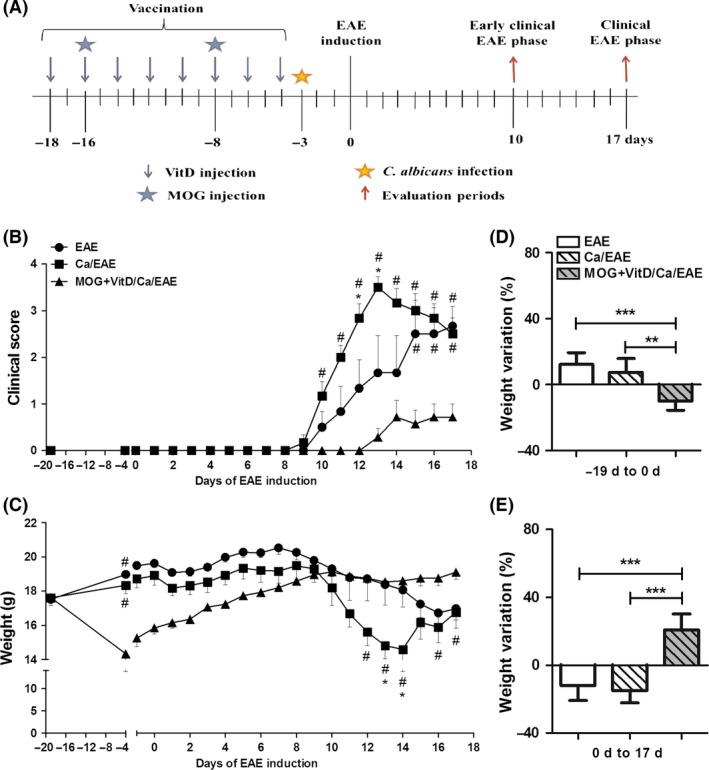
Efficacy of tolerogenic vaccination with MOG+VitD in EAE exacerbated by C. albicans infection. Experimental design (A); vaccination procedure included eight VitD doses administered every other day and two MOG doses injected at days ‐16 and ‐8. One day after last VitD dose, C57BL/6 mice were infected with C. albicans, and 3 days after, they were submitted to EAE induction. Peripheral and local immunological parameters were assessed in early clinical and clinical disease phases, 10th and 17th days after EAE induction, respectively. Clinical score (B) and body weight (C) were daily recorded until the 17th day. The percentage of weight variation was determined during vaccination, that is from day ‐19 to day 0 (D) and during EAE development, that is, from day 0 to day 17 (E). The results are expressed as mean ± SD (6–7 mice/group), one experiment representative of two is shown. (B,C) # indicates difference between vaccinated and nonvaccinated groups; * indicate difference between EAE and Ca/EAE groups. (D,E) **P < 0.01 and ***P < 0.001 indicate difference between vaccinated and nonvaccinated groups.
Mice
Female C57BL/6 mice with 9–11 weeks old were purchased from the University of São Paulo (Ribeirão Preto, SP, Brazil). Mice were allocated in specific pathogen‐free conditions, in cages (maximum 5 mice per cage) with free food and autoclaved tap water in a controlled photoperiod (12‐h/12‐h, dark/light cycle) environment. All procedures were performed in ways that minimize animal suffering. The animals were manipulated according to the ethical principles for animal research adopted by the National Council for the Control of Animal Experimentation (CONCEA). This study was approved by the local Ethics Committee for Animal Experimentation, Univ. Estadual Paulista (UNESP, Botucatu, SP, Brazil; protocol number 351).
Vaccination with MOG Associated with Vitamin D3
Active vitamin D3, 1α,25‐dihydroxyvitamin D3 (VitD) (Sigma‐Aldrich Corporation, St. Louis, MO, USA), was diluted in 15% ethanol and then inoculated intraperitoneally in C57BL/6 mice. Each animal received eight VitD doses (0.1 μg/dose) every other day during 2 weeks (on days 18, 16, 14, 12, 10, 8, 6, and 4 before EAE induction). They also were inoculated with two doses (150 μg/dose) of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) (Genemed Synthesis Inc., San Antonio, TX, USA) that were coadministered on days 16 and 8 19, 20. The vaccination with MOG associated with VitD was represented by MOG+VitD in the text and figures.
Candida albicans Infection
Candida albicans strain FCF 14 (Genbank Accession EF591020) was originally obtained from the mycology collection of the São José dos Campos Dental School (UNESP), and maintained in our mycological collection on Sabouraud dextrose agar (Difco Laboratories, Detroit, MI, USA). For mice infection, C. albicans was cultured on solid media during 24 h at 37°C. The fungal concentration was adjusted to 5 × 107/mL viable yeast cells in sterile saline solution (SSS). Fungal suspension was then inoculated into the lateral tail vein (0.1 mL/animal). For culture stimulation, C. albicans suspension (5 × 107/mL) was killed by heat and pressure during 15 min at 121°C in autoclave.
EAE Induction
Animals were subcutaneously immunized with 25 μL (100 μg) of MOG35–55 peptide emulsified in 25 μL of Complete Freund's Adjuvant (Sigma) containing 4 mg/mL of Mycobacterium tuberculosis. Mice also received two intraperitoneal doses, 0 and 48 h after immunization, of 200 ng of Bordetella pertussis toxin (Sigma). Clinical score and body weight were daily recorded until the 17th day. EAE clinical assessment was performed according to the following criteria: 0, no symptoms; 1, limp tail; 2, hind legs weakness; 3, partially paralyzed hind legs; 4, complete hind leg paralysis; and 5, complete paralysis/death. The percentage of weight loss was calculated considering the day of immunization as day 0.
