Summary
Introduction
Immune dysfunction, promoted by pro‐inflammatory cytokines, plays a pivotal role in neurodegeneration associated with Huntington's disease.
Aims
The aim of this study was to investigate the emerging immunoregulatory and antiinflammatory properties of Sertoli cells in Huntington's disease.
Methods
The experimental R6/2 mouse model of Huntington's disease was treated by a single intraperitoneal injection of microencapsulated prepubertal porcine Sertoli cells and lifespan, motor performance and striatal inflammatory pattern have been evaluated.
Results
The results of this study demonstrated that a single intraperitoneal injection of microencapsulated prepubertal porcine Sertoli cells uniquely improved performances and extended the life expectancy of R6/2 Huntington's disease mice, by immune dysfunction modulation in brain.
Conclusions
This study highlights the immunomodulatory and trophic role of Sertoli cells that could be of help in the treatment of neurodegenerative disorders.
Keywords: Cell transplant, Inflammation, Microcapsules, Motor performance
Introduction
Huntington disease (HD) is a neurodegenerative, autosomal dominantly transmitted disease whose single expanded CAG‐HTT gene mutation results in the synthesis of mutant huntingtin (mHtt), a misfolded protein, with an elongated polyglutamine stretch in the N‐terminus 1. In the brain, progressive striatal atrophy, white matter degeneration (i.e., cortico‐striatal and corpus callosum fibres) and glial activation typically hallmark HD and represent early events in the disease course 2.
mHtt in the striatum is one of the culprits for neurodegeneration. Immune dysfunction, induced by mHtt, is found in both central (CNS) and peripheral immune system. In particular, mHtt induces activation of microglial cells, which primarily mediate neuroinflammation and represent key players in the pathogenesis of neurodegenerative disorders 3.
Testis‐derived Sertoli cells (SC), secreting trophic and immunomodulatory factors that are indispensable for germ cells development, have been used for years in the experimental cell therapy of chronic neurological and inflammatory diseases, with variable extent of success 4, 5.
Materials and Methods
SC Isolation, Culture and Characterization
Specific pathogen‐free (SPF) large white neonatal pigs (20–25 days old) grown in a unique SPF pig colony in Italy, the Experimental Zooprophylactic Institute of Lombardia and Emilia Romagna, were placed in sterile boxes and transported to the surgical suite at the Veterinary Medical College, University of Perugia. SPF pig strains are microbial‐free animals, whose use may be envisioned also in humans. Sertoli cells (SC) were isolated, according to previously established methods, modified in our laboratory 6.
SC Viability and Preparation of Alginate‐Based Microcapsules
Immediately after isolation, at day 3 of culture, SC viability was assessed as previously described 6 and cell culture medium was collected for in vitro treatments. SC were then encapsulated in alginate microcapsules (MC‐SC) according to our method, based on spraying cell suspended in Na alginate solution microdroplets into a Ba chloride bath: the droplets immediately turn into gel microbeads entrapping SC 6.
Apoptosis Assessment
Conditionally immortalized mouse striatal knock‐in cells expressing endogenous levels of wild‐type (STHdh7/7) or mutant huntingtin (STHdh111/111) were purchased from the Coriell Cell Repositories (Coriell Institute for Medical Research, Camden, NJ, USA). Apoptosis evaluation was performed as previously described 7.
Animal Model and Transplantation Protocol
All in vivo experiments were conducted in R6/2 transgenic mice expressing exon 1 of human Htt with approximately 160 ± 10 (CAG) repeats and manifesting first symptoms around week 6, and in wild‐type (WT) littermates, maintained on the B6CBA strain (Jackson Laboratories, Bar Harbor, MI, USA).
Biochemical assessments were carried out on brain tissues of mice euthanized at 12 weeks of age. 10 R6/2 mice used for testing the effect of MC‐SC on animal lifespan were checked daily but otherwise left undisturbed until they died naturally. All experimental protocols were approved by IRCCS Neuromed Animal Care Review Board and by “Ministero della Salute” (permit number: 43/2011‐A).
Mice of each genotype were divided into two experimental groups: (1): n = 6 mice (two males and four females) treated with empty microcapsules (EC); (2): n = 10 (one male and nine females) mice treated with MC‐SC (1 × 106 SC/gram of body weight). EC and MC‐SC were injected into the peritoneum with a syringe (needle gauge: 16 G). All treated animals were monitored weekly throughout 12 weeks. Mice from the same F generation were assigned to experimental groups, such that age and weight were matched.
