Summary
While acute inflammation is a natural physiological response to tissue injury or infection, chronic inflammation is maladaptive and engenders a considerable amount of adverse pain. The chemical mediators responsible for tissue inflammation act on nociceptive nerve endings to lower neuronal excitation threshold and sensitize afferent firing rate leading to the development of allodynia and hyperalgesia, respectively. Animal models have aided in our understanding of the pathophysiological mechanisms responsible for the generation of chronic inflammatory pain and allowed us to identify and validate numerous analgesic drug candidates. Here we review some of the commonly used models of skin, joint, and gut inflammatory pain along with their relative benefits and limitations. In addition, we describe and discuss several behavioral and electrophysiological approaches used to assess the inflammatory pain in these preclinical models. Despite significant advances having been made in this area, a gap still exists between fundamental research and the implementation of these findings into a clinical setting. As such we need to characterize inherent pathophysiological pathways and develop new endpoints in these animal models to improve their predictive value of human inflammatory diseases in order to design safer and more effective analgesics.
Keywords: Animal models, Arthritis, Behavior, Cutaneous pain, Inflammation, Laboratory assessment, Nociception, Visceral pain
Introduction
Under normal conditions, acute inflammation is essential for protecting our bodies from invading pathogens, as well as promoting tissue remodeling and repair. Conversely, chronic inflammation, which lasts for 6 weeks or longer, serves no beneficial purpose and results in tissue damage and pain. Pro‐inflammatory mediators such as prostaglandins, cytokines, chemokines, proteases, neuropeptides, and growth factors are released at sites of inflammation and are capable of sensitizing peripheral pain sensing neurones (see Figure 1) 1, 2. Three organ systems that are particularly susceptible to the development of inflammatory pain are the skin, joints, and gut. Atopic dermatitis, arthritis, and inflammatory bowel disease all pose a serious global burden, and evidence suggests that the prevalence of each is bound to rise in the future if preventative measures are not taken 3, 4, 5. Fortunately, our understanding of the mechanisms that underlie chronic inflammation is constantly growing. Clinical observations have undoubtedly contributed to how we treat inflammatory disease; however, most of the fundamental discoveries regarding inflammatory mechanisms have come from animal models. In vivo models have helped us to elucidate the endogenous molecules involved in initiating and resolving inflammation, in addition to providing us with a better understanding of inflammatory pain. Furthermore, animal models are paramount to testing the efficacy and safety of new chemical entities that have the potential to become novel antiinflammatory analgesics. While animal models are primarily used to help us understand human disease, it is important to be mindful that these discoveries are also applicable to the veterinary field and the treatment of our pets and livestock. The following is a discussion concerning the role animal models play in understanding inflammatory disease and pain. We have also listed different species and tests that can be used in different animal models to study inflammatory pain (see Table 1), as well as the advantages and limitations of these models (see Table 2).
Figure 1.
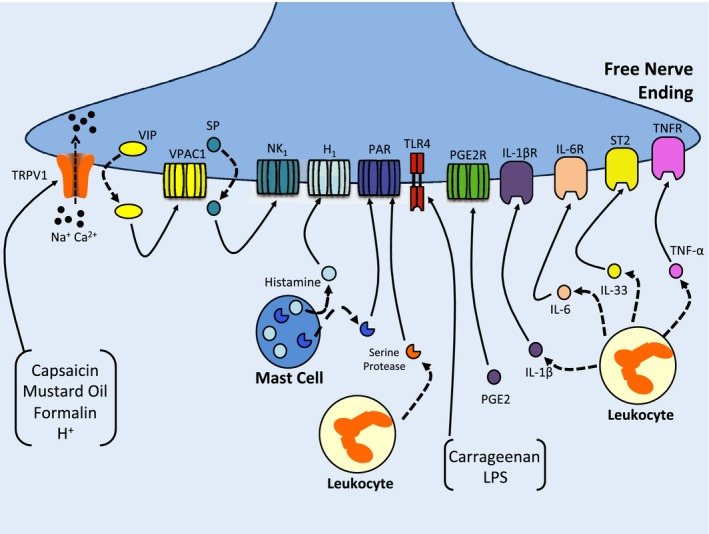
A schematic illustrating peripheral sensitization of a free nerve ending by various inflammatory mediators and irritant substances. Capsaicin, mustard oil, acid, and formalin increase the sensitivity of neurones by opening transient receptor potential vanilloid type 1 (TRPV1) ion channels, which leads to the release of neuropeptides such as substance P (SP) and vasoactive intestinal peptide (VIP). Locally released SP binds to neurokinin 1 (NK1) receptors and VIP binds to VPAC1 receptors. Histamine released from mast cells acts on neuronal H1 receptors. Leukocytes and mast cell release serine proteases which subsequently cleave various proteinase‐activated receptors (PAR) leading to downstream nociceptor modulation. Toll‐like receptor 4 (TLR4) is activated by exogenous substances like carrageenan and lipopolysaccharides (LPS), while leukocyte‐derived cytokines like tumor necrosis factor‐α (TNF‐α), interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), and interleukin‐17 (IL‐17) bind to their respective receptors to enhance pain transmission. Prostaglandin E2 (PGE 2) mainly activates the PGE 2 receptor and sensitizes sensory neurones to environmental stimuli.
Table 1.
Different experimental models, species and tests that can be used for studying inflammatory pain in skin, joint and gut
| Organ | Experimental model | Species | Measurement of pain |
|---|---|---|---|
| Skin | Capsaicin‐induced pain |
Rat 7, 21, 22
Mice 10 Human 23, 34 |
Electrophysiological recordings from skin nerves 22
Response to heat stimuli 22 Pressure algometry 23 von Frey hair algesiometry 11 Grimace scale 159 |
| Mustard oil‐induced pain |
Mice 41
Rat 40 |
Grimace scale 159
Electrophysiology 39 Response to heat or cold stimulation 44, 45 von Frey hair algesiometry 45 Response to electrical stimulation 45 |
|
| Formalin‐induced pain |
Rats 52, 54
Mice 57, 59 Cats 52 |
Grimace scale 159
Time spent in licking/lifting of paw, number of flinches 54 Electrophysiology 54, 55 Ultrasonic vocalization 53, 59 |
|
| Acid‐induced pain |
Rat 71, 73
Human 72, 75 |
Electrophysiology 71
von Frey hair algesiometry 72 |
|
| Joint | FCA‐induced hyperalgesia |
Rat 86, 88, 89, 93
Mice 80, 91, 92 |
Grimace scale 161
Hargreaves test 91 von Frey algesiometry 83, 92 Weight bearing 81, 84 Gait analysis 82, 86 Electrophysiology 85 LABORAS 171 |
| Kaolin‐carrageenan‐induced pain |
Mice 105
Rat 98, 107 Cats 96, 97 |
Grimace scale 161
von Frey algesiometry 98, 105 Weight‐bearing 105 Guarding behavior 107 Vocalization 108 Electrophysiology 97, 109 Hargreaves test 106 |
|
| Collagen‐induced arthritis pain |
Mice 111, 112
Rat 111 |
Hargreaves test 114
von Frey algesiometry 114 LABORAS 114 |
|
| Gut | Capsaicin‐induced visceral pain |
Mice 121, 126
Human 122 |
Measurement of spontaneous behavior (licking, stretching, squashing of abdomen) 121
von Frey algesiometry 121 Electrophysiology 186 |
| Mustard oil‐induced visceral pain | Mice 121, 127 |
Measurement of spontaneous behavior (licking, stretching, squashing of abdomen) 128
von Frey algesiometry 127 Open‐field test 128 Electrophysiology 130 |
|
| Acetic acid‐induced writhing |
Mice 134, 138
Rat 134 |
Grimace scale 159
Electrophysiology 184 Vocalization 184 Hot plate 184 Writhing response 137 |
Table 2.