Fungal Load Determination
Samples from brain and spinal cord were weighted and macerated in 1 mL of SSS. Afterward, 0.1 mL from each tissue homogenate was spread over culture plates containing Sabouraud dextrose agar using a Drigalski T loop. The plates were then sealed and incubated at 37°C for 3 days. The number of colony‐forming units (CFU) was normalized per gram of tissue and logarithmized.
Central Nervous System Mononuclear Cells Isolation
Ten and seventeen days after EAE induction, mice were anesthetized with ketamine/xylazine and perfused with 10 mL of SSS. Brain and spinal cord were collected, macerated, and digested with 2.5 mg/mL of collagenase D (Roche Applied Science, Indianapolis, IN, USA) in 4 mL of RPMI 1640 medium (Sigma) at 37°C for 45 min. Then, suspensions were washed in RPMI and centrifuged at 450 × g at 4°C for 15 min. Cells were resuspended in Percoll (GE Healthcare, Uppsala, Sweden) 37% and gently laid over Percoll 70% in tubes of 15 mL. The tubes were centrifuged at 950 × g for 20 min with centrifuge breaks turned off. After centrifugation, the ring containing mononuclear cells was collected, washed in RPMI, and centrifuged at 450 × g for 10 min. Cells were then resuspended in supplemented RPMI medium (1% gentamicin, 2% glutamine, 1% sodium pyruvate, 1% nonessential amino acids, and 10% of fetal calf serum), counted, and cultured. Pools from two animals were used to get enough cell number to perform the experiments.
Cell Culture Conditions and Cytokine Quantification
Spleen cells were collected and adjusted to 5 × 106 cells/mL in complete RPMI medium (1% gentamicin, 2% glutamine, and 10% of fetal calf serum). CNS‐isolated cells were adjusted to 2 × 105 cells/mL in supplemented RPMI medium. Spleen and CNS‐isolated cells were plated and stimulated with MOG (20 μg/mL and 50 μg/mL, respectively) and with heat‐killed C. albicans (5 yeasts/1 cell). Cytokine levels were evaluated 48 h later by enzyme‐linked immunosorbent assay (ELISA) in culture supernatants using IL‐2, IFN‐γ, and IL‐10 BD OptEIA Sets (Becton, Dickinson and Company, BD, Franklin, San Diego, CA, USA) and IL‐6, IL‐17, TNF‐α, and TGF‐β Duosets (R&D Systems, Minneapolis, MN, USA). The assays were performed according to the manufacturer's instructions.
Flow Cytometry
Lymph nodes (axillary + inguinal) were collected, and the red blood cells were lysed with buffer containing NH4Cl. For regulatory T cells analysis, spleen and lymph node cells were incubated with PerCP‐conjugated anti‐mouse CD3 (clone 145‐2C11, 0.2 μg), FITC‐conjugated anti‐mouse CD4 (clone GK1.5, 0.25 μg), and APC‐conjugated anti‐mouse CD25 (clone PC61.5, 0.13 μg) for 20 min at 4°C. Intracellular FoxP3 transcription factor was detected using PE‐conjugated anti‐mouse FoxP3 (clone FJK‐16s, 0.2 μg) and FoxP3 Staining Set (eBiosciences, San Diego, CA, USA) according to manufacturer's instructions. For myeloid dendritic cells analysis, lymph node cells were incubated with PerCP‐conjugated anti‐mouse F4/80 (clone BM8, 0.25 μg), FITC‐conjugated anti‐mouse CD11c (clone N418, 0.25 μg), APC‐conjugated anti‐mouse MHCII (clone M5/114.15.2, 0.03 μg), and PE‐conjugated anti‐mouse CD86 (clone GL1, 0.13 μg) for 20 min at 4°C. After staining, the cells were washed, resuspended in flow cytometry buffer, and fixed in paraformaldehyde 1%. Flow cytometry was performed using a FACS Canto II (BD) from Institute of Biosciences of Botucatu (UNESP, Botucatu, SP, Brazil), and the data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Histopathology of the Central Nervous System
The histological analysis was performed during clinical EAE phase, that is, 17 days after disease induction. After euthanasia, lumbar spinal cord samples were removed and fixed in 10% neutral buffered formalin. Paraffin slides with 5 μm were stained with hematoxylin and eosin and analyzed with a Nikon microscope. A semiquantitative analysis of CNS inflammation was performed according to the following criteria, adapted from Soellner et al. 21: (0) no infiltrates; (1) partial meningeal infiltration; (2) pronounced meningeal infiltration; and (3) pronounced meningeal and some parenchymal infiltration.
Adoptive Cell Transfer
For adoptive transfer experiment, mice were vaccinated with MOG+VitD as previously described. One day after the end of vaccination, 1.5 × 106 splenocytes from vaccinated mice were adoptively transferred via the retro‐orbital route to naïve mice. A control group received splenic cells from nonvaccinated naïve mice. After 3 days, mice were infected with C. albicans, and after three additional days, they were submitted to EAE induction. Clinical score and body weight were daily recorded until the 10th day. At this time point, CNS‐isolated cells were used to assess cytokine production and lumbar spinal cord was used to assess gene expression.
RT‐PCR Assays
Frozen lumbar spinal cord samples were used for RNA extraction (Trizol reagent; Life Technologies, Carlsbad, CA, USA) and cDNA synthesis according to the manufacturer's recommendations (High Capacity RNA‐to‐cDNA converter kit; Life Techcnologies). Expression of indoleamine 2,3‐dioxygenase (IDO) (Mm00477461_m1) and FoxP3 (Mm00475162_m1) were analyzed in comparison with GAPDH (Mm99999915_g1, housekeeping gene) levels. RT‐PCR reactions were performed using Taqman reagents according to manufacturer's recommendations (Applied Biosystems, Foster City, CA, USA). Expression levels of genes were represented as a relative expression using the method of delta threshold (2−ΔΔCt).