Motor Behaviour Tests and Survival Study
Training and baseline testing for motor function were carried out prior to MC‐SC administration. On this purpose, 8–10 mice per group were examined. General motor function was measured weekly during the entire treatment period. Locomotor behaviour and motor performance were performed using open field, horizontal ladder task and rotarod test as previously described 7.
Protein Lysate Preparation
Dissected brain tissues were homogenized in lysis buffer containing 20 mM Tris, pH 7.4, 1% Nonidet P‐40, 1 mmol/L EDTA, 20 mmol/L NaF, 2 mmol/L Na3V04 and protease inhibitor mixture (Sigma‐Aldrich Corporation, Buchs SG, Switzerland), sonicated with 2 × 10 s pulses and centrifuged for 10 min at 10,000 × g.
Western Blotting Analysis
Western blotting analyses were conducted as previously described 8, using antiphosphorylated p65 (Cell Signalling Technology, Danvers, MA, USA), anti‐iNOS, anti‐COX2 and anti‐GAPDH (Santa Cruz Biotechnology, Dallas, TX, USA) as primary antibodies.
Real‐Time PCR Analysis
Real‐time PCR analyses were conducted as previously described 8.
Statistical Analysis
Two‐way ANOVA followed by Bonferroni post hoc test for multiple comparisons were used to compare treatment groups in the open‐field, rotarod and horizontal ladder tests. Kaplan–Meier curves employing log‐rank test were used to analyse mice survival. Nonparametric Mann–Whitney U‐test was used to analyse cell apoptosis. Two‐tailed t‐tests were employed in all other experiments. All data are expressed as mean ± SD.
Results
Motor Performance Improvement and Lifespan Extension by MC‐SC Treatment
The inhibition of apoptosis in the in vitro model of Huntington disease, STHdh111/111 cells, by factors secreted by SC (Figure 1), led us to evaluate whether a single intraperitoneal SC injection in R6/2 mouse model would improve motor performance and prolong the animals life expectancy.
Figure 1.
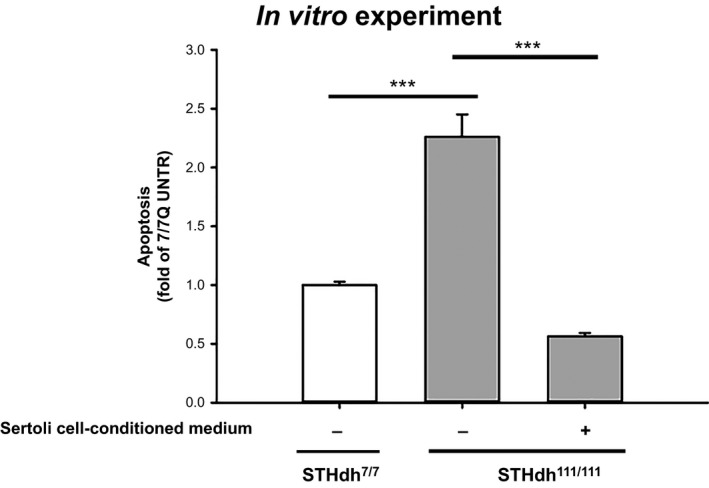
MC‐SC effect in vitro. Analysis of apoptosis in striatal‐derived cell lines, cultured for 6 h in serum‐free medium, in the presence or absence of conditioned medium from 3.5 million Sertoli cells. Data represent the mean ± SD of four experiments, each performed in triplicate. ***P < 0.001.
As transplanted “naked” SC would be impossible to retrieve if necessary, pure populations of isolated specific pathogen‐free (SPF) prepubertal pig Sertoli cells (SC) were enveloped within alginate‐based microcapsules resulting in viable microencapsulated pig SC (MC‐SC) 6.