Advantages and limitations of different experimental models of inflammatory pain
| Organ | Experimental model | Advantages | Limitations |
|---|---|---|---|
| Skin | Capsaicin‐induced pain |
1. Can study both thermal and mechanical hyperalgesia 2. Good translatability 3. Used to study peripheral and central mechanisms of pain in animals and human 4. Contribution of neurogenic inflammation to pain can be studied 5. Used to study desensitization mechanisms |
1. Can lead to Bezhold‐Jarrisch reflex 2. Higher doses produce anti‐ nociceptive effect 3. Neurotoxic at higher doses |
| Mustard oil‐induced pain |
1. Can study both thermal and mechanical hyperalgesia 2. Contribution of neurogenic inflammation to pain can be studied |
1. Moderate translatability 2. Infrequently used in pain studies |
|
| Formalin‐induced pain |
1. Used to study peripheral and central mechanisms of pain 2. Rapid screening of compounds is possible 3. Natural pain response is recorded as animals are unrestrained 4. Biphasic response could be used to differentiate inflammatory and non‐inflammatory pain |
1. NSAIDs and mild analgesics only work at higher doses in this model 198
2. Reliability is moderate 3. Less translatability |
|
| Acid‐induced pain |
1. Used to study heat hyperalgesia 2. Used to study contribution of ASICs to the development of heat and mechanical hyperalgesia |
1. Moderate translatability 2. Less reproducibility |
|
| Joint | FCA‐induced hyperalgesia |
1. Good model to study mechanical hyperalgesia and allodynia 2. Produces persistent pain and mechanical hyperalgesia 3. Closely resembles postoperative pain and persistent injury pain seen in humans 4. NSAIDs show good efficacy in this model |
1. Polyarthritic animals can become quite sick 2. Minimal involvement of immune system 3. Less reliable for mice |
| Kaolin‐carrageenan induced pain |
1. Produces robust inflammatory pain in cats, primates, and rodents 2. Debridement of articular cartilage and synovitis 3. Contribution of neurogenic inflammation in inducing pain can be studied 4. Reproducible 5. Induction is easy |
1. Pain response generated can be severe 2. Pain response typically lasts only 24 h |
|
| Collagen‐induced arthritis pain |
1. Chronic inflammatory pain 2. Pathology is close to human RA patients 3. Progression of pain is gradual 4. Induction is easy and reproducible |
1. Polyarthritis; makes it difficult to assess behavioral pain 2. Time required for arthritis to develop is long 3. Incidence and severity varies among animals 4. Technically demanding |
|
| Gut | Capsaicin‐induced visceral pain |
1. Spontaneous behavioral pain 2. Easy to induce 3. Reproducible 4. Used to study mechanical hyperalgesia 5. Used to study wide range of analgesic compounds |
1. Moderate translatability 2. Intervariability within subjects is high 3. Pain can be severe |
| Mustard oil‐induced visceral pain |
1. Robust pain response 2. Used to study spontaneous pain 3. Used to study primary and secondary hyperalgesia 4. Induction is easy |
1. NSAIDs show moderate efficacy in this model | |
| Acid‐induced writhing |
1. Produces characteristic stretching behavior 2. Good sensitivity to central and peripheral analgesics 3. Induction is easy and reproducible 4. Good translatability 5. Screening of multiple compounds in short period is possible |
1. Poor specificity for drug development 2. Interpretation of results can be problematic |
Models of Cutaneous Pain
Capsaicin‐Induced Pain
Capsaicin is the active irritant found in chili peppers, which belong to the genus Capsicum. Capsaicin is responsible for the hot and zesty taste sensation that we experience from eating spicy foods that contain chili peppers. In addition to its culinary uses, capsaicin has been used as a tool in preclinical and clinical studies, to understand thermal pain mechanisms 6. When 1% of capsaicin is administered locally, C fiber firing is enhanced 7. Intra‐arterial administration of capsaicin over the dose range 2–200 μg also activates C polymodal fibers 8, contributing to hyperalgesia 9, 10, 11. Capsaicin administration activates the nonselective cation channel, transient receptor potential vanilloid‐1 (TRPV1) 9, 12, which is localized on nociceptive free nerve endings and promotes the peripheral release of inflammatory neuropeptides such as calcitonin gene‐related peptide (CGRP), vasoactive intestinal peptide (VIP), and substance P (SP). This phenomenon, by which the peripheral nervous system induces tissue inflammation, is called neurogenic inflammation 13, 14, 15, 16. Moreover, blocking neurokinin 1 (NK1) results in the inhibition of capsaicin‐induced spontaneous pain, suggesting a role for neurogenic inflammation in capsaicin‐induced nociception 17. Inflammatory cytokines are also known to contribute to neurogenic inflammation induced by capsaicin. One particular study observed that intraplantar injection of capsaicin induced acute nociception and an increase in tissue levels of TNF‐α, IL‐1β, and IL‐6 18. Other studies found that injection of TNF‐α, IL‐1β, IL‐6, and IL‐8 produces mechanical hyperalgesia in animals 19, 20.
Multiple studies have consistently shown that capsaicin alters the thermonociceptive threshold of skin 7, 21, 22; however, it should be noted that the effects of capsaicin on nociception depend on the dose and route of administration 6. Acute application of capsaicin (onset 1 min and duration >1 h) results in an initial firing of nociceptors followed by an increased sensitivity to thermal and mechanical stimuli 23, 24, 25, 26. In human volunteers, transdermal application of capsaicin resulted in mechanical hyperalgesia and allodynia, which persisted for 30 min at the site of administration, as well as in the untreated surrounding skin, suggesting that local capsaicin induces both primary and secondary hyperalgesia 25. The investigation by Simone et al. 27 observed dose‐dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin (0.1–100 μg). Further investigation by Torebjork et al. 28 showed that intradermal injection of capsaicin and nerve electrical microstimulation in humans results in central sensitization, which is responsible for the secondary hyperalgesia. Central sensitization refers to an increase in the firing of neurones located in the central nervous system 29 as a result of altered membrane excitability and increased synaptic efficacy 30. This increased responsiveness of neurones primarily results in a hyperalgesic response within an uninjured area. In light of these pro‐nociceptive properties of capsaicin, it has been mainly used to study primary and secondary hyperalgesia. Additionally, this chemical has been used to screen the effect of novel compounds on TRPV1 ion channels 31. This ion channel has been implicated in osteoarthritis pain, dental pain, migraine, chronic inflammatory pain, and neuropathic pain, indicating wide utility of this model 32. Moreover, compounds such as gabapentin, lamotrigine, topiramate 33, diclofenac, hydromorphone, and cannabinoids have been screened for their potential analgesic activity using this model 34, 35. Conversely, repeated dosing or high concentrations of capsaicin deplete thinly myelinated and unmyelinated nociceptive nerve fibers, which results in a loss of responsiveness to painful stimuli 36, 37, 38. This loss of sensory innervation is irreversible when capsaicin is injected into neonatal animals, whereas in adults there is an eventual recovery of afferent nerve density 37. Interestingly, birds are insensitive to capsaicin despite expressing TRPV1 receptors, suggesting structural differences between mammalian and avian TRPV1 39.
