Summary
Aims
Mesial temporal lobe epilepsy (MTLE) is the most common form of drug‐refractory epilepsy. Most of the morphological and electrophysiological features of human MTLE can be reproduced in a mouse by a unilateral intrahippocampal injection of kainate (MTLE mouse model). The effects of antiepileptic drugs (AEDs) on the occurrence of recurrent focal hippocampal seizures in this model remain to be specified. Here, we addressed the pharmacological reactivity of this model to the most commonly used AEDs.
Methods
Using depth electroencephalographical (EEG) recordings, we tested the dose–response effects of acute injection of nine AEDs on the occurrence of hippocampal paroxysmal discharges (HPDs) as well as on ictal and interictal power spectra in the MTLE mouse model.
Results
Valproate, carbamazepine, and lamotrigine dose dependently suppressed HPDs and modified the general behavior and/or EEG activity. Levetiracetam and pregabalin suppressed HPDs at high doses but without any behavioral nor interictal EEG changes. Finally, phenobarbital, tiagabine, vigabatrin, and diazepam suppressed HPDs in a dose‐dependent manner at doses devoid of obvious behavioral effects.
Conclusion
The MTLE mouse model displays a differential sensitivity to AEDs with a greater efficacy of drug that facilitates GABAergic transmission. This model provides an efficient tool to identify new treatment for drug‐resistant forms of focal epilepsies.
Keywords: Animal model, EEG, Fast Fourier transform, Focal paroxysmal discharge, Hippocampus, Quantitative EEG
Introduction
Among the different forms of focal epilepsies, mesial temporal lobe epilepsy (MTLE) is the most common 1. In this syndrome, seizures arise in the temporal lobe and are associated with mild clinical signs. Seizures usually start with an aura characterized by epigastric rising sensations associated with an emotional disturbance (e.g., déjà vu) and autonomic symptoms (e.g., flushing, pallor, tachycardia). Then, the seizure develops with a motionless stare and oro‐alimentary automatisms associated with a progressive clouding of consciousness. Presurgical explorations of MTLE patients with depth electrodes have allowed the characterization of the focal nature of these seizures which generally begin with low‐frequency high‐amplitude spikes in the temporal lobe structures and are followed by high‐frequency low‐amplitude polyspikes 2, 3, 4. The evolution into a secondary generalized convulsive seizure is generally rare. Although these occasional generalized seizures are well controlled by antiepileptic drugs (AEDs), focal seizures on the contrary are or become drug‐resistant in most patients 1. There is therefore a huge need to develop animal models, which display differential responses to AEDs.
We and others have described epileptic discharges observable in the hippocampal structure with rare propagation to the cortex in a mouse model obtained by unilateral injection of kainate (KA) into the dorsal hippocampus 3. Such injection first induced a nonconvulsive status epilepticus lasting several hours and, after 2–3 weeks, spontaneous recurrent hippocampal paroxysmal discharges (HPDs) are recorded in the injected hippocampus concomitant with mild behavioral changes 2, 5, 6, 7. HPDs generally last 15–25 second, remain stable and stereotyped for the animal's life, and occur spontaneously about 45 times per hour when the animals are in a state of quiet wakefulness 8. Similarly to human patients, a neurodegeneration in the hilus of the dentate gyrus as well as in CA1/CA3, along with a dispersion of the dentate gyrus has been described in this model, whereas no significant histological alterations are observed in the contralateral hippocampus 2, 9. Altogether, the MTLE mouse reproduces most of the behavioral, electrophysiological, and histological features of human MTLE, as depicted by European clinical epileptologists 10, 11.
Yet, little is known in this model about the efficacy of AEDs on HPDs. As initially explored, HPDs appear relatively resistant to AEDs such as phenytoin, carbamazepine, and valproate 2, 5, 6, 7, 8. In the present study, we examined the acute effects of nine AEDs (valproate, pregabalin, lamotrigine, carbamazepine, tiagabine, vigabatrin, phenobarbital, diazepam, and levetiracetam), at different doses, to provide a comprehensive pharmacological signature of the MTLE mouse model. In addition, we explored the effects of these compounds, at the lowest effective dose, on interictal and ictal power spectra to further characterize the sensitivity of this model to AEDs.
Materials and Methods
Animals
We used adult C57BL/6J male mice (Janvier, Le‐Genest‐St‐Isle, France) aged between 10 and 12 weeks at the time of surgery. They were housed in individual cages with food and water ad libitum and kept under a 12/12‐h light/dark controlled cycle. All animal procedures were carried out before 2013 and in accordance with the European Community's Council Directive of November 24, 1986 (86/609/EEC), and approved by the local ethics committee. All efforts were made to minimize animal suffering and reduce the number of animals. The total number of animals used in this study can be found in Table 2.
Table 2.