Injection of MC‐SC at the beginning of the symptomatic disease onset (6 weeks of age) significantly extended the R6/2 mice lifespan (Figure 2A) according to Kaplan–Meier survival curve analysis, likely reflecting a neuroprotective effect of the intervention. Moreover, MC‐SC significantly preserved motor function and prevented the progressive and dramatic motor worsening commonly observed in R6/2 mice (Figure 2B, C and D). We observed that MC‐SC beneficial effects on motor coordination were maintained throughout 6 weeks, as assessed by rotarod and horizontal ladder tests (Figure 2B and C), while, spontaneous locomotor activity, maintained for about 3 weeks after the injection, showed a slight worsening, as evaluated by open‐field test (Figure 2D). Starting from the first week and throughout the postintervention follow‐up, MC‐SC‐treated mice performed significantly better than controls. The efficacy of MC‐SC injection on motor symptoms, even at very late stages of the disease, seems to constitute an important therapeutic tool for treating HD.
Figure 2.
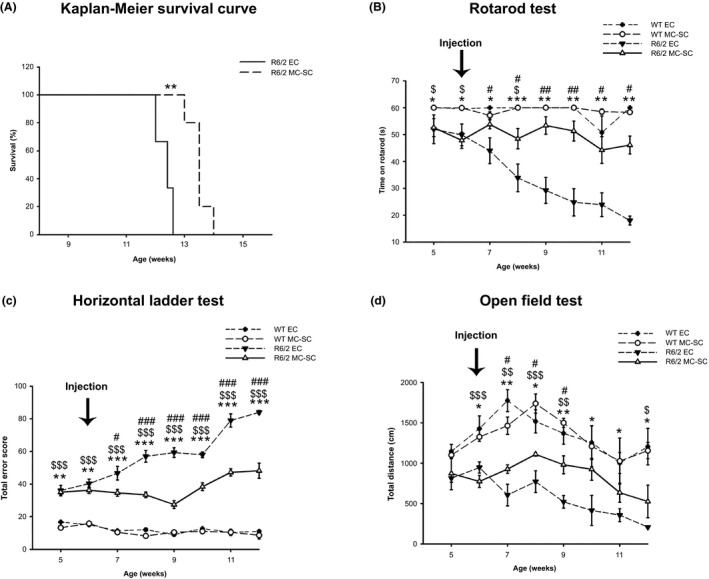
MC‐SC effects in vivo. Lifespan and motor function in R6/2 mice in vivo: (A) Kaplan–Meier probability of survival analysis in MC‐SC‐ and EC‐treated R6/2 mice. N = 5 mice for each group **P < 0.01. (B and C) Analysis of motor coordination, before and after transplantation of either EC or MC‐SC in 6‐week‐old R6/2 mice and WT littermates. (B) latency to fall, on rotarod test and (C) total error score, on horizontal ladder task. Total error score is the cumulative number of footfall errors in the horizontal ladder task. (D) General locomotor activity in the open field in the same mice, before and after transplantation. Each data point represents the average performance ± SD of 6–10 mice for each group. *P < 0.05; **P < 0.001; ***P < 0.0001 (EC‐treated WT versus EC‐treated R6/2); # P < 0.05; ## P < 0.001; ### P < 0.0001 (EC‐treated R6/2 versus MC‐SC‐treated R6/2); $ P < 0.05; $$ P < 0.001; $$$ P < 0.0001 (MC‐SC‐treated WT versus MC‐SC‐treated R6/2).
Reduction of Neuroinflammatory Markers by MC‐SC Treatment
The activation of the immune system and the altered immune response, as the presymptomatic stage of HD, lead to striatal and cortical neurodegeneration 3. Therefore, we analysed striatal levels of pro‐inflammatory molecules (Figure 3A and B). We found that MC‐SC injection induced important changes of the pro‐inflammatory patterns, as shown by the decreased levels of NF‐κB activation, iNOS (inducible nitric oxide synthase) and COX2 (cyclooxygenase 2) protein expression (Figure 3A). Furthermore, we observed that mRNA levels of TNFα and IL‐1β were downregulated (Figure 3B), while levels of the anti‐inflammatory cytokine IL‐10 were significantly upregulated by MC‐SC injection (Figure 3B). We also analysed other cerebral regions, that is hippocampus and cortex and found less pronounced effects of MC‐SC injection on the inflammatory markers (data not shown) confirming that the striatum is primarily involved in the degenerative process 3.
Figure 3.