Mustard Oil‐Induced Pain
Mustard oil is derived from mustard seeds and has a pronounced pungent taste characteristic of the mustard family of plants. It is a chemical irritant that mainly induces inflammation by neurogenic mechanisms 40, 41. Topical application of mustard oil (0.5–20%) onto the ears of mice produces skin inflammation, which is mainly characterized by vasodilatation, increased plasma extravasation, and edema. These changes are blocked by treatment with an NK1 antagonist 41. Similarly, mustard oil application on rat paw skin also triggers local cutaneous inflammation 40. Interestingly, local denervation of nociceptive terminals, using a neurotoxic dose of capsaicin, attenuated mustard oil‐induced inflammation; confirming a neurogenic inflammatory mechanism 40, 41. However, a study by Wong et al. 42 indicated a possible involvement of a nonneurogenic mechanism in mustard oil‐induced inflammation. This study assessed the effect of local anesthetic blockade on edema induced by mustard oil in the temporomandibular joint. It was observed that local anesthetics, which act directly on the neuronal membrane, failed to prevent the development of mustard oil‐induced edema, suggesting a direct action of mustard oil on immune cells or vascular smooth muscle to cause inflammation.
Topical application of mustard oil activates sensory nerve endings (onset 5 min, duration >1 h), which decreases their excitation threshold and hypersensitizes them to thermal and mechanical stimuli 26. Similar to capsaicin, topical application of mustard oil also induces a burning pain sensation in the applied area (primary hyperalgesia) and pain in the surrounding unaffected area (secondary hyperalgesia) 25. Application of mustard oil to oral mucosa is responsible for induction of burning sensation and thermal hyperalgesia 43, 44, 45. Several lines of evidence suggest that TRPA1 is the sole ion channel responsible for this action 46, 47. TRPA1 is involved in inflammatory pain and increases thermal and mechanical sensitivity 47; however, in a recent study comparing TRPV1‐knockout with wild‐type mice it was reported that mustard oil also engages the capsaicin receptor TRPV1 to initiate acute pain responses 48. This model has been mainly utilized to study compounds having an effect on TRPV1 or TRPA1 ion channels 46, 47, 48. It has been shown that adenosine reduces secondary hyperalgesia by mustard oil in human subjects 49. Mustard oil has also been used to promote orofacial inflammation following injection around temporomandibular area 50. Furthermore, injection of mustard oil into craniofacial muscles resulted in nociception, which was significantly reduced by treatment with morphine 51.
Formalin‐Induced Pain
The formalin test has been in existence since 1977, and has been routinely used for testing pain and hyperalgesia 52. This test involves an injection of formalin (0.5–5%) into the plantar surface of an animal paw and produces specific behaviors like paw lifting, flinching, licking, and vocalization 52, 53, 54. Furthermore, formalin injection produces a biphasic response where in phase I (0–5 min) pain results from the direct activation of primary nociceptive afferents, and in phase II (10–40 min) pain involves, at least in part, inflammation‐induced central sensitization in the dorsal horn of the spinal cord 54, 55, 56. Prostaglandins (PGs) are involved in phase II of the formalin model, and cyclooxygenase 2 (COX2)‐specific nonsteroidal antiinflammatory drugs (NSAIDs), which block PG production, have been shown to reduce formalin‐induced pain during phase II 57, 58. A recent study described a role for IL‐33, a pro‐inflammatory cytokine, and its receptor, ST2, in mediating formalin‐induced nociception 59. In this study, IL‐33 induced pain in both phases of the formalin test, and an ST2‐targetted antibody blocked these effects, highlighting the importance of IL‐33 in modulating nociceptive changes both peripherally and centrally in formalin‐treated animals 59. For phase II of the formalin test, there are reports which underscore the importance of ongoing primary afferent input for the development and perpetuation of a pain response in animals 60, 61, 62. In light of these reports it is still under debate whether central sensitization and/or primary afferent input is responsible for the pain response during this phase of the formalin model. An earlier study looked into pain responses from 2 h to 4 weeks after injection of formalin. It was observed that the hyperalgesic response lasted until 4 weeks reaching a second peak from day 7–10 63. The researchers speculated the possibility of spinal microglial activation and peripheral inflammation leading to central sensitization, which would be responsible for the maintenance of this long‐lasting hyperalgesia 63. In addition to the release of various inflammatory mediators, formalin can also activate TRPA1 directly to elicit pain and inflammation 54. This test has been successfully used to assess the efficacy of a variety of compounds like morphine, oxyphenylbutazone, acetylsalicylic acid, corticosteroids 64, diflunisal 65, diacerhein, carbamazepine, topiramate, and gabapentin 66. Antagonists targeting TRPA1 ion channels 54, NMDA receptors 67, and neurokinin‐1 receptors 68 have also been examined. The formalin test has also been used to study orofacial pain, as subcutaneous injection into the lip produces nocifensive behaviors in rats 60, 69. One of the limitations of the formalin test is that it has little translational relevance. Unlike other models of cutaneous sensitization (e.g., capsaicin, UVB, acid), intradermal injection of formalin is not carried out in human volunteers. This model is restricted to animal experiments and is mainly used for studying pain mechanisms rather than analgesic evaluation as it is both reproducible and relatively straightforward to perform.
Acid‐Induced Pain
Inflammatory and ischemic conditions both lower tissue pH 70. During inflammation, several factors promote tissue acidity, including intracellular components released following cell lysis, inflammatory mediators, and pumping of lactic acid by leukocytes 71, 72. A study by Steen et al. 73 found that protons are capable of stimulating cutaneous neurones on their own. Additionally, chemical mediators released during inflammation, combined with the low pH environment, conspire to produce a synergistic effect, resulting in prolonged nociceptor activation 74. Protons produce pain by activating TRPV1 and acid‐sensing ion channels (ASICs) on sensory free nerve endings 75. To date, four different types of ASIC channels have been identified (ASIC1‐4), which are located in the central and peripheral nervous systems 76. Each of these ASIC channels has specific pH sensitivity viz. ASIC1: pH5.8–6.8, ASIC2: pH4.5–4.9, and ASIC3: pH6.4–6.6 76. These ASICs play an important role in the development of chemogenic inflammatory pain 77, neuropathic pain 78, postoperative pain 79, and neurological disease pain 76.