Statistical Analysis
Results were expressed as mean ± standard deviation. To test for the normality of data, results were analyzed by Shapiro–Wilk's test. Comparisons among three groups were made by one‐way ANOVA followed by Tukey's test for parametric variables, and values of P < 0.05 were considered statistically significant. The GraphPad Prism v5.0 Statistical Guide (2007, GraphPad Software Inc., La Jolla, CA, USA) for Windows was used to analyze data and create graphs.
Results
Tolerogenic Vaccination is Efficient even in the Presence of Candida albicans Infection
To investigate whether tolerogenic vaccination with MOG35‐55 in the presence of active vitamin D (MOG+VitD) was efficient even in exacerbated EAE, mice were vaccinated with MOG+VitD strategy and then infected with C. albicans before EAE induction (Figure 1A). Animals were daily monitored for disease development during 17 days. As expected, C. albicans infection triggered a much more severe disease characterized by higher clinical scores (Figure 1B) and more accentuated body weight loss (Figure 1C). Vaccination significantly decreased the incidence of EAE in previously infected mice (Table 1). Animals from MOG+VitD/Ca/EAE group developed a much milder disease with very low clinical scores (Figure 1B) and gained body weight during disease development (Figure 1C). Although vaccinated mice lost body weight during VitD administration (Figure 1D), they subsequently gained weight whereas nonvaccinated mice clearly lost weight (Figure 1E).
Table 1.
EAE incidence, Chi‐squared test (7–13 mice/group), results from two independent experiments were combined
| Groups | Incidence | P value |
|---|---|---|
| EAE | 100% (7/7) | <0.001 |
| Ca/EAE | 100% (11/11) | |
| MOG+VitD/Ca/EAE | 38.5% (5/13) |
Vaccination Downmodulated Peripheral Cytokine Production in Infected EAE Mice
As inverse vaccination has been associated with tolerance induction in EAE models 15, 17, 18, we hypothesized that cytokine production could be decreased in the peripheral lymphoid organs. Therefore, cultures of spleen cell from distinct EAE phases (early clinical and clinical phase) were stimulated with MOG. At the early clinical EAE phase, there was a significant downmodulation of Interleukin (IL)‐17, Tumor Necrosis Factor (TNF)‐α, Interferon (IFN)‐γ, and IL‐2 production (Figure 2B–E) in the MOG+VitD/Ca/EAE group in comparison with the Ca/EAE group. In this stage, no difference was observed in IL‐6 and IL‐10 production (Figure 2A,F), and Transforming Growth Factor (TGF)‐β levels were lower in the Ca/EAE group in comparison with the two other experimental groups (Figure 2G). Nonetheless, a very distinct scenario was observed when cytokine levels were evaluated in cultures from the clinical EAE phase. Even though vaccination determined a significant decrease in IL‐17 production (Figure 2B) in the previously infected mice, the production of other proinflammatory cytokines, IL‐6, TNF‐α, IFN‐γ, and IL‐2, was similar between MOG+VitD/Ca/EAE and Ca/EAE groups (Figure 2A,C–E). In addition, there was no difference in the levels of IL‐10 and TGF‐β levels between these two groups (Figure 2F,G). The comparison between the two clinical time points in the nonvaccinated EAE groups revealed a decrease in IL‐6, IL‐17, and IL‐2 production and an increase in TGF‐β production (Figure 2A,B,E,G). In the vaccinated group, with exception of IL‐6 and IL‐10, there was no difference in the levels of cytokines between the two time points (Figure 2B–E,G).
Figure 2.
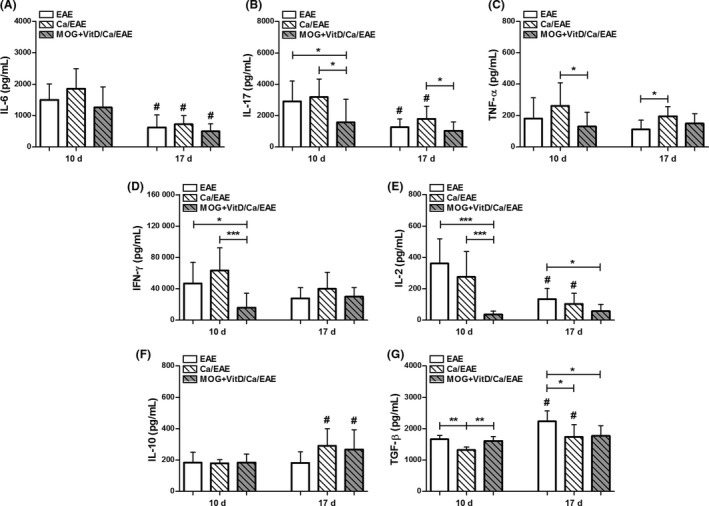
Vaccination downmodulated peripheral cytokine production in infected EAE mice. C57BL/6 mice were vaccinated with MOG+VitD, infected with C. albicans, and then submitted to EAE induction. IL‐6 (A), IL‐17 (B), TNF‐α (C), IFN‐γ (D), IL‐2 (E), IL‐10 (F), and TGF‐β (G) levels were measured in spleen cell cultures (5 × 106 cells/mL) stimulated with MOG (20 μg/mL) at early clinical (10th day) and clinical (17th day) EAE phases. The results are expressed as mean ± SD (6–14 cultures/group), and results from two independent experiments were combined. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate difference among groups. # indicates difference between time points of analysis.
