Summary
Aims
We aimed to evaluate early recanalization postintravenous (i.v.) tissue plasminogen activator (t‐PA) by digital subtraction angiography (DSA) in acute ischemic stroke (AIS) with large vessel occlusion (LVO).
Methods
We performed baseline CT angiography to identify LVO in AIS. Recanalization pre‐ and post‐intra‐arterial therapy (IAT) was categorized to none, partial, and global recanalization (GR). Modified Rankin Scale score ≤2 at 3 months was considered a favorable outcome.
Results
Among 1610 patients with AIS, 286 received IV t‐PA. Of these, 55 patients with LVO were included. The median time from IV t‐PA to DSA was 120 min (interquartile range, 79–152). Recanalization post‐IV t‐PA was observed in seven patients (12.7%). By occlusion sites, the recanalization rates were as follows: extracranial internal carotid artery 2 of 14 (14.3%); intracranial internal carotid artery 3 of 24 (12.5%); M1 of middle cerebral artery 3 of 39 (7.7%); M2 of middle cerebral artery 1 of 40 (2.5%); vertebral artery 0 of 4; and basilar artery 0 of 7. GR post‐IAT was associated with favorable outcomes (odds ratio: 8.6; 95% confidence interval, 1.5–48.0; P = 0.014).
Conclusion
Early recanalization assessed by DSA post‐IV t‐PA is rarely observed in acute ischemic stroke patients with LVO.
Keywords: Digital subtraction angiography, Ischemic stroke, Large vessel occlusion, Recanalization, Thrombolysis
Introduction
Intravenous (i.v.) tissue plasminogen activator (t‐PA) improves clinical outcome of patients with acute ischemic stroke (AIS) 1. However, large vessel occlusion (LVO) responds poorly to t‐PA, leading to reduced recanalization rates and unfavorable clinical outcomes 2, 3, 4. Recently, updated evidence from several trials (MR CLEAN, ESCAPE, EXTEND‐IA, etc.) showed that combined IV t‐PA and intra‐arterial (IA) intervention offered both superior recanalization and clinical outcomes in cases of LVO 5, 6, 7, 8, 9. Early recanalization post‐IVT in each arterial segment as well as whole arterial tree was rarely assessed by digital subtraction angiography (DSA) in real world. Multiple grading scales, such as Thrombolysis in Myocardial Infarction (TIMI) scale and Thrombolysis in Cerebral Infarction (TICI) scale, have been used to evaluate the recanalization status, only showing modest inter‐rater agreement though 10, 11. In our study, we aimed to perform a simplified grading scale to assess recanalization success based on baseline CT angiography (CTA) followed by DSA in the cohort of AIS patients with LVO. We also explored the relationship between recanalization success and clinical outcomes.
Materials and Methods
Subjects
This was a retrospective study designed to analyze a prospectively collected data set of consecutive patients admitted for AIS from June 2010 to December 2013. Patients in current clinical trials were not included in our study. Human Ethics Committee in Royal Melbourne Hospital has approved the use of human subjects for our study. All patients' information was anonymized and de‐identified prior to analysis. Therefore, patient consent was not obtained. Our hospital was centrally located in the Greater Melbourne metropolitan covering a population of 4.2 million. Since 2007, a Code Stroke system has been implemented in our stroke service with a rapid notification to the stroke team by Emergency Department. In addition, combined IV‐IA therapy was not broadly employed until 2010. All patients were examined, and National Institute of Health Stroke Scale (NIHSS) scores were obtained by trained neurologists or trained personnel before IV t‐PA administration. Baseline information was collected, including demographics and vascular risk factors (hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, smoking, as well as history of ischemic heart disease and stroke). The decision in administering IV t‐PA was modeled according to the inclusion criteria of European Cooperative Acute Stroke Study III 12, including patients older than 80 years if otherwise healthy and without contraindications.
For anterior circulation ischemic stroke, we followed protocol whereby patients were selected if the ischemic area was less than 1 of 3 of the middle cerebral artery territory 13, 14.In addition, other criteria were as follows: (1) acute anterior ischemic stroke within 4.5 h of stroke ictal onset or acute posterior circulation stroke within 24 h of onset; (2) NIHSS > 4 at presentation; (3) LVO on CTA of intracranial internal carotid artery (i‐ICA, i.e., C2‐C7), M1 of middle cerebral artery (M1‐MCA), M2 of MCA (M2‐MCA), vertebral artery (VA), and basilar artery (BA),with or without the occlusion of extracranial ICA (e‐ICA, i.e., C1); (4) prestroke modified Rankin Scale (mRS) score of <2.