Models of Joint Pain
Freund's Complete Adjuvant‐Induced Hyperalgesia
Freund's complete adjuvant (FCA) is a routinely used model to study chronic inflammatory pain in rodents 80, 81, 82. Intra‐articular injection of FCA (heat‐killed Mycobacterium tuberculosis in paraffin oil) results in localized edema, as well as mechanical and thermal hyperalgesia 83, 84, 85, 86. Immunological reactions develop 7 days after injection of FCA, which leads to tissue swelling. Therefore, pain studies usually follow 2 treatment regimens: 0–8 days (prophylactic regimen) and post day 8 (therapeutic regimen) 87. Interestingly, FCA also produces articular hypoemia, which nicely models the hypoxic environment found in rheumatoid arthritic joints 88. It has been shown in previous studies that FCA injection elicits the production and release of various inflammatory mediators such as PGE2, nitric oxide, leukotriene B2, TNF‐α, IL‐2, and IL‐17 89. These pro‐inflammatory mediators cause synovitis, polyarticular inflammation, bone resorption, periosteal bone, and proliferation and can result in joint degeneration 87. In addition, these pro‐inflammatory cytokines play an important role in inducing joint pain by causing sensitization of neurones either directly or by indirect means, that is via release of prostaglandins 1. Extra‐articular manifestation resembles those of RA patients. However, the cartilage damage observed in this model is less severe than human RA and is therefore not recommended for the study of this aspect of the disease. The inflammatory mediators released into the joint are also responsible for sensitizing articular nociceptive afferents, resulting in arthritic pain. Injection of FCA also causes activation of TRPV1 ion channels that participate in the joint inflammation process 90. A study by Keeble et al. 91 investigated the effect of intra‐articular FCA in wild‐type and TRPV1‐knockout mice. Knee swelling and vascular hyperpermeability were significantly reduced in TRPV1‐knockout mice, as compared to wild‐type mice. Interestingly, leukocyte accumulation and TNF‐α levels were similar in WT and TRPV1‐knockout mice. To explain this effect, the authors argued that TRPV1 may be acting as a modulator instead of primary contributor. The mechanical hyperalgesia was correlated with knee swelling and was reduced in TRPV1‐knockout mice. A further investigation by Fernandes et al. 92 identified a role for TRPA1, as well as TRPV1, in FCA‐induced monoarthritis; however, the contribution of TRPA1 was moderate. The inflammatory neuropeptide substance P also plays an important role in FCA‐induced joint inflammation and pain. When animals were treated with an NK1 antagonist, FCA‐induced hyperalgesia and inflammation were significantly reduced 93. Based on these results, it is clear that FCA induces the expression of several inflammatory mediators, which promote joint pain. Because of the robust nociceptive responses observed in this model, FCA is one of the better models for studying inflammatory joint pain. It should be noted that the model described here is distinct from the hindpaw FCA injection model, which is used to assess cutaneous inflammatory pain. Both models, however, are highly utilized in academic and industrial settings to study novel antiarthritic compounds and potential analgesics. Several compounds such as dexamethasone, methotrexate, NSAIDs, and biological agents 94, 95 which are used clinically had previously shown good efficacy in the FCA model.
Kaolin–Carrageenan‐Induced Monoarthritis
The kaolin–carrageenan arthritis model is a widely used monoarthritic model of acute joint inflammation. This model produces a well‐defined pattern of inflammation and pain and has been established in primates, cats 96, 97, and rodents 98, 99. Kaolin, also known as China clay, is an inorganic substance that is composed of hydrated aluminum silicate (H2Al2Si2O8.H2O). When injected into the joint, as an aqueous suspension, it produces fine debridement of the articular cartilage and irritates the synovial membrane. λ‐Carrageenan type IV (carrageenan) is a sulfated polysaccharide, derived from Irish moss, and so named after the Irish town of Carragheen. Carrageenan produces an acute inflammatory response by first activating cell surface expressed Toll‐like receptor 4 (TLR4). TLR4 activation leads to the phosphorylation of BCL10, which then activates nuclear factor kappa‐light‐chain enhancer of activated B cells (NF‐κB). This chain of events results in leukocyte infiltration and the release of cellular mediators that promote local inflammation 100.
Kaolin–carrageenan‐induced joint inflammation has a well‐defined time course, where signs of inflammation are apparent 1–3 h following injection, peak and plateau around 5–6 h, and are maintained for 7 days or more 101. This inflammatory phase is characterized by hyperemia, edema and immune cell infiltration 102, 103, 104. In addition to inflammation, this model also produces a number of behavioral and neuronal responses that are characteristic of joint pain. Animals show decreased weight bearing on the inflamed limb, decreased withdrawal thresholds to von Frey hairs 105, noxious heat stimuli 106, increased guarding 107, and pain‐induced vocalizations 108. Furthermore, articular afferents and dorsal horn neurones show increased electrical activity following intra‐articular injection of kaolin–carrageenan 97, 98, 99, 109, 110. Because of the well‐defined and consistent pain and inflammation that this model produces, it is frequently used to test new antiinflammatory and analgesic compounds.
Collagen‐Induced Arthritis
Collagen‐induced arthritis (CIA) is the most commonly used model for studying novel disease modifying drugs for rheumatoid arthritis (RA) in rodents. In this model, an emulsion is made using type II collagen and FCA which is injected into the base of the tail 111, 112. This FCA emulsion acts as an antigen repository which causes slow systemic leakage of antigen thereby triggering an autoimmune response leading to deposition of immune complexes onto the surface of joint tissues. Others features of this model include symmetrical joint involvement which is less observed in the adjuvant monoarthritis model, prominent synovitis, bone resorption, and periosteal proliferation 87. The cartilage damage and lesions produced after injection of CIA are comparable to those observed in human RA 87. However, extra‐articular manifestations are less apparent in this model as compared to the adjuvant model. For many years, this model was used for screening the antiinflammatory potential of compounds; however, studies conducted in the past few years have shown utility of this model for testing analgesics. Inflammation observed in this model primarily involves T and B cells which promote synovial hyperplasia or thickening, cartilage erosion, swelling, and reduced mobility of the joints 113.
Inglis et al. 114 have shown a correlation between inflammation (paw swelling and clinical score) and mechanical and thermal hyperalgesia in the CIA model. A positive correlation exists between inflammation and hyperalgesia up to 10 days after the onset of arthritic symptoms. Simultaneously, the expression of TNFα and IL‐1β also decreased around that time, which highlights the role of these mediators as algogens. It has been suggested that different cytokines cause sensitization of neurones by receptor‐associated kinases, by promoting ion‐channel phosphorylation, or by altering downstream signaling 1. Astrocytes are known to play a supportive role in maintaining chronic pain 115, and CIA‐injected animals have increased levels of lumbar spinal cord astrocytes 114. Finally, the expression of activation transcription factor‐3 (ATF‐3), a marker of nerve damage, was found to be increased in DRGs supplying collagen‐injected ankles indicating peripheral neuropathy in this inflammatory model 114. Compounds from different drug classes such as corticosteroids, NSAIDs, methotrexate, and biologics have shown better efficacy in this model compared to other models of joint inflammation 94, 95. Alternative compounds that modify different cellular targets such as Janus kinase 116, histone deacetylase 6 117, MAPK 118, and chemokine receptors 119 have also been successfully screened using the CIA model.