MOG+VitD Vaccination and Candida albicans Infection Lead to Expansion of CD25+FoxP3+ T cells and Myeloid Dendritic Cells in Lymph Nodes
We next tested whether this peripheral cytokine downmodulation was concomitant with alterations in the proportion of T cells and myeloid Dendritic Cells (DCs). The percentage of CD3+CD4+ T cells in the lymph nodes was significantly elevated in the Ca/EAE group (Figure 3A) compared with uninfected EAE group. In the vaccinated group, the percentage of CD3+CD4+ T cells was lower than in the Ca/EAE group at both early clinical and clinical EAE phases (Figure 3A). Even though the three experimental groups presented elevated percentages of regulatory T cells (CD3+CD4+CD25+FoxP3+) at the clinical phase compared with the early clinical EAE phase, the values were significantly higher in Ca/EAE and MOG+VitD/Ca/EAE groups than in the EAE control group (Figure 3B). The CD25+FoxP3+/CD3+CD4+ ratio was higher in the MOG+VitD/Ca/EAE group in comparison with nonvaccinated groups during the early clinical EAE phase (Figure 3C). In this EAE phase, the percentage of DCs (F4/80‐CD11c+CD86+MHC II+) was similar in the three experimental groups (Figure 3D). During clinical EAE phase, even though the percentage of DCs was higher in both infected groups (Ca/EAE and MOG+VitD/Ca/EAE) in comparison with the EAE group, vaccination decreased the percentage of myeloid DCs in MOG+VitD/Ca/EAE group compared with Ca/EAE group (Figure 3D).
Figure 3.
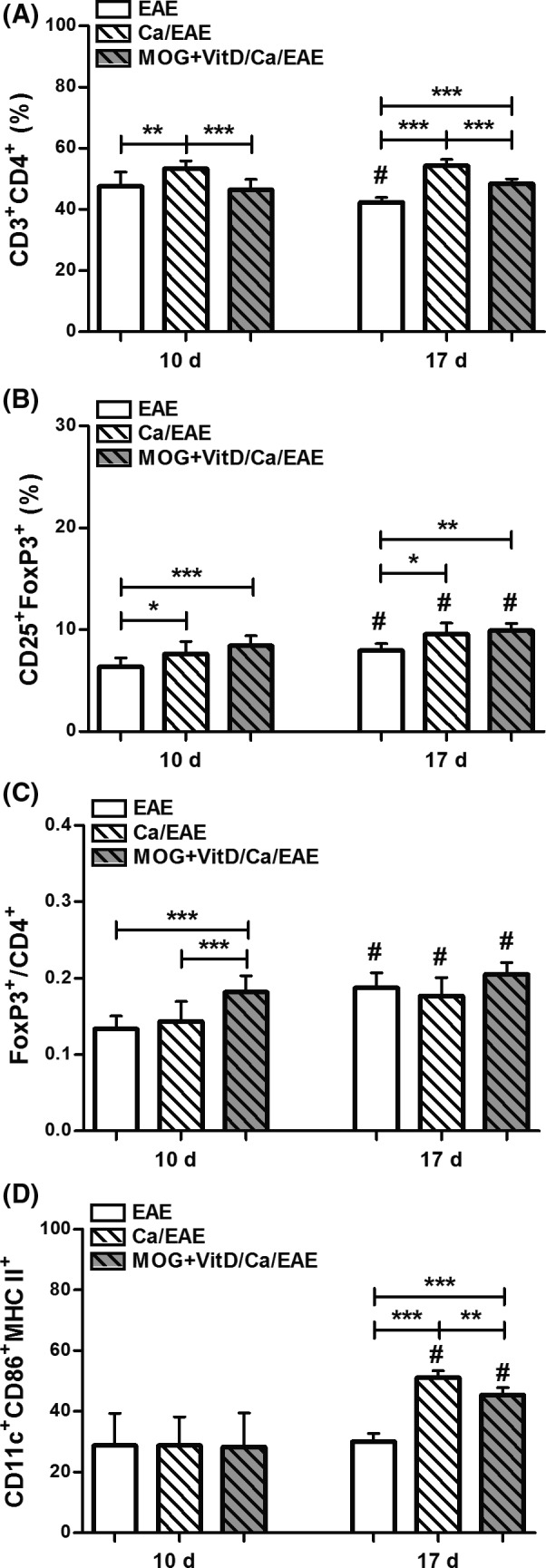
Expansion of regulatory FoxP3+ T cells in MOG+VitD‐vaccinated mice. C57BL/6 mice were vaccinated with MOG+VitD, infected with C. albicans, and then submitted to EAE induction. The percentage of T cells and dendritic cells (DCs) was determined in a pool of axillary and inguinal lymph node cells at early clinical (10th day) and clinical (17th day) EAE phases. Percentage of CD3+ CD4+ cells (A) in 100,000 acquired events and CD25+FoxP3+ cells (B) in total CD3+ CD4+ cells. Ratio between percentage of FoxP3+ cells and CD4+ cells (C). Percentage of CD86+ MHC II + (D) in F4/80‐ CD11c+ DCs in 500,000 acquired events. The results are expressed as mean ± SD (6–14 samples/group), and results from two independent experiments were combined. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate difference among groups. #indicates difference between time points of analysis.