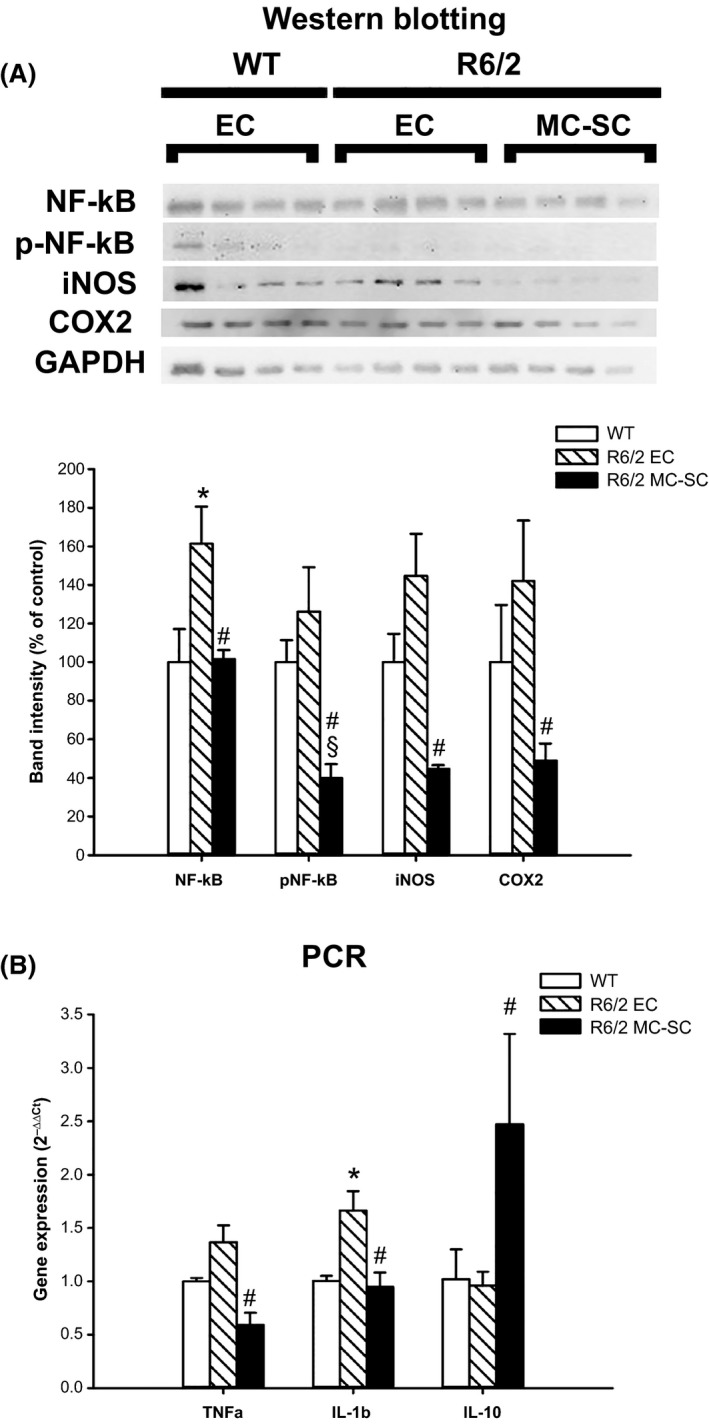
MC‐SC effects on striatal inflammatory pattern. Striatal pro‐inflammatory molecules pattern: (A) Protein expression of, NF‐κB, phospho‐p65 (pNF‐κB), COX2 and iNOS in the striatum of WT and R6/2 mice transplanted with either EC or MC‐SC. Bar graph represents the mean ± SD of densitometric analysis of the bands compared to GAPDH. (B) mRNA levels of TNF‐α, IL‐1β and IL‐10 expressed as 2−ΔΔCt. Data represent the mean ± SD of n = 4 mice for each group. *P < 0.05 versus WT; # P < 0.05 versus R6/2 EC; § P < 0.05 versus WT.
Discussion
Our data provide clear evidence of the neuroprotective and anti‐inflammatory effects of a single intraperitoneal injection of microencapsulated prepubertal porcine Sertoli cells, as shown by improved functional performance and extension of R6/2 HD mice lifespan. Indeed, disease progression was dramatically slowed down until animal death.