Effects of valproate (100, 200, 300, and 400 mg/kg), pregabalin (10, 50, and 100 mg/kg), lamotrigine (30, 60, and 90 mg/kg), carbamazepine (25, 50, 75, and 100 mg/kg), tiagabine (0.3, 0.6, and 1.2 mg/kg), vigabatrin (25, 50, 75, and 100 mg/kg), diazepam (0.5, 1, 2, and 3 mg/kg), and levetiracetam (200, 400, 600, and 800 mg/kg) on the cumulated duration and the number of hippocampal paroxysmal discharges (HPD)
| Antiepileptic drugs | Dose (mg/kg) | n | Cum. Dur. of HPDs (second)/h | F | P (vs. vehicle) | Nb HPDs | F | P (vs. vehicle) |
|---|---|---|---|---|---|---|---|---|
| Valproate | 0 | 8 | 864 ± 172 | F 4.39 = 14.1, P < 0.001 | 35 ± 2 | F 4.39 = 16.3, P < 0.001 | ||
| 100 | 8 | 742 ± 68 | NS | 41 ± 4 | NS | |||
| 200 | 8 | 581 ± 49 | NS | 37 ± 5 | NS | |||
| 300 | 8 | 342 ± 82 | P = 0.027 | 26 ± 7 | NS | |||
| 400 | 8 | 29 ± 13 | P = 0.004 | 3 ± 1 | P < 0.001 | |||
| Pregabalin | 0 | 8 | 612 ± 125 | F 3.31 = 14.8, P < 0.001 | 41 ± 6 | F 3.31 = 25.6, P < 0.001 | ||
| 10 | 8 | 577 ± 110 | NS | 32 ± 3 | NS | |||
| 50 | 8 | 301 ± 92 | P = 0.005 | 17 ± 6 | P < 0.001 | |||
| 100 | 8 | 219 ± 67 | P = 0.002 | 14 ± 4 | P < 0.001 | |||
| Lamotrigine | 0 | 7 | 530 ± 112 | F 3.27 = 5.6, P = 0.007 | 32 ± 6 | F 3.27 = 6.6, P = 0.003 | ||
| 30 | 7 | 924 ± 50 | P = 0.012 | 75 ± 4 | P = 0.002 | |||
| 60 | 7 | 525 ± 215 | NS | 46 ± 15 | NS | |||
| 90 | 7 | 163 ± 94 | P = 0.02 | 17 ± 10 | P = 0.162 | |||
| Carbamazepine | 0 | 8 | 851 ± 104 | F 4.39 = 14.5, P < 0.001 | 37 ± 3 | F 4.39 = 9.8, P < 0.001 | ||
| 25 | 8 | 967 ± 83 | NS | 44 ± 2 | NS | |||
| 50 | 8 | 678 ± 88 | NS | 48 ± 4 | NS | |||
| 75 | 8 | 386 ± 108 | P = 0.004 | 26 ± 6 | NS | |||
| 100 | 8 | 151 ± 92 | P < 0.001 | 10 ± 5 | P = 0.002 | |||
| Tiagabine | 0 | 8 | 778 ± 111 | F 3.31 = 17.0, P < 0.001 | 45 ± 3 | F 3.31 = 20.1, P < 0.001 | ||
| 0.3 | 8 | 364 ± 83 | P = 0.031 | 27 ± 4 | P = 0.009 | |||
| 0.6 | 8 | 152 ± 101 | P = 0.01 | 10 ± 6 | P = 0.002 | |||
| 1.2 | 8 | 66 ± 66 | P = 0.002 | 4 ± 4 | P < 0.001 | |||
| Vigabatrin | 0 | 7 | 636 ± 107 | F 4.34 = 16.9, P < 0.001 | 34 ± 2 | F 4.34 = 24.3, P < 0.001 | ||
| 25 | 7 | 554 ± 81 | NS | 35 ± 3 | NS | |||
| 50 | 7 | 231 ± 41 | P = 0.004 | 16 ± 3 | P = 0.002 | |||
| 75 | 7 | 160 ± 54 | P = 0.008 | 12 ± 3 | P = 0.003 | |||
| 100 | 7 | 28 ± 13 | P = 0.001 | 3 ± 1 | P < 0.001 | |||
| Diazepam | 0 | 6 | 559 ± 88 | F 4.29 = 11.8, P < 0.001 | 37 ± 7 | F 4.29 = 14.5, P < 0.001 | ||
| 0.5 | 6 | 580 ± 77 | NS | 42 ± 4 | NS | |||
| 1 | 6 | 258 ± 72 | P = 0.014 | 22 ± 5 | P = 0.006 | |||
| 2 | 6 | 195 ± 153 | P = 0.047 | 14 ± 9 | NS | |||
| 3 | 6 | 131 ± 104 | P = 0.008 | 10 ± 7 | P = 0.003 | |||
| Phenobarbital | 0 | 6 | 691 ± 77 | F 3.23 = 17.5, P < 0.001 | 46 ± 5 | F 3.23 = 12.3, P < 0.001 | ||
| 20 | 6 | 301 ± 59 | P = 0.003 | 26 ± 5 | NS | |||
| 40 | 6 | 178 ± 81 | P < 0.001 | 15 ± 6 | P = 0.003 | |||
| 60 | 6 | 27 ± 17 | P < 0.001 | 1 ± 1 | P < 0.001 | |||
| Levetiracetam | 0 | 8 | 637 ± 83 | F 4.39 = 19.4, P < 0.001 | 41 ± 5 | F 4.39 = 18.9, P < 0.001 | ||
| 200 | 8 | 626 ± 46 | NS | 36 ± 2 | NS | |||
| 400 | 8 | 717 ± 108 | NS | 43 ± 6 | NS | |||
| 600 | 8 | 225 ± 81 | P = 0.027 | 19 ± 7 | NS | |||
| 800 | 8 | 50 ± 46 | P < 0.001 | 2 ± 1 | P < 0.001 |
Values are given as mean ± SEM. Data were analyzed using one‐way repeated‐measures ANOVAs and Bonferroni's post hoc comparisons (significance threshold P = 0.05). n: number of mice. NS: nonsignificant.
Drugs
Pregabalin (Pfizer, New‐York, NY, USA), levetiracetam (UCB pharma, Brussels, Belgium), vigabatrin (Lundbeck, Valby, Denmark), phenobarbital (Sanofi‐Aventis, Paris, France), diazepam (Roche, Basel, Switzerland), tiagabine (Cephalon, Frazer, PA, USA), lamotrigine (GSK, Brentford, UK), carbamazepine (Novartis, Basel, Switzerland), and valproate (Sigma‐Aldrich, France) were dissolved in NaCl 0.9% on the day of experiment. The primary target of each AED used in this study is summarized in Table 1. They were all injected intraperitoneally (i.p.) in a volume of 10 mL/kg with doses determined according to the literature and pilot experiments 9, 12, 13.
Table 1.