Models of Visceral Pain
Capsaicin‐Induced Visceral Pain
Patients suffering from inflammatory bowel disease (IBD), such as Crohn's disease and ulcerative colitis, frequently experience visceral pain. Assessing visceral pain is challenging, as the primary afferent neurones in the gut run closely with the enteric nervous system raising the possibility of interaction between the two nervous systems 120. The visceral pain observed in response to intraluminal capsaicin injection is poorly localized much like human visceral pain 121, 122. Attempts have been made to develop models of visceral pain, which would allow the evaluation of inflammatory mechanisms and potential therapies. It has been suggested that hypersensitization of visceral afferent neurones is one of the major factors responsible for chronic pain states in IBD patients. As such, capsaicin has been used to gain insights into the mechanism which contribute to visceral pain. Studies have shown that intraluminal administration of capsaicin causes an increase in behavioral response by animals, which is blocked by TRPV1 antagonists highlighting the role of capsaicin‐sensitive afferents in mediating visceral pain 123. Other reports have shown great promise of TRPV1 as a pharmacological target for the treatment of visceral pain 124, 125. Chemostimulation of the colon using capsaicin (0.03–0.3%) has been used to induce visceral pain in vivo 121. Intracolonic administration of capsaicin evokes spontaneous pain behaviors, which are characterized by writhing, stretching and licking of the abdomen, as well as reduced self‐grooming 126. Morphine dose dependently blocks these nociceptive responses 121. Intracolonic administration of capsaicin also induces mechanical hyperalgesia, which results in increased responsiveness to von Frey hairs (force, 1–32 mN) applied to the abdomen 121. In addition to a hyperalgesic response, capsaicin also produces low‐grade inflammation in the colon, which was evident by increased plasma extravasation. This pro‐inflammatory effect of capsaicin was neurogenic, as denervation of the colon abolished this response. Furthermore, NK1‐receptor‐knockout mice failed to show any behavioral signs of pain, suggesting that tachykinins are involved in capsaicin‐induced gut inflammation 121, 126.
Mustard Oil‐Induced Visceral Pain
Similar to capsaicin, intracolonic injection of mustard oil (0.25–2.5%) produces visceral pain, referred pain, and inflammation 121. Mustard oil injection causes stimulation of peripheral nociceptors and spinal dorsal horn neurones to evoke the primary pain response and referred hyperalgesia, respectively 126, 127. In a recent study, the activity of two NSAIDs, ketorolac and ketoprofen, as well as morphine were tested against mustard oil‐induced nociception. Morphine was able to block spontaneous pain behaviors and referred hyperalgesia tested using von Frey hairs 121. The NSAIDs, however, were only able to block mustard oil‐induced spontaneous pain behaviors. NSAIDs also reduced plasma extravasation, indicating an antiinflammatory effect of these drugs in this model 127. The algesic and inflammatory effects of intracolonic mustard oil have been found to be TRPV1 dependent, suggesting a common pathway with capsaicin 128. Intracolonic administration of mustard oil results in the release of various pro‐inflammatory cytokines, chemokines, and neuropeptides, which suggests its utility as a model of colonic inflammatory pain 129. This model produces visceral hypersensitivity and permanent sensitization of dorsal horn neurones, which are also observed in patients suffering from irritable bowel syndrome (IBS) 130, 131. For example, a recent study has shown that targeting TNF‐α produces a modest reduction in pain scores in patients suffering from Crohn's disease 132.
Acetic Acid‐Induced Writhing
Intraperitoneal injection of chemical irritants has been used for over 40 years to induce visceral pain in animals 133, 134, 135. Substances such as phenylbenzoquinone or acetic acid produce characteristic writhing responses following injection 133, 134. These writhing responses include abdominal stretching, flinching, licking, and motor incoordination 135. For induction of a writhing response in unanesthetized animals, an intraperitoneal injection of dilute acetic acid (0.6–9%V/V) or phenylquinone (0.3–0.9%) is made at a fixed dose (0.2 ml/mouse or 0.5–2.5 ml/rat) or in some cases a weight‐adjusted dose (10 ml/kg) is used 136. Cytokines 137, prostaglandins 138, and bradykinin 139 are all released following intraperitoneal injection of acetic acid and are capable of sensitizing visceral nociceptive afferents. Inhibition of the pro‐inflammatory cytokines TNF‐α and IL‐1β results in the inhibition of visceral hyperalgesia produced by injection of acetic acid 140. Furthermore, administration of antiinflammatory cytokines (IL‐4, IL‐10 and IL‐13) also produces an antinociceptive effect by downregulating the release of eicosanoids in this model 141. A study conducted by Pavao‐de‐Souza. 142 looked at the activation of different downstream signaling pathways in the spinal cord after acetic acid injection 142. Intrathecal administration of inhibitors of extracellular signal‐regulated kinase (ERK), Jun N‐terminal Kinase (JNK), p38, phosphatidylinositol 3‐kinase (PI3K) reduced nociceptive responses in a dose‐dependent manner 142. This model has been mainly used as a screening tool to characterize analgesic activity of opioid compounds 143 and NSAIDs 144, 145. Some of the limitations of this test include lack of specificity as some nonanalgesic drugs also show efficacy. Irritation of nongastrointestinal tissues has also been reported in this model 136.
Assessment of Inflammatory Pain
von Frey Hair Tactile Sensitivity
One method that is used to test peripheral and central sensitization associated with inflammatory pain is von Frey hair mechanosensitivity. von Frey hairs are a graded series of thin, calibrated filaments that bend to exert a specific mechanical force 146. Normal animals show a paw withdrawal response when stimulated by a von Frey hair applied to the plantar surface of the paw. von Frey hairs with a low bending force elicit a tactile withdrawal in naïve animals; however, filaments with higher forces could produce a nocifensive response. Inflamed tissues are hypersensitive to von Frey hairs, leading to a reduction in withdrawal threshold (i.e., they respond to a hair which normally does not evoke a withdrawal response). In the periphery, inflammation can result in changes in voltage‐gated sodium and calcium channel expression and a heightened firing frequency of nociceptors 147. Furthermore, changes within the CNS have also been attributed to heightened von Frey hair sensitivity following inflammation. Central sensitization can involve altered pain signal processing in the spinal cord 148 and brain 149, insufficient descending inhibitory signals 150, excessive descending facilitatory signals 151, and changes in synaptic plasticity in the anterior cingulate cortex 152. Measuring the changes in sensitivity to plantarly applied von Frey hairs allows researchers to investigate referred pain during inflammation, and how this secondary pain response develops and responds to drug treatment. There are some limitations to this technique including the potential of experimenter bias, that is, the withdrawal response nocifensive or a startle reflex. An additional limitation is that paw withdrawal thresholds have the propensity to vary between animals because of repeated application of multiple of hairs. To overcome these limitations, electronic von Frey hairs with a calibrated force transducer and dynamic plantar aesthesiometers have been developed. These instruments are capable of recording force and latency to withdrawal following application of a single fine metal monofilament with increasing force, thus avoiding the need for repeated application of multiple filaments. Also, the rate of increasing force is applied at a constant ramp speed which reduces experimenter bias 153.