Vessel Assessment
All eligible patients underwent CTA of neck and brain following noncontrast CT (4.5 mm slices) before or immediately after t‐PA bolus, if there were no contradictions, such as a past history of renal dysfunction and previous allergic reaction to contrast agents.
CT angiography was obtained via 16‐slice multidetector scanner (Siemens, Erlangen, Germany) using 70 mL contrast at 5 mL/s, 100 kV, and 200 mA with slice thickness of 0.75 mm. Image acquisitions were auto‐triggered by the appearance of contrast media in the ascending aorta. Occlusions were categorized into six segments: e‐ICA, i‐ICA, M1‐MCA, M2‐MCA, VA, and BA. A segment was defined as occluded if it contributed to the present AIS and did not opacify on CTA.
According to the initial angiogram, occlusive segments on baseline CTA were dichotomized as: (1) persistent occlusion—no continuous contrast material going through these segments; and (2) recanalization—continuous contrast material through the previously occluded segments. Of note, recanalization of M2‐MCA was specified by opacification of all M2‐MCA branches. This grading scale was consistent with the definition of occlusion on CTA. The angiograms were assessed by two independent observers (P.M. and B.Y.) separately and who were blinded to clinical outcomes. Patients were categorized to three groups: (1) no recanalization (NR)—all previously occluded segments remain occluded; (2) partial recanalization (PR)—not all previously occluded segments were patent; and (3) global recanalization (GR)—all previously occluded segments were patent. In the circumstances of occluded proximal ICA coinciding with opacified MCA on baseline CTA, contralateral ICA angiography would be attempted to confirm whether the ipsilateral MCA was patent.
Intra‐arterial thrombolysis or thrombectomy was used either alone or in combination, and a permanent stent was used in severe stenotic e‐ICA only if necessary to access to distal occlusion or to prevent acute reocclusion. Urokinase up to 500,000 units was used during IA thrombolysis. In our stroke center, stent retrievers (Solitaire FR, Covidien/eV3, Plymouth, MN, USA) were used in approximately 80% of AIS cases treated with combined IV‐IA. The endpoint of intervention was mTICI ≥ 2b. The procedure would be abandoned beyond 8 h of stroke onset except for patients with BA occlusion. The final DSA acquisitions were assessed by mTICI.
Outcomes
The primary outcome was recanalization of any segment post‐t‐PA. Secondary outcomes were favorable outcomes, death at 30 days and 3 months due to any cause, and symptomatic intracerebral/intracranial hemorrhage (SICH) in the three patient groups defined by the pattern of recanalization as aforementioned. The definition of favorable outcomes was that mRS scores at 3 months were 0–2 or equal to premorbidity. SICH was defined as local or remote parenchymal hemorrhage type 2 within 36 h of treatment, combined with a neurological deterioration of ≥4 points compared with baseline NIHSS or the lowest NIHSS value between baseline and 24 h 15.
Statistics
The numerical variables were presented as medians and interquartile ranges (IQR) and tested by Mann–Whitney U‐test where applicable. Categorical variables were presented as percentages and were evaluated using Fisher exact test where applicable. The inter‐rater agreement on the recanalized status of each segment post‐IV t‐PA was assessed using κ value. The relationships between potential factors and outcomes were estimated as odds ratios (ORs) with corresponding 95% confidence interval (CI) using logistic regression modeling. We set significance level at alpha of 0.05, and all tests were 2‐tailed. Statistical analysis was performed with the Stata/SE 12.0 statistical package (StataCorp LP, College Station, TX, USA).
Results
We collected 286 (17.8%) consecutive patients who received IV t‐PA of 1610 patients with AIS admitted to our hospital between 2010 and 2013. Of 286 patients, 223 (80%) had CTA at baseline which identified LVO in 150 (67.3%) cases, in which 76 (50.6%) underwent combined IV‐IA therapy (Figure 1). A total of 55 (72.4%) patients were included in the analyses, while 21 (27.6%) were recruited in EXTEND‐IA trial. Demographics and vascular risk factors are shown in Table S1. Compared with the patients who were excluded from our study, included ones had younger median age (69 vs. 77; IQR, 58–77 and 67–85, respectively; P < 0.0001), higher median NIHSS score at baseline (16 vs. 11; IQR, 11–20 and 6–18, respectively; P = 0.003), and shorter median time from onset to treatment (OTT) (126 min vs. 132 min; IQR, 105–172 and 101–188, respectively; P = 0.82). Eight (14.6%) of 55 had AIS in posterior circulation.