List of the antiepileptic drugs (AED) used in this study with their primary known targets (adapted from 20) chemical name and dosage form
| AEDs | Primary target | Chemical name | Dosage form |
|---|---|---|---|
| Valproic acid | Voltage‐gated Na+ channel/T‐type Ca2+ channel | Sodium 2‐propylpentanoate | Valproic acid sodium salt |
| Lamotrigine | Voltage‐gated Na+ channel | 6‐(2,3‐dichlorophenyl)‐1,2,4‐triazine‐3,5‐diamine | Powder |
| Carbamazepine | Voltage‐gated Na+ channel | 5H‐dibenzo[b,f]azepine‐5‐carboxamide | Powder |
| Levetiracetam | SV2A | (S)‐2‐(2‐Oxopyrrolidin‐1‐yl)butanamide | Liquid solution (100 mg/mL) |
| Pregabalin | α 2 δ subunit of the voltage‐dependent Ca2+ channel | (S)‐(+)‐4‐Amino‐3‐(2‐methylpropyl)butanoic acid | Powder |
| Tiagabine | GABA reuptake | (R)‐1‐[4,4‐bis(3‐methylthiophen‐2‐yl)but‐3‐enyl] piperidine‐3‐carboxylic acid | Powder |
| Vigabatrin | GABA transaminase | (RS)‐4‐aminohex‐5‐enoic acid | Powder |
| Diazepam | GABAA | 7‐chloro‐1‐methyl‐5‐phenyl‐3H‐1,4‐benzodiazepin‐2‐one | Liquid solution (5 mg/mL) |
| Phenobarbital | GABAA | 5‐Ethyl‐5‐phenyl‐1,3‐diazinane‐2,4,6‐trione | Liquid solution (20 mg/mL) |
Surgery
KA Injection
Under general anesthesia (xylazine, 4 mg/kg, and chloral hydrate, 400 mg/kg, i.p.), mice were stereotaxically injected with KA (50 nL of a 20 mM KA solution dissolved in NaCl solution (0.9%); Sigma‐Aldrich, Lyon, FR, USA) into the right dorsal hippocampus (AP = −2, ML = −1.5, DV = −2 mm from bregma) as previously described 2, 6, 10, 14.
Electrode Implantation
After KA injection, mice were implanted with a bipolar electrode, made of two twisted polyester‐insulated stainless steel wires, into the injected hippocampus. Three monopolar surface electrodes were implanted over the left and right frontoparietal cortex and the cerebellum (reference electrode). The five electrodes were then soldered to a female connector (SLR 50Z, Fischer, France) and fixed to the skull with dental cement.
For the assessment of the antiepileptic effect of vigabatrin, a telemetric system was used. Mice were implanted with a bipolar electrode into the injected hippocampus and soldered to the tips of the telemetric transmitter. Two surface electrodes (0.64‐mm‐diameter stainless steel insulated wire, PhysioTel F20‐EET; DSI, St. Paul, Minnesota) were inserted into the left and right frontoparietal cortex. The radiofrequency transmitter was inserted subcutaneously.
Electroencephalographical Recordings
Animals were used between 4 and 6 weeks after the intrahippocampal injection and electrodes implantation.
EEG was recorded using three different digital acquisition systems: Coherence 3NT (Natus, San Carlos, CA, USA, 512 Hz sampling rate, low pass filter: 120), SystemPlus Evolution (Micromed France, Mâcon, France. 512 Hz sampling rate, low pass filter: 150 Hz, high pass filter: 0.008 Hz), or DataQuest/Neuroscore (DSI, St. Paul, MN, USA, 500 Hz sampling rate, no filters). Animals were freely moving for EEG recordings.
After a habituation period (2 h), a 20‐min EEG recording was performed to serve as reference. The vehicle or a given dose of AED was injected, and the EEG was recorded for 1 h except for lamotrigine (between 1 and 2 h postinjection) and vigabatrin (between 3 and 4 h postinjection). A crossover protocol was applied where each animal has its own control. Animals were injected in a random order with the vehicle or the different doses of AEDs. Drug conditions were counterbalanced. At least 3 days between injections (1 week for vigabatrin) was allowed. For each compound, a different group of animals was used.
Hippocampal Paroxysmal Discharges Quantification
Hippocampal paroxysmal discharges quantifications were performed manually by EEG experts unaware of the recording conditions using Coherence software (Natus, San Carlos, CA, USA). HPDs were defined as rhythmic high‐amplitude sharp waves (more than two times the background activity) lasting 5 seconds or more, and separated by at least 1 second as previously reported 6, 8. The cumulated duration and number of HPDs were calculated during the different drug conditions and compared to the vehicle injection.
FFT and Morlet Wavelet Calculations
Signal analyses were achieved using a dedicated toolbox developed in‐house and running in MATLAB (Mathworks, Natick, MA, USA). Morlet wavelets were computed in the 1–50 Hz frequency range with a resolution of 0.1 Hz, using a time window of 1‐second duration moved every 0.1 second. FFT analyses were performed in the 1–128 Hz frequency range with a frequency resolution of 0.5 Hz, using a sliding window of 2‐second duration with 90% overlap over the periods of interest.
Interictal periods were defined as EEG portions of at least 10‐seconds duration devoid of any artifact, epileptic spike, or HPDs. Interictal power spectra were calculated in the baseline recording (20 min) and in the 10–30 min posttreatment period. The power change of each frequency band between these two time frames was then calculated. A longer (1 h) posttreatment time frame was used for the delayed and long‐lasting drugs (lamotrigine and vigabatrin) to match their peak effects.
To assess whether the drugs altered the power of the HPDs, we compared the power of the baseline HPDs to the power of the residual HPDs. We chose to use a 1‐h posttreatment time frame (10–70 min postinjection, except for lamotrigine, 60–120 min, and vigabatrin 180–240 min, instead of 10–30 min for the interictal analysis) to include a sufficient number of HPDs to perform the analysis.
Histological Controls
Upon completion of the experiments, all mice were injected with a lethal dose of pentobarbital (pentobarbital sodium, Céva Santé Animale, France, 60 mg/kg, i.p.). Their brains were removed, frozen, and cut into 20‐μm‐thick coronal sections and stained with cresyl violet. Histological analyses were performed to verify (1) correct location of bipolar electrodes in dorsal hippocampus, (2) cell loss in CA1, CA3, and hilus regions, and (3) 2‐ to 3‐fold increase of dentate gyrus surface, as compared to the contralateral side, in agreement with previous reports 2, 6, 8, 15.
Statistical Analysis
All data were expressed as mean ± SEM of cumulated duration and number of HPDs. The AED's effects were compared to the corresponding vehicle's using a repeated‐measures analysis of variance followed by Bonferroni's post hoc comparisons using SigmaStat 3.1 software (Systat Software Inc., Chicago, IL, USA). Statistical significance of power change per frequency band was determined by comparing the mean of the power change to a hypothetical value (0) using a nonparametric test for related samples (Wilcoxon). The significance threshold was set at P = 0.05.