Hindlimb Weight Bearing
Spontaneous pain is a characteristic of many inflammatory diseases 1; therefore, measuring nonevoked painful behaviors in animal models is essential in understanding this clinically relevant phenomenon. One technique used to measure spontaneous‐like pain is hindlimb incapacitance, in which the difference in weight bearing between a quadruped's hindlimbs is assessed 154. Normally, rodents distribute their weight equally between both hindlimbs; however, following the induction of unilateral hindlimb inflammation, rodents tend to favor the noninflamed leg. This shift in weight bearing can be measured in stationary animals (static weight bearing; SWB), as well as freely mobile animals (dynamic weight bearing; DWB). SWB requires that the animal rear on both hindlimbs while standing on two force plates, one under each hindpaw. When measuring DWB, the animal is placed in a chamber carpeted with a pressure sensitive flooring pad, which allows weight bearing to be assessed during movement. In both techniques, the weight borne by each hindlimb is measured, and the difference between the two is calculated. Hindlimb incapacitance measurements are useful in testing weight bearing changes in models of inflammatory arthritis, osteoarthritis, neuropathic pain, hindpaw dermatitis, and bone cancer pain 154, 155. Potential limitations of this technique are that it only permits the assessment of hindlimb inflammatory pain, some animals may experience restraining stress, and repeated testing could modify behavior 156.
Grimace Scale
Facial expression is an important element of communicating pain between individuals. In 1978, Ekman and Friesen developed a facial action coding system, a tool to characterize distinct emotions in human 157. Facial expressions have also been effectively used for the detection of pain in noncommunicative humans such as neonates and patients with cognitive impairment 158, 159. The expression of pain facially is not only restricted to humans, but is conserved in many other sentient species 159, 160. This physical aspect of nonhuman pain has been developed into a grimace scale using a standardized behavioral coding system to measure spontaneous pain in rodents 159, 161. In this test, five facial features (orbital tightening, nose bulge, cheek bulge, ear position, and whisker position change) are scored by blinded coders on a three‐point scale, where 0 = not present, 1 = moderately present, and 2 = severe. To test the reliability of this scale, various nociceptive assays were performed and pain severity was compared to the grimace score. It was observed that the grimace scale produced consistent and reliable pain scores across all behavioral assays tested. Grimace has been successfully used to demonstrate analgesia in rodents where a linear correlation was observed between dose of morphine and a reduction in grimace score following intraplantar or intra‐articular zymosan and cyclophosphamide‐induced bladder cystitis 159, 161.One of the potential disadvantages of the grimace score is that it can be very time‐consuming; however, this limitation has been mitigated by the use of Rodent Face Finder® software, which automatically generates photographs from video thereby saving a significant amount of processing time. Another limitation of this technique is that it is only effective in assessing acute pain, as animals with chronic pain learn to adapt and do not produce facial grimacing 161. Nevertheless, as this test is capable of capturing spontaneous pain, it could be used for prioritizing compounds in the later stages of drug discovery.
Hargreaves Test
Hypersensitivity to noxious thermal stimuli is a commonly observed during cutaneous inflammation, and the Hargreaves test was designed to assess this response in rodents. In this test, a movable heat source is placed under a glass floor upon which the animal is standing. The thermode is then positioned underneath the plantar surface of the hindpaw and radiant heat is gradually increased at a fixed rate until the animal feels pain and withdraws its paw 162. This test allows researchers to measure thermal hyperalgesia in an unrestrained animals, therefore providing less stressful testing conditions for the animals 163. One limitation of the Hargreaves test is that repeated measurements can affect withdrawal latencies 163, since rodents learn rapidly and quickly adapt to a changing environment 163.
Randall‐Selitto Paw Pressure Test
The Randall‐Selitto paw pressure test is used to measure the cutaneous mechanical hyperalgesia in rats 164. In this test, the paw or tail of the rat is placed on a circular platform and a linearly increasing force is applied to the tissue with a dome‐shaped plastic tip 165. A similar system has been developed to assess mechanonociception in experimental models of arthritis where calibrated forceps are oriented along the joint line and an increasing compression force is applied. When the mechanical force reaches a painful threshold, the rat either withdraws its hindlimb or vocalizes. At this point, the force application is stopped and the nociceptive withdrawal threshold is recorded. The entire procedure is repeated 2–3 times and a mean value is calculated. Care must be taken not to exceed the maximum force as this could result in tissue injury and inflammation. It is possible to discriminate between left and right hindlimb responses using this test, and it also allows multiple measurements within the same animal. Since the test involves manually restraining the animal, stress‐induced analgesia could diminish the magnitude of the pain _measured 166. While this test is practicable for assessing inflammatory pain in the extremities, it not useful for measuring visceral pain.
Locomotor Activity
Most of the tests that are designed to study pain mechanisms and evaluate analgesic activity of compounds are based on evoked responses to an external stimulus, involve restraining animals, and are very much experimenter‐dependent 167. In an attempt to overcome these issues, Matson et al. 168 developed the reduction in spontaneous activity by adjuvant (RSAA) test, which assesses spontaneous animal behaviors in a novel environment. The animals are placed in a Plexiglass box, and the walls of this box are fitted with sensors to detect changes in locomotion. Two activity measures, total distance travelled and vertical activity (rearing), are recorded before and after the administration of an inflammatory agent. It has been shown that administration of these agents results in a decrease in locomotor activity and rearing. Moreover, treatment with morphine, ibuprofen, rofecoxib, celecoxib, piroxicam, and dexamethasone all reversed these locomotor deficits, suggesting a usefulness of this technique to evaluate analgesic activity 168, 169. This test allows animals to be unrestrained which helps in reducing stress and there is no need for subjective behavioral scoring 168. Repeated testing may not suitable as this test relies on the novelty of the environment and the exploratory nature of rodent animals 167.
Another useful tool used to study pain‐induced behavior changes in rodents is the Laboratory Animal Behaviour Observation, Registration and Analysis System (LABORAS). This is an automated system which is capable of detecting changes in the behavior and locomotor activity of rodents by assessing changes in vibration signatures produced by their movement 170. This system discriminates between different behaviors such as eating, drinking, grooming, climbing, resting, and locomotion, which are all compromised during pain 170. This system has been successfully used to detect pain behaviors after induction of collagen‐induced arthritis 114 and adjuvant‐induced cutaneous inflammation 171.
Changes in burrowing activity is another behavioral readout designed to capture spontaneous‐like pain in experimental animals. Burrowing is a natural phenomenon in rodents in which the animals dig holes or tunnels to create a domicile or a place of refuge. This behavior can be quantified by measuring the difference in the known weight of bedding placed in a habitation tube and the weight which has been excavated during a period of burrowing 172, 173. This assay has been successfully used to predict the analgesic efficacy of compounds in models of knee joint inflammation 174, 175, osteoarthritis 176, colitis 177, and peripheral nerve injury 178. This assay offers good sensitivity, is easy to perform, inexpensive, and requires minimum experimenter training 172, 173. High variability between animals is one limitation of this method; however, this can be somewhat mitigated by training the animals prior to recording. Furthermore, it is uncertain whether changes in burrowing activity are attributable to pain or whether the animal is demonstrating anxiety or depression.
Electrophysiological Recording of Nerves
Tissue inflammation leads to an increase in neuronal excitability and a decrease in activation threshold in response to environmental stimuli. Therefore, studying the electrical activity of nociceptors using exquisite electrophysiological techniques can offer an objective means of assessing nociception. Nerve recordings may be carried out in the periphery (primary afferent neurones, dorsal root ganglia), in the dorsal horn of the spinal cord, or in supraspinal regions involved in pain perception (e.g., thalamus, somatosensory cortex, amygdala). Nerve recordings can be either extracellular or intracellular with extracellular recording being the most commonly used approach for pain studies. Extracellular recordings are carried out in vivo and can take the form of either single unit recordings from discrete axon bundles, or compound action potentials from whole nerve preparations. In vitro intracellular recordings can be carried out on intact or dissociated neurones, intact dorsal root ganglia, or isolated tissue slices. In vivo intracellular recordings are possible with whole neurones, single axons, or dorsal root ganglia. These electrophysiological approaches have dramatically advanced our understanding of the neuronal mechanisms responsible for the generation of evoked and spontaneous pain.