Less Proinflammatory Activity in the Central Nervous System of Vaccinated Infected EAE Mice
To ascertain whether the vaccination procedure was also efficiently reducing the cellular infiltration in the CNS, stained lumbar spinal cord sections were semiquantitatively evaluated concerning the degree of inflammation during the clinical EAE phase (Figure 4A). Whereas the EAE and Ca/EAE groups presented a similar degree of inflammation, MOG+VitD/Ca/EAE presented a much less pronounced inflammatory process as illustrated in Figure 4B. The number of leukocytes per gram of CNS (brain plus spinal cord) was lower in vaccinated group compared with nonvaccinated groups in the early clinical EAE phase (Figure 4C). This difference was not observed when this comparison was made at the clinical EAE phase. Despite the lower inflammation in the CNS in previously vaccinated mice, they presented the same amount of fungus at the CNS as nonvaccinated mice at both disease phases (Figure 4D). In spite of this reduced inflammation, it was possible to recover enough cells to be stimulated in vitro with MOG to assess cytokine production. During the early clinical EAE phase, Ca/EAE group produced elevated levels of proinflammatory cytokines while vaccinated group showed downmodulation of these cytokines (Figure 5A–E). During the clinical EAE phase, when EAE and Ca/EAE presented similar clinical scores, comparable levels of IL‐6, IL‐17, and TNF‐α were detected, as showed in Figure 5(A–C). On the other hand, the amount of these same mediators was significantly reduced in the MOG+VitD/Ca/EAE group in comparison with nonvaccinated groups. The production of IL‐2 and IL‐10 followed a similar pattern, that is, lower levels were observed in the MOG+VitD/Ca/EAE group (Figure 5E,F). No difference was observed in TGF‐β levels (data not shown).
Figure 4.
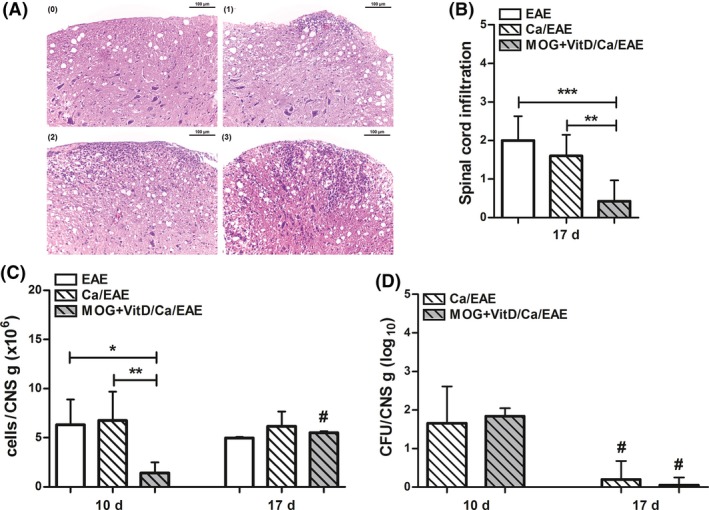
CNS inflammatory infiltration and fungal load. C57BL/6 mice were vaccinated with MOG+VitD, infected with C. albicans, and then submitted to EAE induction. Lumbar spinal cord inflammation was evaluated according to the following criteria (A): (0) no infiltrates; (1) partial meningeal infiltration; (2) pronounced meningeal infiltration, and (3) pronounced meningeal and some parenchymal infiltration. This semiquantitative analysis was used to assess the inflammatory infiltration (B) during the clinical EAE phase. The number of leukocytes (C) and fungal burden (D) was determined per gram of CNS tissue (in a pool of brain and spinal cord) at early clinical (10th day) and clinical (17th day) EAE phases. The results are expressed as mean ± SD (6–14 mice/group), and results from two independent experiments were combined. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate difference among groups. # indicates difference between time points of analysis.
Figure 5.
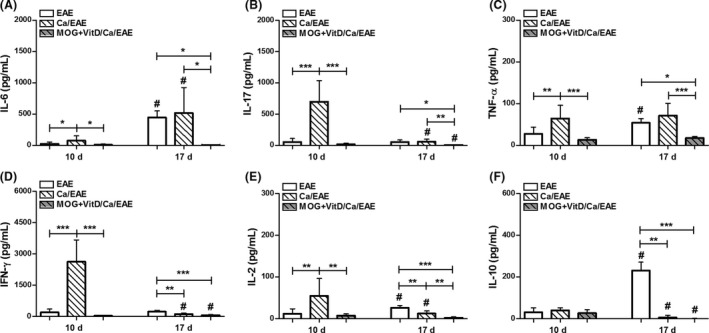
CNS cytokine production. C57BL/6 mice were vaccinated with MOG+VitD, infected with C. albicans, and then submitted to EAE induction. IL‐6 (A), IL‐17 (B), TNF‐α (C), IFN‐γ (D), IL‐2 (E), and IL‐10 (F) levels were measured CNS cell culture (2 × 105 cells/mL) stimulated with MOG (50 μg/mL) at early clinical (10th day) and clinical (17th day) EAE phases. The results are expressed as mean ± SD (4–11 cultures/group), and results from two independent experiments were combined. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate difference among groups. # indicates difference between time points of analysis.
Splenocytes from Vaccinated Mice Transfer Protection against EAE Development
In order to test whether the protection induced by vaccination with MOG+VitD was associated with an effect over the specific cellular immune response and not the result of a nonspecific effect of VitD, we evaluated the ability of splenocytes from vaccinated naïve mice to suppress EAE (Figure 6A). Adoptive transfer of MOG+VitD splenocytes obtained from previously vaccinated mice reduced clinical EAE manifestations in mice infected before encephalomyelitis induction. Until early clinical EAE phase, Ca/EAE mice recipient of MOG+VitD splenocytes showed no signs of paralysis and no body weight loss in comparison with control recipient Ca/EAE mice (Figure 6B,C). Considering that FoxP3, IDO, and cytokines are factors usually involved in VitD immunomodulation 22, the relative mRNA expression for FoxP3 and IDO and also the production of cytokines were assessed in the CNS. An increased expression of FoxP3 was detected in the lumbar spinal cord from Ca/EAE mice recipient of MOG+VitD splenocytes in comparison with mice from control recipient group (Figure 6D), and no difference was observed in IDO expression (Figure 6E). Additionally, the significantly lower production of TNF‐α and IFN‐γ (Figure 6F,G) provide evidence that the vaccination with MOG plus with vitamin D induced tolerance to CNS antigen that reduced EAE severity.