Results
Spontaneous Recurrent Seizures in the MTLE Mouse
Four weeks after intrahippocampal KA injection, spontaneous recurrent HPDs (Figure 1A), lasting on average 15–20 seconds with a frequency of 3–11 Hz, were recorded in the ipsilateral injected hippocampus. They consist of slow rhythmic high‐voltage sharp waves, subsequently followed by low‐amplitude discharge of spikes and polyspikes in the absence of any paroxysmal activities on the cortex, similar to previous studies 2, 6, 16 (Figure 1A). Most HPDs were observed during a state of quiet wakefulness and were associated with behavioral arrest and/or facial automatisms. In this study, we chose to focus specifically on HPDs as they (1) are the most prominent feature of epileptic hippocampal signal 10 and (2) their reduction is the principal endpoint of AEDs. Isolated spikes and short bursts of spikes were observed and considered as interictal activities. They were not quantified in this study.
Figure 1.
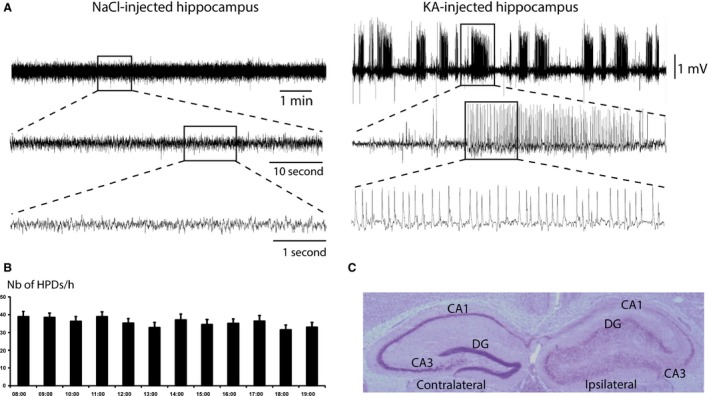
(A) Electroencephalographical recordings from NaCl‐injected (left) and kainate‐injected (KA, right) hippocampi in freely moving C57Bl6/j mice. Signal from NaCl‐injected hippocampus was recorded during quiet wakefulness 28 days following NaCl administration. Signal from the KA‐injected hippocampus was recorded 28 days following a status epilepticus induced by the administration of kainate (KA). The signal displays a series of spontaneous hippocampal paroxysmal discharges (HPDs). (B) Histogram of HPDs occurrence (number ± SEM) per hour between 08:00 am and 08:00 pm. (C) Nissl‐stained section from MTLE mouse 8 weeks after intrahippocampal KA injection. The injected hippocampus is on the right side. DG: dentate gyrus; CA1: Cornu Ammonis 1; CA3: Cornu Ammonis 3.
Telemetric EEG recordings from 8 am to 8 pm during three consecutive days (n = 9 mice, 6–8 weeks after KA injection) showed a stable mean number of HPDs per hour during daytime (Figure 1B). Among these nine mice, three displayed 1–2 generalized convulsive seizures (i.e., paroxysmal activities spreading to the cortex lasting 1 or 2 min) during this recording period. Using tethered EEG recording (1 h/week) in six to 14 mice, we found that HPDs were stable during the experimental periods with a recurrence of 38 ± 3/h in agreement with previous reports 14.
Histological controls revealed neuronal loss in the ipsilateral CA1, CA3, and hilus areas as well as a dispersion of the granule cell layer of the dentate gyrus restricted to the ipsilateral side, whereas no gross morphological modifications were observed in the contralateral side (Figure 1C), as previously described 2, 6, 8, 15, 17.
Effects of Antiepileptic Drugs
The effects of each AED were always compared to their respective vehicles.
Valproate
Valproate (100, 200, 300, and 400 mg/kg, i.p.) induced a dose‐dependent decrease in both the number and cumulated duration of HPDs (Figure 2; Table 2). A significant reduction of 60% and 26% of the cumulated duration and number of HPDs, respectively, was observed at 300 mg/kg. At this dose, valproate induced a significant decrease in hippocampal theta power during interictal epochs (Table 3), whereas no power change was observed during HPDs, when compared to baseline. No behavioral effect was seen at this dose. At 400 mg/kg, we observed a reduction of 96% and 91% in cumulated duration and number of HPDs, respectively. At this dose, animals remained immobile and appeared lethargic.
Figure 2.
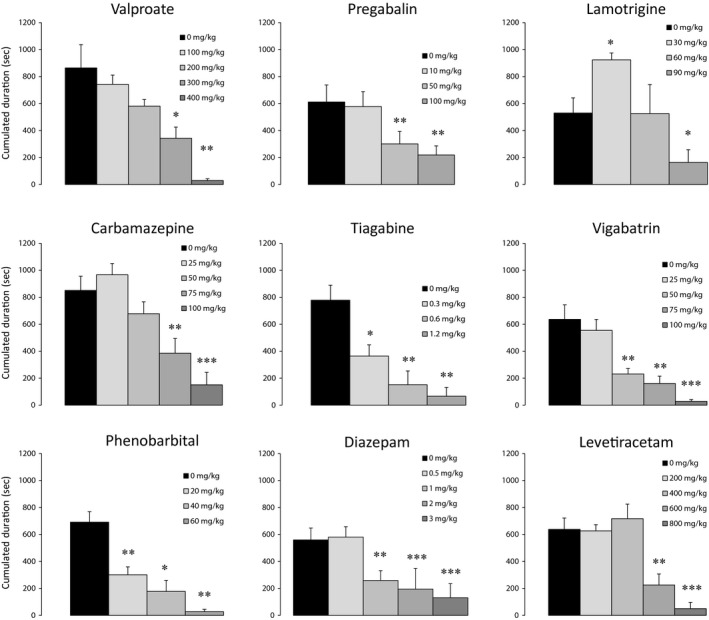
Dose–response effect of acute injections of valproate, pregabalin, lamotrigine, carbamazepine, tiagabine, vigabatrin, phenobarbital, diazepam, and levetiracetam on the cumulated duration of hippocampal paroxysmal discharges (HPD; mean ± SEM). *P < 0.05, **P < 0.01 and ***P < 0.001 (see Table for details).
Table 3.