The superficial location of cutaneous afferents make them an attractive and accessible population of nerve fibers from which to record. Following a focal injury, skin develops two zones of inflammatory pain: the first is restricted to the precise site where the injury occurred (primary hyperalgesia) while the second extends into the area surrounding the initial insult (secondary hyperalgesia). Primary hyperalgesia is attributable to the sensitization of nociceptor nerve endings in the skin whereas secondary hyperalgesia arises due to plasticity changes occurring in the central nervous system. Thus, skin is an appealing organ in which to assess inflammatory pain at different levels of the pain pathway.
Electrophysiological recordings have also been performed on animal knee joint primary afferents 179, 180, 181, 182. Single unit extracellular recordings provide persuasive subjective information regarding joint nociceptor biology and responsiveness to inflammatory mediators. Numerous studies have shown that articular administration of pro‐inflammatory chemicals causes a reduction in afferent mechanical threshold and increased firing in response to joint movement 183.
Electrophysiological approaches have also been utilized to investigate the mechanisms of visceral pain. Electrophysiological recordings from nociceptive dorsal horn neurones have been used to give insights into the neuronal mechanisms involved in the generation of abdominal pain in irritable bowel syndrome and colitis 130. Intraperitoneal injection of noxious agents such as acetic acid or infectious agents such as Citrobacter rodentium can sensitize visceral extrinsic neurones leading to increased afferent firing that encodes gastrointestinal pain 184, 185. The generation of referred hyperalgesia is an important characteristic of visceral pain. A study by Sanoja et al. 186 assessed the electrical activity of “ON‐like” cells in the rostral ventromedial medulla (RVM) following intracolonic instillation of capsaicin. Electrophysiological recording of “ON‐like” RVM neurones showed a long‐lasting sensitization phenomenon and this altered firing pattern encodes referred hyperalgesia.
In the spinal cord, recordings are made from neurones located in laminae I and II of the dorsal horn. Electrophysiological measurements can be performed on an in vivo preparation or on slices of spinal tissue, although these latter experiments must be carried out within hours of tissue excision and plating. Spinal recordings are useful to determine the effect of inflammatory mediators on central sensitization mechanisms and their effect on central neurotransmission. Supraspinally, pan cortical recording of the somatosensory cortex has been carried out in response to peripheral inflammation 187. In order to gain insight into the affective aspects of inflammatory pain, in vivo recordings have also been carried out on specific nuclei of the amygdala 188. Thus, recording from different regions of the brain allows us to explore how inflammatory pain signals are processed into a negative sensory experience.
Reasons for the Translational Gap between Animal Models and Human Inflammatory Pain
Animal models have successfully been able to predict clinical efficacy of commonly used treatments for inflammatory pain including acetaminophen, ibuprofen, morphine, corticosteroids, and biologics 51, 87, 189, 190, 191, 192, 193, 194, 195. However, there are still a number of compounds which show great efficacy in preclinical models but fail during clinical trials. For example, substance P antagonists 196 and an endocannabinoid hydrolysis inhibitor 197 showed great promise in animal models of disease, but were found wanting in the clinic. These reports imply that there are profound limitations with animal models which question their translational veracity. Some of the reasons for the discrepancy between humans and animal models include differences in how we assess pain, mismatches in the time required for pain development, and interspecies variation in pharmacokinetic/pharmacodynamic properties of drugs.
There are intrinsic differences in perception of pain between animals and humans. Animals lack the ability to describe their pain intensity and can only manifest nociception in behavior or show withdrawal reflexes to noxious stimuli. As such, current animal models of inflammatory pain and our interpretation thereof are primarily based on generating disturbances in neurosensation. On the other hand, human pain is multifaceted encompassing emotional, cognitive, affective, and psychosocial components which can impact pain reporting 198, 199. Clearly, there is a need for the development of new endpoints or animal models which are capable of recapitulating multiple features of human pain states. Comorbidities are also a prominent part of human disease which are often overlooked in animal models. Patients suffering from rheumatoid arthritis, for example, are at higher risk of cardiovascular disease and gastrointestinal inflammation. A greater focus should therefore be placed on animal models that incorporate comorbidities across different species.
Our interpretation of pain in animals and humans is different. In preclinical models, pain is mainly assessed by recording changes in the withdrawal threshold to mechanical or thermal stimuli. In contrast, the severity and quality of human pain is predominantly assessed by using subjective rating scales like the visual analog scale (VAS), verbal rating scale (VRS), or the numerical rating scale (NRS) 200. These rating scales essentially are a series of questions designed to capture self‐reported clinical pain which is subjective and inherently leads to variability. Therefore, pain responses captured in animals will not mimic the expressed feelings of pain in humans. Another important difference between animal and human pain is the time required for the pain to develop. In the case of animal models, it usually takes from hours to weeks whereas in humans persistent pathological pain lasts for months or years 199. Factors such as cost and ethics preclude long‐term pain studies in animals and as such it is impractical to model the precise temporal aspects of chronic human pain.
Another important characteristic that has largely been underestimated is the disparity in pharmacokinetics/pharmacodynamics (PK/PD) between species. Pharmacokinetic studies generate information about the drug concentrations at target and nontarget sites, metabolism and elimination pattern. Pharmacodynamic studies mainly assess the pharmacological effect of drug at available concentrations. It should be noted that a correlation exists between these two disciplines and they are interdependent 201. However, several studies have shown that pharmacokinetic parameters differ between species which makes extrapolation of animal data to humans challenging 202, 203. These differences could impact the pharmacological efficacy and safety of a drug in different species. For example, analgesics such as celecoxib and indomethacin exhibit differences in dose, plasma exposure, and bioavailability between rat and human 204. It is essential, therefore, to recognize important pharmacokinetic and pharmacodynamics parameters between species and between different pain states. This consideration would improve the predictive capacity and translatability of future preclinical studies.