Figure 1.
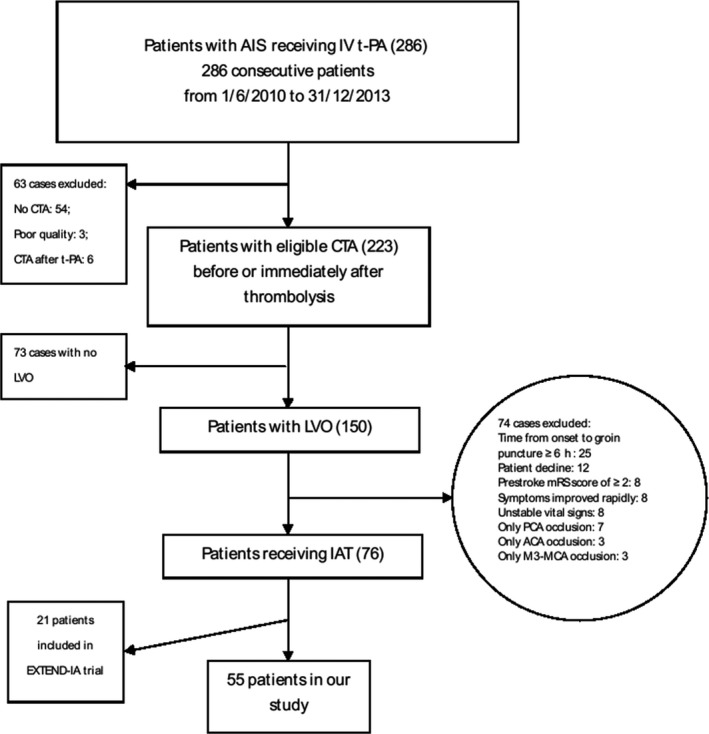
Flowcharts of patient inclusion and exclusion. AIS, acute ischemic stroke; IV, intravenous; t‐PA, tissue plasminogen activator; CTA, computed tomographic angiography; LVO, large vessel occlusion; mRS, modified Rankin Scale; PCA, posterior cerebral artery; ACA, anterior cerebral artery; MCA, middle cerebral artery; IAT, intra‐arterial therapy; EXTEND‐IA, EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra‐Arterial therapy.
Overall, 128 occlusive segments in 55 patients were identified by CTA at baseline. Between two observers, the agreement on recanalization status visualized by angiogram was excellent (κ = 0.94, P < 0.0001) and final assessment results were achieved by consensus. The median time from t‐PA bolus to baseline angiogram was 120 (IQR: 79–152) min. Recanalization rates of occlusive segments were listed in Table 1. Of those segments, recanalization after IV therapy was seen in two occlusive e‐ICA (2 of 14, 14.3%), followed by i‐ICA (3 of 24, 12.5%), M1‐MCA (3 of 39, 7.7%), M2‐MCA (1 of 40, 2.5%), VA (0 of 4, 0), and BA (0 of 7, 0) (P = 0.47).
Table 1.
Recanalization of occlusive arterial segments in patients with combined IV‐IA therapy
| Occlusive segment | Recanalization, n (%) | ||
|---|---|---|---|
| Post‐IV t‐PA | Post‐IA intervention | All | |
| e‐ICA (n = 14) | 2 (14.3) | 10 (71.4) | 12 (85.7) |
| i‐ICA (n = 24) | 3 (12.5) | 16 (66.7) | 19 (79.2) |
| M1‐MCA (n = 39) | 3 (7.7) | 26 (66.7) | 29 (74.4) |
| M2‐MCA (n = 40) | 1 (2.5) | 25 (62.5) | 26 (65.0) |
| VA (n = 4) | 0 (0) | 3 (75) | 3 (75) |
| BA (n = 7) | 0 (0) | 7 (100) | 7 (100) |
IV, intravenous; IA, intra‐arterial; t‐PA, tissue plasminogen activator; e‐ICA, extracranial internal carotid artery; i‐ICA, intracranial internal carotid artery; MCA, middle cerebral artery; VA, vertebral artery; BA, basilar artery.