Quantitative EEG modifications of power spectra for hippocampal paroxysmal discharges (HPDs) and interictal periods induced by AEDs. For each AED, the dose chosen is the lowest inducing a significant antiepileptic effect (see Table 2). Modifications of power were calculated as percent change between the posttreatment and the pretreatment periods for each animal
| AED | Dose (mg/kg) | n | Postdrug HPD power changes | Postdrug interictal power changes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ | θ | α | β | γ | Minutes posttreatment | δ | θ | α | β | γ | Minutes posttreatment | |||
| Valproate | 300 | 6 | ns | ns | ns | ns | ns | 10–70 | ns | ↓ | ns | ns | ns | 10–30 |
| Pregabalin | 50 | 8 | ns | ns | ns | ↓ | ↓ | 10–70 | ns | ns | ns | ns | ns | 10–30 |
| Lamotrigine | 90 | 7 | ns | ns | ns | ↓ | ↓ | 60–120 | ns | ↓ | ↓ | ↓ | ↓ | 60–120 |
| Carbamazepine | 75 | 8 | ↑ | ↑ | ↑ | ↑ | ns | 10–70 | ns | ns | ns | ↑ | ↑ | 10–30 |
| Tiagabine | 0.3 | 8 | ns | ns | ns | ns | ↓ | 10–70 | ns | ns | ns | ns | ns | 10–30 |
| Vigabatrin | 50 | 7 | ns | ns | ns | ns | ns | 180–240 | ns | ns | ns | ns | ns | 180–240 |
| Diazepam | 1 | 5 | ↓ | ns | ns | ns | ns | 10–70 | ↑ | ↑ | ns | ↑ | ↑ | 10–30 |
| Phenobarbital | 20 | 6 | ns | ns | ns | ↓ | ns | 10–70 | ns | ns | ns | ns | ns | 10–30 |
| Levetiracetam | 600 | 6 | ns | ns | ns | ns | ns | 10–70 | ns | ns | ns | ns | ns | 10–30 |
The EEG signals from some animals included in Table 2 were not used for quantitative EEG analyses because they displayed too many movement artifacts. Statistical significance (threshold P = 0.05) of power change per frequency band was determined by comparing the actual mean of the power change to a hypothetical value (0) using a one‐sample t‐test (for normally distributed data) or a signed‐rank Wilcoxon test (for nonnormally distributed data). ns: nonsignificant; ↑: significant power increase; ↓: significant power decrease.
Pregabalin
Pregabalin (10, 50, and 100 mg/kg, i.p.) induced a dose‐dependent reduction in both cumulated duration and number of HPDs. 50 mg/kg significantly decreased cumulated duration and number of HPDs by 60% and 65%, respectively, while 100 mg/kg reduced their cumulated duration and number by 72% and 73%, respectively (Figure 2 and Table 2). No obvious behavioral changes were observed at these doses. At 50 mg/kg, pregabalin did not change the interictal EEG power spectrum, but decreased the power of beta and gamma component of HPDs (Table 3).
Lamotrigine
Lamotrigine was reported to have a maximal effect around 1 h after injection 16, 18. Therefore, we analyzed the effect of this AED between 60 and 120 min postinjection. Lamotrigine (30, 60, and 90 mg/kg, i.p.) showed a biphasic effect on HPDs that we never observed with any other AED. At 30 mg/kg, we observed a significant increase in both HPD cumulated duration and number versus saline (73% and 140%, respectively), whereas we saw no effect at 60 mg/kg. At these doses, no obvious modification of the animals' behavior was seen. At the highest dose (90 mg/kg), the cumulated duration of HPDs was significantly reduced (70%) (Figure 2 and Table 2). However, at this dose, a decrease in all frequency bands power except delta was seen along with the occurrence of numerous interictal spikes (Figure 3B and Table 3). In addition, the gamma power of posttreatment HPDs was significantly decreased (44%). At this dose, animals walked with difficulty and displayed body tremors.
Figure 3.
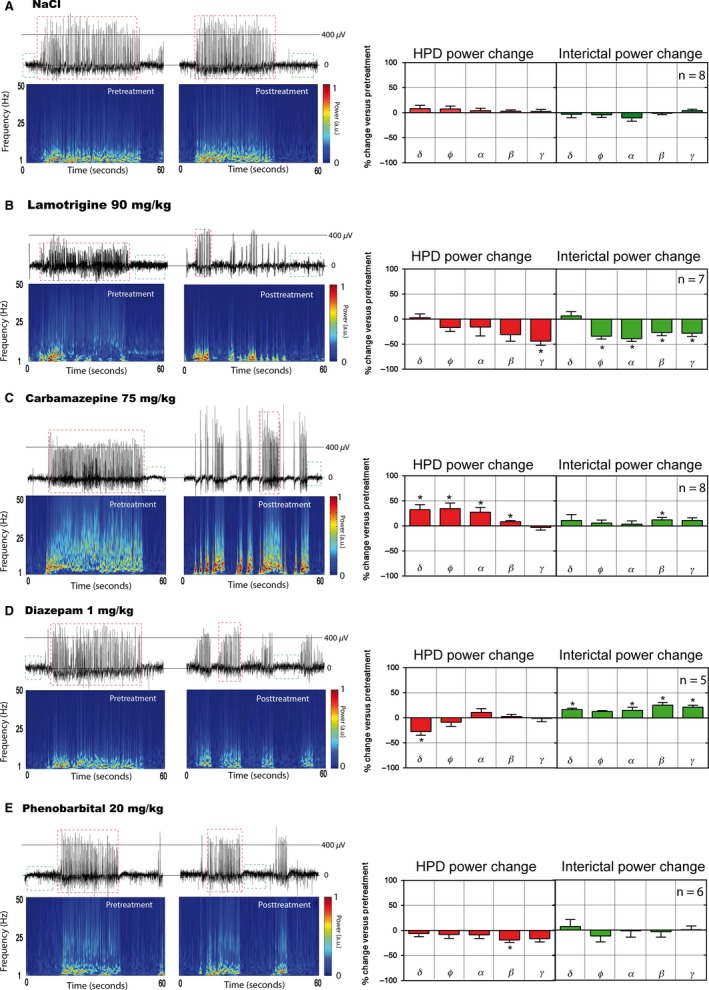
Examples of hippocampal EEG signals and time–frequency maps obtained in freely moving MTLE mice before (left panels) following treatments (right panels) with (A) NaCl 0.9%, (B) lamotrigine 90 mg/kg, (C) carbamazepine 75 mg/kg, (D) diazepam 1 mg/kg, and (E) phenobarbital 20 mg/kg. Each animal has its own control. For each EEG recording, epochs containing hippocampal paroxysmal discharges (HPDs) (such as those within red dashed squares) and artifact‐free spike‐free interictal signal (such as those within green dashed squares) were manually noted. For all drugs except lamotrigine, the power of the different frequency bands of HPDs and interictal epochs were calculated using fast Fourier transform for pretreatment (20 min) and 10–30 min (interictal) or 10–70 min (HPDs) posttreatment time ranges (lamotrigine: 60–120 min for both interictal epochs and HPDs, see Table 3). Power is expressed as arbitrary units (a.u.) ranging from 0 (minimum power in the map) to 1 (maximum power in the map). The effects of each compound on the spectral power of HPDs (red histograms) and interictal epochs (green histograms) are shown as percent change from baseline for each frequency band: delta (δ: 1.5–4 Hz), theta (θ: 6–8.5 Hz), alpha (α: 8.5–12.5 Hz), beta (β: 12.5–30 Hz), gamma (γ: 30–135 Hz). Statistical significance of power change per frequency band was determined by comparing the mean of the power change to a hypothetical value (0) using a one‐sample t‐test. *P < 0.05.