Figure 6.
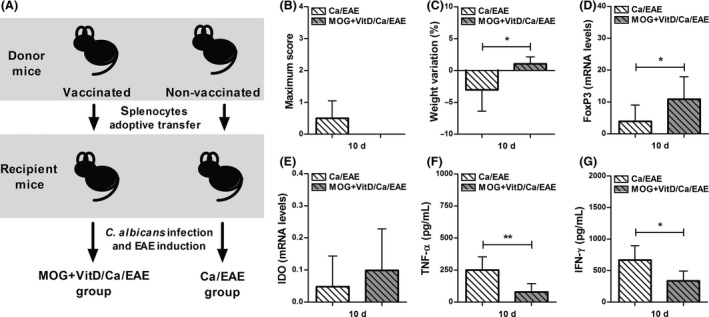
Splenocytes from vaccinated mice transfer protection against EAE development. Experimental design (A); vaccination procedure included eight VitD doses administered every other day and two MOG doses injected at days ‐16 and ‐8. One day after last VitD dose, splenocytes were adoptively transferred (1.5 × 106 cells/mouse) into C57BL/6‐naïve mice. Recipient mice were infected with C. albicans 3 days before EAE induction. Disease development was followed during the early clinical EAE phase to assess maximum clinical score (B) and percentage of weight variation (C). Expression of FoxP3 (D) and IDO (E) were analyzed in lumbar spinal cord. The TNF‐α (F) and IFN‐γ (G) levels were measured in CNS cell culture stimulated with MOG. The results are expressed as mean ± SD (6 mice/group), and one experiment is shown. *P < 0.05 and **P < 0.01 indicate difference between groups.
Discussion
Our research team has investigated strategies to prevent or to treat autoimmune diseases. We recently described that the association of the myelin oligodendrocyte glycoprotein (MOG35‐55) with active vitamin D3 (VitD) is highly efficient as a precocious treatment for EAE 19 and also as a prophylactic vaccination 20. We also focus our work on the effect of infectious agents on EAE development. Whereas Staphylococcus aureus infection attenuated this experimental disease 23, C. albicans triggered a much more accentuated encephalomyelitis 14. In this context, we questioned whether prophylaxis with MOG+VitD could be also effective to reduce EAE in the presence of C. albicans infection.
Clinical, immunological, and histopathological findings contributed to conclude that vaccination with MOG+VitD can decrease EAE severity even in the presence of C. albicans that is a disease aggravator. To get evidences for the mechanism that could be involved in this protective effect, we evaluated cytokine production, the percentage of regulatory T cells (Tregs) and myeloid DCs during both the early clinical (10th day) and clinical (17th day) EAE phases. Even though only more recently explored, alterations present at the EAE onset or early clinical disease phase are relevant to determine the involvement and kinetics of different cell types and their mediators in disease aggravation or disease control 24, 25. The relevance of this early analysis was very clear in our study. Furthermore, in this case, the results obtained during the clinical disease phase could be misunderstood because the acute phase in the EAE control group coincided with a recovery phase in the Ca/EAE group.
In a general way, proinflammatory cytokine production by splenocytes was significantly downmodulated in the vaccinated group during the early clinical EAE. Lower levels of IL‐17 were maintained during the clinical phase. Similar alterations were triggered by subcutaneous inverse vaccination with nano/microparticles loaded with MOG35‐55 in EAE; this procedure also decreased the secretion of IL‐17 and IFN‐γ induced by MOG35‐55 in splenic T cell cultures 18. Additionally, during the early clinical EAE, the MOG+VitD/Ca/EAE group produced more TGF‐β than the Ca/EAE group. This finding seems relevant because the production of TGF‐β has been implicated in the efficacy of prophylactic and therapeutic strategies in EAE 26 and also in MS 27. Several studies have shown that the TGF‐β suppressor role has been associated, at least in part, to its ability to inhibit activation and maturation of DCs 28, 29 and to induce transcription of FoxP3 30. Surprisingly, we found no differences in the amount of myeloid DCs (F4/80‐CD11c+CD86+MHC II+) among experimental groups during this disease phase. A reduced amount of myeloid DCs was expected based on our previous results 19 and also in the extensive literature disclosing the ability of vitamin D3 to interfere with DCs maturation 31, 32. Differently from expected, we observed an expansion of myeloid DCs in infected groups in comparison with the EAE group during the clinical EAE phase. Also the MOG+VitD/Ca/EAE group showed a lower amount of myeloid DCs in comparison with Ca/EAE group. The increased percentage of myeloid DCs was attributed to C. albicans immune response 33 because the comparison between infected groups revealed that vaccination decreased the percentage of myeloid DCs.
Considering that active vitamin D3 is able to generate tolerogenic DCs that promote the induction of regulatory T (Treg) cells 34, we investigated whether there was an expansion of Treg cells in vaccinated group. As expected, as Candida infection per se results in a FoxP3+ cells expansion 35, the infected groups showed an elevated percentage of Treg cells (CD3+CD4+CD25+FoxP3+) in the lymph nodes at both disease phases. Interestingly, the vaccinated group exhibited lower percentage of CD4+ cells. The ratio between the number of FoxP3+ and CD4+ cells indicated that this relationship was significantly more elevated in the MOG+VitD/Ca/EAE group in comparison with Ca/EAE group. Together, these results suggest that Treg cells expansion contributed to the protective effect of MOG+VitD. A similar protective effect mediated by a tolerogenic vaccination was already described. Treatment with MOG‐DNA vaccines reduces the clinical and histopathological signs of EAE when administered in both prophylactic and therapeutic settings through dampening of antigen‐specific proinflammatory Th1 and Th17 immune responses and expansion of Treg in the periphery 36.