Early recanalization post‐IV t‐PA was only observed in 7 patients (12.7%) comprised of 2 (3.6%) with global recanalization and 5 with partial recanalization (9.1%). Baseline characteristics between patients with recanalization and ones with no recanalization were statistically similar (Table S2).
Recanalization after IV‐IA treatment was observed in 46 patients (83.4%), of whom 38 (69.1%) achieved global recanalization. The median time from initial angiogram to end of procedure was 74 (IQR: 49–93) min. Compared with patients with no recanalization, ones with global recanalization were more likely to achieve favorable outcomes at 3 months (OR, 8.6; 95% CI, 1.5–48.0; P = 0.014), which remained significant adjusted for age and baseline NIHSS score (adjusted OR, 6.5; 95% CI, 1.1–39.5; P = 0.041); partial recanalization was not associated with favorable outcomes (OR, 1.2; 95% CI, 0.1–11.0; P = 0.89). Favorable outcomes were observed in a higher proportion of patients with global recanalization and excellent reperfusion (OR, 13.5; 95% CI, 2.5–72.6; P = 0.002), compared with patients with both incomplete recanalization and poor reperfusion (Table 2).
Table 2.
Relationships between clinical outcomes and recanalization status postcombined IV‐IA therapy in multivariable logistic regression
| Recanalization status | SICH | Death at 30 days | Death at 3 months | mRS ≤ 2 at 3 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | P | No. (%) | OR (95% CI) | P | No. (%) | OR (95% CI) | P | No. (%) | OR (95% CI) | P | |
| NR or PR co‐occurring with mTICI < 2b (n = 12)a | 1 (8.3) | 1 | … | 5 (41.7) | 1 | … | 6 (50.0) | 1 | … | 2 (16.7) | 1 | … |
| GR co‐occurring with mTICI ≥ 2b (n = 37) | 1 (2.7) | 0.3 (0.02–5.3) | 0.42 | 5 (13.5) | 0.2 (0.05–0.97) | 0.045 | 5 (13.5) | 0.2 (0.04–0.7) | 0.013 | 27 (73.0) | 6.5 (1.1–39.5) | 0.041 |
Reference group in regression model. IV, intravenous; IA, intra‐arterial; NR, no recanalization—all previously occlusive segments were still blocked; PR, partial recanalization—not all previously occlusive segments were patent; GR, global recanalization—all previously occlusive segments were patent; excellent reperfusion indicates modified Thrombolysis in Cerebral Infarction scale achieves 2b or 3; SICH, symptomatic intracerebral/intracranial hemorrhage; OR, odds ratio; CI, confidence interval; NA, not available due to insufficient numbers; mRS, modified Rankin Scale.
Discussion
Our study showed that early recanalization post‐IV t‐PA in either any arterial segment or whole arterial tree was rarely observed in AIS patients with LVO. Global recanalization postcombined IV‐IA therapy was associated with good outcomes.
Early recanalization rate after IV t‐PA has been reported previously utilizing different imaging modalities and grading scales. In a CTA‐proven occlusion cohort of 127 patients, recanalization (more than TIBI 3 or TIMI 2) was, respectively, observed in 32.6% of 46 patients assessed by TCD and 11.7% of 103 performed by DSA (i‐ICA: 4.4%; M1‐MCA: 32.3%; M2‐MCA: 30.8% and BA: 4%) 2. Few studies used DSA to assess early recanalization after IV t‐PA. A DSA study of 31 patients with LVO on initial CTA found early recanalization (TICI 2 or 3) in 7 (22.6%) patients, among whom recanalization rate of ICA or proximal MCA, distal MCA and BA was 12.5%, 27.3% and 50%, respectively 16.
Our study showed that the rate of recanalization post‐IV t‐PA was 14% in e‐ICA, followed by 12.5% in i‐ICA, 7.7% in M1‐MCA, and 2.5% in M2‐MCA. Surprisingly, it appeared that recanalization was more frequent in the occlusion of larger caliber vessels, although the trend was not significant. The larger caliber vessels, actually, were more proximal arteries. It was possible that thrombus in proximal artery (e.g., ICA) could move forward to distal branches (e.g., MCA) after IV t‐PA. We also showed a low recanalization rate of distal MCA. This finding was contrary to findings in previous studies. Distal MCA was often evaluated to be occluded in the scenario of proximal MCA occlusion due to absent antegrade flow. Recently, a 4D‐CTA study demonstrated that occlusion extended from M1‐MCA to M2‐MCA in 29% of patients with isolated MCA occlusion 17. In our study, M2‐MCA was considered to be patent only if two or three branches are all open. By this definition, recanalization of M2‐MCA was less than expected.