Carbamazepine
Carbamazepine (25, 50, 75, and 100 mg/kg, i.p.) induced a dose‐dependent reduction in the number and cumulated duration of HPDs (Figure 2) which was significant at the highest two doses with a reduction of 50% and 84% in the cumulated duration of HPDs, respectively (Table 2). A reduction in the number of HPDs was also observed which was significant only at 100 mg/kg (Table 2) associated with a reduction in locomotion, lethargy, and drowsiness. At 75 mg/kg, we observed numerous interictal spikes along with an increased power of beta oscillations (Figure 3C and Table 3). At this dose, residual posttreatment HPDs were strikingly increased in amplitude and power compared with the reference period (Figure 3C and Table 3).
Tiagabine
We observed a dose‐dependent reduction in both number and cumulated duration of HPDs following tiagabine injection (0.3, 0.6, and 1.2 mg/kg), which was significant at all doses tested. At 1.2 mg/kg, a reduction of 91% and 89% in HPD cumulated duration and number was obtained (Figure 2, Table 2). No modification of animals' behavior was observed at any of the doses used. In addition, at the lowest antiepileptic dose tested (0.3 mg/kg), tiagabine did not change the interictal EEG power spectrum, but decreased the power of gamma oscillations of HPDs (Table 3).
Vigabatrin
Because of its long‐lasting mode of action 19, we analyzed the effects of vigabatrin (25, 50, 75, and 100 mg/kg, i.p.) on HPDs between 3 and 4 h postinjection and a minimum of 1 week was allowed between two injections.
We observed a significant dose‐dependent suppression of cumulated duration and number of HPDs, which was significant at 50 (72% and 52% suppression, respectively), 75 (75% and 70% suppression, respectively), and 100 mg/kg (96% and 93%, respectively; Figure 2 and Table 2). No spectral EEG change and no gross modifications of animals' behavior were seen at 50 mg/kg.
Diazepam
Following injection of diazepam (0.5, 1, 2, 3 mg/kg, i.p.), we observed a dose‐dependent reduction in cumulated duration of HPDs, with a maximal effect at 3 mg/kg (77% and 76% suppression, respectively) (Figure 2, Table 2). A significant decrease in the cumulated duration of HPDs was observed at 1, 2, and 3 mg/kg. The number of HPDs was, however, significantly decreased at 1 and 3 mg/kg (Table 2). No gross modifications of animals' behavior were seen at any of the doses used. At 1 mg/kg (i.e., the lowest dose inducing significant suppression), we observed an increase in all frequency bands power (except alpha) during the interictal epochs (Figure 3D). By contrast, we saw a decrease in the delta power on posttreatment residual HPDs associated with a decrease in amplitude (Figure 3D).
Phenobarbital
Injection of phenobarbital (20, 40, and 60 mg/kg, i.p.) significantly reduced both cumulated duration and number of HPDs in a dose‐dependent way. The maximal effect was observed at 60 mg/kg (96% suppression for both) (Figure 2 and Table 2). No gross modification of animals' behavior was seen at any of the doses used. After the injection of 20 mg/kg, we only observed a significant reduction in the beta band power (19%) during residual HPDs (Figure 3E).
Levetiracetam
Levetiracetam (200, 400, 600, and 800 mg/kg, i.p.) induced a dose‐dependent suppression of both cumulated duration and number of HPDs, with a significant effect on the cumulated duration at 600 mg/kg and 800 mg/kg (65% and 92% reduction, respectively) (Figure 2, Table 2). The number of HPDs was significantly decreased only at 800 mg/kg. At this dose, numerous interictal spikes appeared on the EEG recording (data not shown). At the doses used, we observed no changes in interictal and ictal spectral power, nor in animals' behavior.
Discussion
Stability and Reliability of Spontaneous Hippocampal Paroxysmal Discharges
Accumulated evidence shows that hippocampal paroxysmal discharges (HPDs) recorded in the MTLE mouse model are reminiscent of the focal seizures observed in MTLE patients 2, 5, 6, 9, 12, 14, 15. Indeed, these recurrent discharges develop in the injected hippocampus and become stereotyped 2–3 weeks later with a steady duration of about 15–25 second 6, 17. HPDs are associated with mild behavioral changes and rarely generalize (less than once a day during daytime) in agreement with other reports 18 and clinical data 10, 11. Our data also confirmed the reproducibility and stability of HPDs in the MTLE mouse model and therefore their relevance for preclinical evaluation of AEDs.
Differential Effects of Antiepileptic Drugs on Hippocampal Paroxysmal Discharges in the MTLE Mouse Model
In the present study, we found that all AEDs tested acutely reduced the occurrence of HPDs. However, our seizure quantifications associated with spectral analysis of ictal and interictal epochs suggest that the AEDs used here can be classified into three main categories, with AEDs showing: (1) significant effects only at high doses associated with major effects on the different frequency bands (i.e., valproate, lamotrigine, and carbamazepine) and/or gross motor impairments, (2) significant suppression of HPDs only at relatively high doses with minor effects on frequency bands (i.e., levetiracetam, pregabalin) and behavior, and (3) significant suppression of HPDs at relatively low doses (i.e., phenobarbital, diazepam, tiagabine, and vigabatrin).