This protective effect was clearly confirmed when the CNS was analyzed concerning the degree of inflammation and cytokine production. A semiquantitative evaluation showed that the inflammatory process in the lumbar spinal cord was very discrete in the vaccinated group comparing with the two other experimental groups. In spite of this, enough cells were isolated from the CNS to evaluate cytokine production. By comparing cytokine levels produced by the same number of cells, very low amounts of encephalitogenic cytokines were detected during both early clinical and clinical disease phases. This lower production of proinflammatory cytokines was not due to a high local production of IL‐10 or TGF‐β. It could be, at least in part, associated with the downmodulation of peripheral MOG‐specific immunity by the vaccination with MOG+VitD. In this context, a lower amount of encephalitogenic T cells would be available to reach the CNS. As the fungal burden recovered from the CNS was similar in vaccinated and nonvaccinated groups, we concluded that protection was not related to a local fungal clearance.
To ascertain that this protective effect was due to an active process mediated by peripheral cells, we transferred splenocytes from mice previously vaccinated with MOG+VitD to naïve mice before both C. albicans infection and EAE induction. This adoptive cell transference was able to avoid early clinical disease manifestations. In comparison with mice that received splenocytes from naïve mice and were already sick 10 days after EAE induction, the ones that received splenocytes from vaccinated mice did not lose weight neither presented paralysis at this time point. This protection mediated by these splenic cells was associated with a decreased TNF‐α and IFN‐γ production in the CNS. Most of the immunoregulatory ability of vitamin D in EAE has been linked to tolerogenic DCs that produce IDO which results in Treg expansion 21. Considering that both, DCs and Treg, can be isolated from CNS of patients with MS and EAE mice 37, we initially compared the amounts of IDO and FoxP3 mRNA expression in lumbar spinal cord samples from the two groups. A similar amount of IDO mRNA expression was detected. However, a higher FoxP3 mRNA expression was detected in the CNS from protected mice, suggesting that transferred regulatory T cells are moving from the periphery to the CNS.
Conclusion
Together, our data indicate that C. albicans infection is not able to disrupt the efficacy of tolerogenic vaccination with MOG+VitD in EAE. Even in the presence of this aggravating infectious agent, the vaccination was able to reduce the clinical signs of EAE by downregulating cellular immune response toward MOG35–55 in both periphery and CNS. The adoptive transfer of splenocytes from mice vaccinated with MOG+VitD reinforced this cellular protective effect. Vaccination with MOG+VitD is therefore very effective. The potential efficacy as vaccines or therapies by associations between specific autoantigens and vitamin D in other autoimmune pathologies deserves full investigation.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
Financial support for this study was provided by São Paulo Research Foundation (FAPESP, grant number 2012/12540‐7). TFCFS received a FAPESP scholarship (grant number 2013/14353‐2).
References
- 1. Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo‐controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 2. Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 2015;14:406–419. [DOI] [PubMed] [Google Scholar]
- 3. Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J Clin Cases 2015;3:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson AP, Rodgers JM, Goings GE, Miller SD. Characterization of oligodendroglial populations in mouse demyelinating disease using flow cytometry: Clues for MS pathogenesis. PLoS One 2014;9:e107649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Procaccini C, De Rosa V, Pucino V, Formisano L, Matarese G. Animal models of Multiple Sclerosis. Eur J Pharmacol 2015;759:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mendel I, Rosbo NK, Ben‐Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H‐2b mice: Fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol 1995;25:1951–1959. [DOI] [PubMed] [Google Scholar]
- 7. Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B‐cell‐deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol 1998;161:4480–4483. [PubMed] [Google Scholar]
- 8. Koh DR, Fung‐Leung WP, Ho A, Gray D, Acha‐Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8‐/‐ mice. Science 1992;256:1210–1213. [DOI] [PubMed] [Google Scholar]
- 9. Baron JL, Madri JA, Ruddle NH, Hashim G. Janeway Jr.CA. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med 1993;177:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson S Jr, Taylor BV, van der Mei I. The role of epidemiology in MS research: Past successes, current challenges and future potential. Mult Scler 2015;21:969–977. [DOI] [PubMed] [Google Scholar]
- 11. Murthy JM, Sundaram C. Fungal infections of the central nervous system. Handb Clin Neurol 2014;121:1383–1401. [DOI] [PubMed] [Google Scholar]
- 12. Benito‐León J, Pisa D, Alonso R, Calleja P, Díaz‐Sánchez M, Carrasco L. Association between multiple sclerosis and Candida species: Evidence from a case‐control study. Eur J Clin Microbiol Infect Dis 2010;29:1139–1145. [DOI] [PubMed] [Google Scholar]
- 13. Pisa D, Alonso R, Jiménez‐Jiménez FJ, Carrasco L. Fungal infection in cerebrospinal fluid from some patients with multiple sclerosis. Eur J Clin Microbiol Infect Dis 2013;32:795–801. [DOI] [PubMed] [Google Scholar]