Despite imbalances in baseline characteristics including age, stroke severity, and OTT, early recanalization rate in our study was lower than that reported in the literature, ranging from 22.6% to 51.3% 2, 16, 18, 19, 20, 21, 22. The differing recanalization rates were likely caused by variable measurements owing to the utilization of different imaging tools and grading scales. TCD was a noninvasive tool for recanalization evaluation in real‐time post‐IV thrombolysis. However, it is operator‐dependent and insensitive to visualization of distal vessels and infeasible in patients with suboptimal acoustic windows 23, 24. Therefore, recanalization status may not be reflected precisely by TCD. Furthermore, accuracy of recanalization rate was also hindered by inequality between pre‐ and post‐thrombolytic scales. In certain DSA studies, TIMI/TICI system was used after IV t‐PA to evaluate the status of baseline CTA‐proven occlusion that was assumed to be equal to grade 0 or 1 in TIMI/TICI scale 2, 16, which was inappropriate due to recanalization differing from reperfusion 25.
Based on the high degree of agreement (100%) on arterial occlusion between CTA and DSA 26, we adopted a simple criterion (“persistent occlusion/recanalization”) of arterial patency to assess status of each arterial segment on pre‐ and post‐thrombolytic angiography. This method was simple to perform and showed excellent inter‐rater reliability (κ = 0.94) compared with either arterial occlusive lesion (κ = 0.39) or TIMI (κ = 0.40) or TICI (κ = 0.45) 10, 11.
To improve global cerebral circulation rather than merely one segment, we assessed, in addition to recanalization status of focal segments, also that of the whole arterial tree. Our study did not show an association of patency of all occlusive segments (i.e., global recanalization) post‐IV t‐PA with clinical good outcomes. This may be due to the small number of cases with recanalization leading to Type 2 error. However, global recanalization after IV‐IA treatment, as expected, was associated with good outcomes. Conversely, if not all segments were recanalized (i.e., incomplete recanalization), favorable outcome was unlikely. Incomplete recanalization could be associated with clot burden, which was approximately measured as the number of arterial occlusions 27. Previous studies showed that incomplete recanalization might lead to reocclusion associated with clinical deterioration, which could be a possible explanation for our findings 28, 29.
Digital subtraction angiography was the only tool for assessing recanalization post‐IV t‐PA in our study. Furthermore, same scale was applied to pre‐ and post‐thrombolytic angiograms. Our study maintained consistency in measurements of recanalization status which may improve the accuracy of recanalization rate. In addition, we developed a simple and feasible grading scale for the evaluation of global recanalization status after treatment, which was associated with clinical outcomes. However, our study had several limitations. It was a retrospective analysis of prospective data and not all patients treated with IV t‐PA underwent angiogram, leading to a potential for bias in the selection of subjects. Patients in our study were younger and had more severe stroke than the excluded patients with AIS. Our sample size was small. As a result, the number of patients with recanalization post‐IV t‐PA may not be adequate to demonstrate the association with clinical outcomes. In addition, adjustment for age and baseline stroke severity in analyzing the association of recanalization status with favorable outcomes at 3 months may be unstable statistically due to insufficient sample size. Given the invasive nature of DSA, baseline arterial status was measured by CTA in our study. However, CTA does not provide real‐time flow hemodynamic information. Therefore, using CTA and DSA at different time points may cause a potential error in the definition of occlusion.
Conclusions
The rate of early recanalization postintravenous t‐PA demonstrated by DSA was low in acute ischemic stroke patients with large vessel occlusion identified by baseline CTA in our center. This information may be useful for the consideration of patients for intra‐arterial therapy.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Table S1. Baseline Characteristics of Study Population.
Table S2. Baseline Characteristics of Patients with Different Status of Recanalization post t‐PA.
Acknowledgments
We gratefully acknowledge the entire stroke team and all neuroradiologists in Royal Melbourne Hospital.