Acute administrations of valproate, carbamazepine, and lamotrigine suppressed HPDs but at higher doses than the ones active in other animal models of epilepsy 9, 12. At these doses, we observed obvious behavioral effects, such as drowsiness and reduced locomotion. These three AEDs also modified the power spectrum of interictal EEG, although with different patterns. In previous studies on the MTLE mouse model, no suppressive effect of carbamazepine and valproate was obtained 2, 5, 7, 20 using lower doses of valproate (300 mg/kg) and carbamazepine (20–40 mg/kg). Our data are also in line with reports indicating that high doses of valproate and carbamazepine are necessary to obtain an antiepileptic effect in models of focal epilepsies 13, 18. These three AEDs are known to primarily block presynaptic sodium channels, and our data are in agreement with reports showing that phenytoin, which also acts on such channels, is ineffective on HPDs 2, 19.
High doses of levetiracetam and pregabalin (600–800 mg/kg and 50–100 mg/kg, respectively) were necessary in our study to significantly suppress HPDs. At these doses, no obvious behavioral alterations nor interictal EEG spectral changes were observed. However, in other models of focal epilepsy, lower doses of levetiracetam and pregabalin were shown to be effective 12, 21. These two AEDs target either synaptic vesicles (SV2a) or voltage‐dependent calcium channels, respectively 20. These observations confirm and further extend the recently published data showing that levetiracetam is effective on the MTLE mouse model only at high doses 18.
In contrast with the two categories above, tiagabine, vigabatrin, diazepam, and phenobarbital all reduced the occurrence of HPDs at low doses, an effect that was dissociated from observable behavioral and spectral effects. Our data further extend the initial observations that these four AEDs suppress focal seizures in this model in the same dose range reported in other animal models 2, 5, 19, 22. It is important to note that all these four AEDs potentiate the GABAergic neurotransmission by acting on the GABAA receptor or the metabolism of GABA 1, 20.
Our study therefore suggests that the MTLE mouse model is more sensitive to drug acting on the GABAergic system and less sensitive to drugs acting on voltage‐dependent sodium channels. The greater effectiveness of the AEDs that potentiate GABAergic transmission is somewhat in disagreement with electrophysiological data that showed depolarizing effects of GABAA receptor activation in respective tissues from MTLE patients 23 but in agreement with recent studies 3. This “depolarizing GABA” hypothesis in epilepsy is, however, quite debated 22. In the MTLE mouse model, we have recently shown that injections of GABAA agonists significantly suppress HPDs despite a dysregulation of chloride transporters expression (V. Duveau, B. Pouyatos, K. Bressand, C. Bouyssières, T. Chabrol, Y. Roche, A. Depaulis and C. Roucard, submitted).
Effects of AEDs on Ictal and Interictal Quantitative EEG Changes
We next tested the effect of AEDs on the ictal and interictal power spectra at the lowest effective dose. We found that pregabalin, lamotrigine, tiagabine, and phenobarbital decreased the power in the beta and/or gamma bands during HPDs, in line with data in patients and animal models of epilepsy 24. By contrast, carbamazepine induced an increased power of residual HPDs, effect not seen on HPDs in the literature. However, an increase in the number of interictal spikes and bursts of spikes was reported in the alumina‐gel monkey model 25 and human MTLE patients 26. Whether carbamazepine facilitates short synchronizations of large assembly of neurons within seizure generators remains to be tested. Interictal power spectrum analysis showed various effects of AEDs on frequency bands. This qEEG analysis may help to predict effects of new compounds on neuronal oscillations and represent an objective way to evaluate possible associated effects of novel molecules. Clinical data assessing the effects of AEDs on power spectrum are sparse, sometimes contradictory and based on scalp EEG, unlike our data based on hippocampal depth recordings. It is interesting to note, however, that most of the interictal quantitative EEG changes induced by AEDs in the epileptic hippocampus are in agreement with patients' scalp EEG data 24, 27, 28.
The Use of MTLE Mouse Model for Preclinical Evaluation of New Antiepileptic Drugs
Despite the development of more than 20 new AEDs since the 1970s, there is still a substantial unmet need for drug‐resistant epileptic patients. The NIH/NINDS/AES Workshop on animal models recommended the development of drug‐resistant chronic limbic epilepsy models with highly recurrent seizures as screening tools 29, 30. These recommendations were confirmed by the Working Group on the Anticonvulsant Screening Program in 2012 (www.ninds.nih.gov/research/asp/asp_working_group_report_022712). Most existing animal models of chronic epilepsy are time‐consuming and labor‐intensive, because of the rare occurrence of spontaneous focal seizures. All the methodological advantages of the MTLE mouse model are similar to those described in genetic models of absence epilepsy such as the GAERS or the WAG/Rij 31 Here, using the MTLE mouse model, we could record frequent HPDs (about 45/per h) allowing a reliable quantification of discharges. A fast evaluation of new AEDs upon acute injection is therefore possible. In addition, analysis of ictal and interictal periods using quantitative EEG analysis allows a better characterization of each AED and identifies the potential effect of AEDs on the activity of the brain. In future studies, combinations of drugs could be tested to examine potentiating or synergistic effects. Using appropriate protocols (isobolography), the MTLE mouse model should help identifying the most effective drug associations. Finally, the MTLE mouse model provides objective and reliable readouts which should facilitate the early selection and validation of lead compounds. The Anticonvulsant Screening Program (ASP) is using the MTLE mouse model associated with a medium‐throughput protocol.