Soluble factors, such as cytokines (i.e., TNFα and IL‐1β), prostaglandins synthesized by COX2, and nitric oxide, synthesized by iNOS, are increased in several mouse models of HD and in the serum of patients with HD. Collectively, these factors are deemed to be responsible for neuronal dysfunction and degeneration 3. Notably, we confirmed that brain lysates of R6/2 mice exhibited significantly higher levels of IL‐1β as compared to wild‐type controls. IL‐1β directly induces neurotoxicity via activation of the NF‐κB pathway. Of interest is the evidence that the pro‐inflammatory NF‐κB signalling pathway is upregulated by mHtt, thereby possibly contributing to neuroinflammation 3. We showed that intraperitoneal injection of MC‐SC reduced NF‐κB activation. Typical of neuroinflammation is the presence of overactivated microglial cells, also shown in the R6/2 mouse model 3. It is likely that MC‐SC‐derived immunomodulatory factors 4 acted on microglial cells preventing their overactivation, thereby decreasing pro‐inflammatory molecule levels.
Moreover, defective regulation of growth factors, such as brain‐derived neurotrophic factor (BDNF) and glial‐derived neurotrophic factor (GDNF), affects CNS function and contributes to the pathogenesis of HD 2. It is known that intrastriatal injection of “naked” SC enhances survival of dopaminergic neurons and induces an extensive neurite outgrowth by GDNF, a SC secretory product 4.
It has been recently demonstrated that cerebral vasculature is damaged in R6/2 mice and in patients with HD leading to blood‐brain barrier leakage 9. Moreover, a functional CNS lymphatic system, able to carry both fluid and immune cells from the cerebrospinal fluid, has been recently discovered 10. These results might underpin the effects of intraperitoneal‐transplanted MC‐SC on brain function, although the hypothesis that Sertoli cells may exert beneficial effects in periphery, thus improving the disease progression, cannot be at present ruled out and will be the object of further researches.
In conclusion, we showed for the first time that a single intraperitoneal injection of SPF MC‐SC in R6/2 mice remarkably prolonged the animals lifespan with concomitant improvement of their quality of life, as assessed by motor function analysis, at least in part, by reducing striatal inflammation. On the whole, these results corroborate the novel concept that SC can be envisioned as a cellular complex that secretes a cocktail of bioactive molecules. These are associated with immunomodulatory and trophic properties that synergistically act on multiple fronts.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by Mr. Gary Harlem (Altucell Inc. 3 Astor Court, Dix Hills, New York, NY for licensing and funding) and Consorzio Interuniversitario per Trapianti di Organo, Rome, Italy.
The first two authors contributed equally to this work.
References
- 1. Squitieri F. Neurodegenerative disease: ‘fifty shades of grey’ in the Huntington disease gene. Nat Rev Neurol 2013;9:421–422. [DOI] [PubMed] [Google Scholar]
- 2. Rikani AA, Choudhry Z, Choudhry AM, et al. The mechanism of degeneration of striatal neuronal subtypes in Huntington disease. Ann Neurosci 2014;21:112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellrichmann G, Reick C, Saft C, Linker RA. The role of the immune system in Huntington's disease. Clin Dev Immunol 2013;2013:541259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez AI, Willing AE, Saporta S, et al. Effects of Sertoli cell transplants in a 3‐nitropropionic acid model of early Huntington's disease: A preliminary study. Neurotox Res 2003;5:443–450. [DOI] [PubMed] [Google Scholar]
- 5. Fallarino F, Luca G, Calvitti M, et al. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J Exp Med 2009;206:2511–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luca G, Mancuso F, Calvitti M, et al. Long‐term stability, functional competence, and safety of microencapsulated specific pathogen‐free neonatal porcine Sertoli cells: A potential product for cell transplant therapy. Xenotransplantation 2015;22:273–283. [DOI] [PubMed] [Google Scholar]
- 7. Di Pardo A, Amico E, Favellato M, et al. FTY720 (fingolimod) is a neuroprotective and disease‐modifying agent in cellular and mouse models of Huntington disease. Hum Mol Genet 2014;23:2251–2265. [DOI] [PubMed] [Google Scholar]
- 8. Bellezza I, Grottelli S, Mierla AL, et al. Neuroinflammation and endoplasmic reticulum stress are coregulated by cyclo(His‐Pro) to prevent LPS neurotoxicity. Int J Biochem Cell Biol 2014;51:159–169. [DOI] [PubMed] [Google Scholar]
- 9. Drouin‐Ouellet J, Sawiak SJ, Cisbani G, et al. Cerebrovascular and blood‐brain barrier impairments in Huntington's disease: Potential implications for its pathophysiology. Ann Neurol 2015;78:160–177. [DOI] [PubMed] [Google Scholar]
- 10. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]