References
- 1. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–25. [PubMed] [Google Scholar]
- 2. Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real‐world experience and a call for action. Stroke 2010;41:2254–8. [DOI] [PubMed] [Google Scholar]
- 3. Smith WS, Tsao JW, Billings ME, et al. Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit Care 2006;4:14–7. [DOI] [PubMed] [Google Scholar]
- 4. Yoshimura S, Egashira Y, Sakai N, et al. Retrospective nationwide survey of acute stroke due to large vessel occlusion in Japan: a review of 1,963 patients and the impact of endovascular treatment. Cerebrovasc Dis 2011;32:219–26. [DOI] [PubMed] [Google Scholar]
- 5. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 6. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med 2015;372:1009–18. [DOI] [PubMed] [Google Scholar]
- 7. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. [DOI] [PubMed] [Google Scholar]
- 8. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. [DOI] [PubMed] [Google Scholar]
- 9. Saver JL, Goyal M, Bonafe A, et al. Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med 2015;372:2285–95. [DOI] [PubMed] [Google Scholar]
- 10. Bar M, Mikulik R, Jonszta T, et al. Diagnosis of recanalization of the intracranial artery has poor inter‐rater reliability. AJNR Am J Neuroradiol 2012;33:972–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaha M, Roy C, Estrade L, et al. Inter‐ and intraobserver agreement in scoring angiographic results of intra‐arterial stroke therapy. AJNR Am J Neuroradiol 2014;35:1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bluhmki E, Chamorro A, Davalos A, et al. Stroke treatment with alteplase given 3.0‐4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol 2009;8:1095–102. [DOI] [PubMed] [Google Scholar]
- 13. Kalafut MA, Schriger DL, Saver JL, et al. Detection of early CT signs of >1/3 middle cerebral artery infarctions: interrater reliability and sensitivity of CT interpretation by physicians involved in acute stroke care. Stroke 2000;31:1667–71. [DOI] [PubMed] [Google Scholar]
- 14. Fields JD, Khatri P, Nesbit GM, et al. Meta‐analysis of randomized intra‐arterial thrombolytic trials for the treatment of acute stroke due to middle cerebral artery occlusion. J Neurointerv Surg 2011;3:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study. Lancet 2007;369:275–82. [DOI] [PubMed] [Google Scholar]
- 16. Lee KY, Han SW, Kim SH, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre‐ and post‐thrombolytic angiography in acute ischemic stroke patients. Stroke 2007;38:192–3. [DOI] [PubMed] [Google Scholar]
- 17. Rohan V, Baxa J, Tupy R, et al. Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke 2014;45:2010–7. [DOI] [PubMed] [Google Scholar]
- 18. del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–86. [DOI] [PubMed] [Google Scholar]
- 19. Kimura K, Iguchi Y, Shibazaki K, et al. Early recanalization rate of major occluded brain arteries after intravenous tissue plasminogen activator therapy using serial magnetic resonance angiography studies. Eur Neurol 2009;62:287–92. [DOI] [PubMed] [Google Scholar]
- 20. Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 1992;42:976–82. [DOI] [PubMed] [Google Scholar]
- 21. Yeo LL, Paliwal P, Teoh HL, et al. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol 2013;70:353–8. [DOI] [PubMed] [Google Scholar]
- 22. Zangerle A, Kiechl S, Spiegel M, et al. Recanalization after thrombolysis in stroke patients: predictors and prognostic implications. Neurology 2007;68:39–44. [DOI] [PubMed] [Google Scholar]
- 23. Alexandrov AV, Demchuk AM, Burgin WS, et al. Ultrasound‐enhanced thrombolysis for acute ischemic stroke: phase I. Findings of the CLOTBUST Trial. J Neuroimaging 2004;14:113–17. [PubMed] [Google Scholar]
- 24. Tsivgoulis G, Ribo M, Rubiera M, et al. Real‐time validation of transcranial Doppler criteria in assessing recanalization during intra‐arterial procedures for acute ischemic stroke: an international, multicenter study. Stroke 2013;44:394–400. [DOI] [PubMed] [Google Scholar]
- 25. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol 2005;26:1012–21. [PMC free article] [PubMed] [Google Scholar]
- 27. Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009;30:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology 2002;59:862–7. [DOI] [PubMed] [Google Scholar]
- 29. Qureshi AI, Siddiqui AM, Kim SH, et al. Reocclusion of recanalized arteries during intra‐arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2004;25:322–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Study Population.
Table S2. Baseline Characteristics of Patients with Different Status of Recanalization post t‐PA.