In conclusion, the MTLE mouse model offers the possibility to determine a differential pharmacological profile after acute treatment with different AEDs, which suggests a better efficacy for drug potentiating GABAergic transmission. This specific pharmacological reactivity further confirms the predictivity of this model to study the physiopathology of MTLE and should offer the possibility to detect new compounds able to suppress focal seizures in human patients.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This study was supported by the Ligue Française Contre l'Epilepsie (bourse LFCE/Pfizer to K. Bressand), La Métro, FEDER, the Région Rhône‐Alpes (project FUI, Rhenepi), and INSERM. The study on pregabalin was supported by Pfizer. The authors would like to thank Pr P. Kahane, Drs C. Deransart, and O. David, as well as C. Dumont and D. Delotterie for their scientific and technical help.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1. Kwan P, Schachter SC, Brodie MJ. Drug‐resistant epilepsy. N Engl J Med 2011;365:919–926. [DOI] [PubMed] [Google Scholar]
- 2. Riban V, Bouilleret V, Pham‐Le B, Fritschy J. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience 2002;112:101–111. [DOI] [PubMed] [Google Scholar]
- 3. Krook‐Magnuson E, Armstrong C, Oijala M, Soltesz I. On‐demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun 2013;4:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chabardès S, Kahane P, Minotti L, et al. The temporopolar cortex plays a pivotal role in temporal lobe seizures. Brain 2005;128:1818–1831. [DOI] [PubMed] [Google Scholar]
- 5. Klein S, Bankstahl M, Löscher W. Inter‐individual variation in the effect of antiepileptic drugs in the intrahippocampal kainate model of mesial temporal lobe epilepsy in mice. Neuropharmacology 2014;90C:53–62. [DOI] [PubMed] [Google Scholar]
- 6. Heinrich C, Lähteinen S, Suzuki F, et al. Neurobiology of Disease. Neurobiol Dis 2011;42:35–47. [DOI] [PubMed] [Google Scholar]
- 7. Gouder N, Fritschy J‐M, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia 2003;44:877–885. [DOI] [PubMed] [Google Scholar]
- 8. Duveau V, Madhusudan A, Caleo M, Knuesel I, Fritschy J‐M. Impaired reelin processing and secretion by Cajal‐Retzius cells contributes to granule cell dispersion in a mouse model of temporal lobe epilepsy. Hippocampus 2011;21:935–944. [DOI] [PubMed] [Google Scholar]
- 9. Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 2001;47:217–227. [DOI] [PubMed] [Google Scholar]
- 10. Guillemain I, Kahane P, Depaulis A. Animal models to study aetiopathology of epilepsy: What are the features to model? Epileptic Disord 2012;14:217–225. [DOI] [PubMed] [Google Scholar]
- 11. Depaulis A, Hamelin S. Animal models for mesiotemporal lobe epilepsy: The end of a misunderstanding? Rev Neurol 2015;171:217–226. [DOI] [PubMed] [Google Scholar]
- 12. Rowley NM, White HS. Comparative anticonvulsant efficacy in the corneal kindled mouse model of partial epilepsy: Correlation with other seizure and epilepsy models. Epilepsy Res 2010;92:163–169. [DOI] [PubMed] [Google Scholar]
- 13. Leclercq K, Matagne A, Kaminski RM. Low potency and limited efficacy of antiepileptic drugs in the mouse 6 Hz corneal kindling model. Epilepsy Res 2014;108:675–683. [DOI] [PubMed] [Google Scholar]
- 14. Langlois M, Polack PO, Bernard H, et al. Involvement of the Thalamic Parafascicular Nucleus in Mesial Temporal Lobe Epilepsy. J Neurosci 2010;30:16523–16535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maroso M, Balosso S, Ravizza T, et al. Interleukin‐1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 2011;8:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castel‐Branco MM, Falcão AC, Figueiredo IV, Caramona MM. Lamotrigine pharmacokinetic/pharmacodynamic modelling in rats. Fund Clin Pharm 2005;19:669–675. [DOI] [PubMed] [Google Scholar]
- 17. Maroso M, Balosso S, Ravizza T, et al. Toll‐like receptor 4 and high‐mobility group box‐1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 2010;16:413–419. [DOI] [PubMed] [Google Scholar]
- 18. Klein S, Bankstahl M, Löscher W. Inter‐individual variation in the effect of antiepileptic drugs in the intrahippocampal kainate model of mesial temporal lobe epilepsy in mice. Neuropharmacology 2015;90:53–62. [DOI] [PubMed] [Google Scholar]
- 19. Jung MJ, Lippert B, Metcalf BW, Böhlen P, Schechter PJ. gamma‐Vinyl GABA (4‐amino‐hex‐5‐enoic acid), a new selective irreversible inhibitor of GABA‐T: Effects on brain GABA metabolism in mice. J Neurochem 1977;29:797–802. [DOI] [PubMed] [Google Scholar]
- 20. Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti‐epileptic drug discovery and development. Nat Rev Drug Discov 2013;12:757–776. [DOI] [PubMed] [Google Scholar]
- 21. Vartanian MG, Radulovic LL, Kinsora JJ, et al. Activity profile of pregabalin in rodent models of epilepsy and ataxia. Epilepsy Res 2006;68:189–205. [DOI] [PubMed] [Google Scholar]
- 22. Bregestovski P, Bernard C. Excitatory GABA: How a correct observation may turn out to be an experimental artifact. Front Pharmacol 2012;3:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 2002;298:1418–1421. [DOI] [PubMed] [Google Scholar]
- 24. Arzy S, Allali G, Brunet D, Michel CM, Kaplan PW, Seeck M. Antiepileptic drugs modify power of high EEG frequencies and their neural generators. Eur J Neurol 2010;17:1308–1312. [DOI] [PubMed] [Google Scholar]
- 25. Lockard JS, Levy RH. Carbamazepine plus stiripentol: Is polytherapy by design possible? Epilepsia 1988;29:476–481. [DOI] [PubMed] [Google Scholar]
- 26. Marciani MG, Gigli GL, Stefanini F, et al. Effect of carbamazepine on EEG background activity and on interictal epileptiform abnormalities in focal epilepsy. Int J Neurosci 1993;70:107–116. [DOI] [PubMed] [Google Scholar]
- 27. Clemens B, Ménes A, Piros P, et al. Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Res 2006;70:190–199. [DOI] [PubMed] [Google Scholar]
- 28. Claus S, Leijten F, Kallansee P, et al. An electro‐encephalogram beta gap after induction with diazepam: A localization method in epileptogenic lesions. Clin Neurophysiol 2009;120:1235–1244. [DOI] [PubMed] [Google Scholar]
- 29. Klitgaard H, Matagne A, Gobert J, Wülfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol 1998;353:191–206. [DOI] [PubMed] [Google Scholar]
- 30. Stables JP, Bertram E, Dudek FE, et al. Therapy discovery for pharmacoresistant epilepsy and for disease‐modifying therapeutics: summary of the NIH/NINDS/AES models II workshop. 2003. pages 1472–8. [DOI] [PubMed]
- 31. Depaulis A, David O, Charpier S. The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J Neurosci Meth 2016;260:159–174. [DOI] [PubMed] [Google Scholar]