- 14. Fraga‐Silva TF, Mimura LA, Marchetti CM, et al. Experimental autoimmune encephalomyelitis development is aggravated by Candida albicans infection. J Immunol Res 2015;2015:635052; DOI: 10.1155/2015/635052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mannie MD, Blanchfield JL, Islam SMT, Abbott DJ. Cytokine‐neuroantigen fusion proteins as a new class of tolerogenic, therapeutic vaccines for treatment of inflammatory demyelinating disease in rodent models of multiple sclerosis. Front Immunol 2012;3:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinman L. Inverse vaccination to silence immunity to myelin in multiple sclerosis. Can J Neurol Sci 2010;37 (Suppl 2):S49–S58. [DOI] [PubMed] [Google Scholar]
- 17. Islam SMT, Curtis AD, Taslim N, Wilkinson DS, Mannie MD. GM‐CSF‐neuroantigen fusion proteins reverse experimental autoimmune encephalomyelitis and mediate tolerogenic activity in adjuvant‐primed environments: Association with inflammation‐dependent, inhibitory antigen presentation. J Immunol 2014;193:2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cappellano G, Woldetsadik AD, Orilieri E, et al. Subcutaneous inverse vaccination with PLGA particles loaded with a MOG peptide and IL‐10 decreases the severity of experimental autoimmune encephalomyelitis. Vaccine 2014;32:5681–5689. [DOI] [PubMed] [Google Scholar]
- 19. Chiuso‐Minicucci F, Ishikawa LL, Mimura LA, et al. Treatment with Vitamin D/MOG Association Suppresses Experimental Autoimmune Encephalomyelitis. PLoS One 2015;10:e0125836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mimura LAN, Chiuso‐Minicucci F, Fraga‐Silva TFC, et al. Association Of Myelin Peptide With Vitamin D Prevents Autoimmune Encephalomyelitis Development. Neuroscience 2016;317:130–140. [DOI] [PubMed] [Google Scholar]
- 21. Soellner IA, Rabe J, Mauri V, Kaufmann J, Addicks K, Kuerten S. Differential aspects of immune cell infiltration and neurodegeneration in acute and relapse experimental autoimmune encephalomyelitis. Clin Immunol 2013;149:519–529. [DOI] [PubMed] [Google Scholar]
- 22. Farias AS, Spagnol GS, Bordeaux‐Rego P, et al. Vitamin D3 induces IDO+ tolerogenic DCs and enhances Treg, reducing the severity of EAE. CNS Neurosci Ther 2013;19:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. França TG, Chiuso‐Minicucci F, Zorzella‐Pezavento SF, et al. Previous infection with Staphylococcus aureus strains attenuated experimental encephalomyelitis. BMC Neurosci 2014;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun 2010;24:641–651. [DOI] [PubMed] [Google Scholar]
- 25. Thomé R, Bombeiro AL, Issayama LK, et al. Exacerbation of autoimmune neuro‐inflammation in mice cured from blood‐stage Plasmodium berghei infection. PLoS One 2014;9:e110739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu CY1, Guo SD, Yu JZ, et al. Fasudil mediates cell therapy of EAE by immunomodulating encephalomyelitic T cells and macrophages. Eur J Immunol 2015;45:142–152. [DOI] [PubMed] [Google Scholar]
- 27. Mirshafiey A, Mohsenzadegan M. TGF‐beta as a promising option in the treatment of multiple sclerosis. Neuropharmacology 2009;56:929–936. [DOI] [PubMed] [Google Scholar]
- 28. Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF‐beta and vitamin D3 utilize distinct pathways to suppress IL‐12 production and modulate rapid differentiation of human monocytes into CD83 + dendritic cells. J Immunol 2005;174:2061–2070. [DOI] [PubMed] [Google Scholar]
- 29. Fogel‐Petrovic M, Long JA, Misso NL, Foster PS, Bhoola KD, Thompson PJ. Physiological concentrations of transforming growth factor beta1 selectively inhibit human dendritic cell function. Int Immunopharmacol 2007;7:1924–1933. [DOI] [PubMed] [Google Scholar]
- 30. Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF‐{beta} for the differentiation of Foxp3+CD4+ regulatory T cells from peripheral Foxp3‐ precursors. Blood 2007;110:4293–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adorini L, Penna G. Dendritic cell tolerogenicity: A key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol 2009;70:345–352. [DOI] [PubMed] [Google Scholar]
- 32. Pedersen AW, Claesson MH, Zocca MB. Dendritic cells modified by vitamin D: Future immunotherapy for autoimmune diseases. Vitam Horm 2011;86:63–82. [DOI] [PubMed] [Google Scholar]
- 33. Maher CO, Dunne K, Comerford R, et al. Candida albicans stimulates IL‐23 release by human dendritic cells and downstream IL‐17 secretion by Vδ1 T cells. J Immunol 2015;194:5953–5960. [DOI] [PubMed] [Google Scholar]
- 34. Penna G, Roncari A, Amuchastegui S, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25‐dihydroxyvitamin D3. Blood 2005;106:3490–3497. [DOI] [PubMed] [Google Scholar]
- 35. Whibley N, Maccallum DM, Vickers MA, et al. Expansion of Foxp3(+) T‐cell populations by Candida albicans enhances both Th17‐cell responses and fungal dissemination after intravenous challenge. Eur J Immunol 2014;44:1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fissolo N, Costa C, Nurtdinov RN, et al. Treatment with MOG‐DNA vaccines induces CD4+CD25+FoxP3+ regulatory T cells and up‐regulates genes with neuroprotective functions in experimental autoimmune encephalomyelitis. J Neuroinflammation 2012;9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xie ZX, Zhang HL, Wu XJ, Zhu J, Ma DH, Jin T. Role of the Immunogenic and Tolerogenic Subsets of Dendritic Cells in Multiple Sclerosis. Mediators Inflamm 2015;2015:513295; DOI: 10.1155/2015/513295. [DOI] [PMC free article] [PubMed] [Google Scholar]