Concluding Remarks
Patients suffering from chronic inflammatory conditions live in a considerable amount of pain over which we have limited control. The establishment of experimental models and different techniques to assess pain have played a significant role in advancing our knowledge about peripheral and central mechanisms of pain. Furthermore, these models have been successfully used to discover new molecular and cellular targets which form the basis of future analgesic drugs. These inflammatory models should be continually refined so as to improve their translatability to human conditions. In addition, novel ways of assessing and interpreting pain in animal models are required to help complement and supplement the current battery of techniques used to detect nociception.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth 2001;87:3–11. [DOI] [PubMed] [Google Scholar]
- 2. Lipnik‐Stangelj M. Mediators of inflammation as targets for chronic pain treatment. Mediators Inflamm 2013;2013:783235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014;69:3–16. [DOI] [PubMed] [Google Scholar]
- 4. Widdifield J, Paterson JM, Bernatsky S, et al. The epidemiology of rheumatoid arthritis in Ontario, Canada. Arthritis Rheumatol 2014;66:786–793. [DOI] [PubMed] [Google Scholar]
- 5. Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am 2002;31:1–20. [DOI] [PubMed] [Google Scholar]
- 6. O'Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the mystery of capsaicin: A tool to understand and treat pain. Pharmacol Rev 2012;64:939–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kenins P. Responses of single nerve fibres to capsaicin applied to the skin. Neurosci Lett 1982;29:83–88. [DOI] [PubMed] [Google Scholar]
- 8. Szolcsanyi J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra‐violet irradiation. J Physiol 1987;388:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat‐activated ion channel in the pain pathway. Nature 1997;389:816–824. [DOI] [PubMed] [Google Scholar]
- 10. Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000;288:306–313. [DOI] [PubMed] [Google Scholar]
- 11. Kim HK, Schattschneider J, Lee I, Chung K, Baron R, Chung JM. Prolonged maintenance of capsaicin‐induced hyperalgesia by brief daily vibration stimuli. Pain 2007;129:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain‐producing stimuli. Neuron 1998;21:531–543. [DOI] [PubMed] [Google Scholar]
- 13. Lembeck F, Holzer P. Substance p as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol 1979;310:175–183. [DOI] [PubMed] [Google Scholar]
- 14. Petermann J, Fischer JA. Coexistence of tachykinins and calcitonin gene‐related peptide in sensory nerves in relation to neurogenic inflammation. Fernstrom Found Ser 1985;6:149–157. [Google Scholar]
- 15. Foreman JC. Peptides and neurogenic inflammation. Br Med Bull 1987;43:386–400. [DOI] [PubMed] [Google Scholar]
- 16. Lin Q, Li D, Xu X, Zou X, Fang L. Roles of trpv1 and neuropeptidergic receptors in dorsal root reflex‐mediated neurogenic inflammation induced by intradermal injection of capsaicin. Mol Pain 2007;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Cang CL, Kawasaki Y, et al. Neurokinin‐1 receptor enhances trpv1 activity in primary sensory neurons via PKCepsilon: A novel pathway for heat hyperalgesia. J Neurosci 2007;27:12067–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saade NE, Massaad CA, Ochoa‐Chaar CI, Jabbur SJ, Safieh‐Garabedian B, Atweh SF. Upregulation of proinflammatory cytokines and nerve growth factor by intraplantar injection of capsaicin in rats. J Physiol 2002;545:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 1992;107:660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin‐1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 1988;334:698–700. [DOI] [PubMed] [Google Scholar]
- 21. Carter RB, Francis WR. Capsaicin desensitization to plasma extravasation evoked by antidromic c‐fiber stimulation is not associated with antinociception in the rat. Neurosci Lett 1991;127:43–45. [DOI] [PubMed] [Google Scholar]
- 22. Lynn B, Ye W, Cotsell B. The actions of capsaicin applied topically to the skin of the rat on c‐fibre afferents, antidromic vasodilatation and substance p levels. Br J Pharmacol 1992;107:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Culp WJ, Ochoa J, Cline M, Dotson R. Heat and mechanical hyperalgesia induced by capsaicin. Cross modality threshold modulation in human c nociceptors. Brain 1989;112(Pt 5):1317–1331. [DOI] [PubMed] [Google Scholar]
- 24. Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain 1991;47:285–294. [DOI] [PubMed] [Google Scholar]
- 25. Koltzenburg M, Lundberg LE, Torebjork HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain 1992;51:207–219. [DOI] [PubMed] [Google Scholar]
- 26. Ren K, Dubner R. Inflammatory models of pain and hyperalgesia. ILAR J 1999;40:111–118. [DOI] [PubMed] [Google Scholar]
- 27. Simone DA, Baumann TK, LaMotte RH. Dose‐dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain 1989;38:99–107. [DOI] [PubMed] [Google Scholar]
- 28. Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin‐induced secondary hyperalgesia in humans. J Physiol 1992;448:765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woolf CJ. Evidence for a central component of post‐injury pain hypersensitivity. Nature 1983;306:686–688. [DOI] [PubMed] [Google Scholar]
- 30. Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petho G, Izydorczyk I, Reeh PW. Effects of trpv1 receptor antagonists on stimulated icgrp release from isolated skin of rats and trpv1 mutant mice. Pain 2004;109:284–290. [DOI] [PubMed] [Google Scholar]
- 32. Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor trpv1: 10 years from channel cloning to antagonist proof‐of‐concept. Nat Rev Drug Discov 2007;6:357–372. [DOI] [PubMed] [Google Scholar]
- 33. Paudel KR, Bhattacharya S, Rauniar G, Das B. Comparison of antinociceptive effect of the antiepileptic drug gabapentin to that of various dosage combinations of gabapentin with lamotrigine and topiramate in mice and rats. J Neurosci Rural Pract 2011;2:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petersen KL, Rowbotham MC. A new human experimental pain model: The heat/capsaicin sensitization model. NeuroReport 1999;10:1511–1516. [DOI] [PubMed] [Google Scholar]
- 35. Reddy KS, Naidu MU, Rani PU, Rao TR. Human experimental pain models: A review of standardized methods in drug development. J Res Med Sci 2012;17:587–595. [PMC free article] [PubMed] [Google Scholar]
- 36. Szolcsanyi J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J Physiol (Paris) 1977;73:251–259. [PubMed] [Google Scholar]
- 37. Jancso G, Kiraly E, Jancso‐Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 1977;270:741–743. [DOI] [PubMed] [Google Scholar]
- 38. Nagy JI, Iversen LL, Goedert M, Chapman D, Hunt SP. Dose‐dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci 1983;3:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jordt SE, Julius D. Molecular basis for species‐specific sensitivity to “hot” chili peppers. Cell 2002;108:421–430. [DOI] [PubMed] [Google Scholar]
- 40. Lippe IT, Stabentheiner A, Holzer P. Participation of nitric oxide in the mustard oil‐induced neurogenic inflammation of the rat paw skin. Eur J Pharmacol 1993;232:113–120. [DOI] [PubMed] [Google Scholar]
- 41. Inoue H, Asaka T, Nagata N, Koshihara Y. Mechanism of mustard oil‐induced skin inflammation in mice. Eur J Pharmacol 1997;333:231–240. [DOI] [PubMed] [Google Scholar]
- 42. Wong JK, Haas DA, Hu JW. Local anesthesia does not block mustard‐oil‐induced temporomandibular inflammation. Anesth Analg 2001;92:1035–1040. [DOI] [PubMed] [Google Scholar]
- 43. Carstens E, Mitsuyo T. Neural correlates of oral irritation by mustard oil and other pungent chemicals: A hot topic. Chem Senses 2005;30(Suppl 1):i203–i204. [DOI] [PubMed] [Google Scholar]
- 44. Albin KC, Carstens MI, Carstens E. Modulation of oral heat and cold pain by irritant chemicals. Chem Senses 2008;33:3–15. [DOI] [PubMed] [Google Scholar]
- 45. Merrill AW, Cuellar JM, Judd JH, Carstens MI, Carstens E. Effects of trpa1 agonists mustard oil and cinnamaldehyde on lumbar spinal wide‐dynamic range neuronal responses to innocuous and noxious cutaneous stimuli in rats. J Neurophysiol 2008;99:415–425. [DOI] [PubMed] [Google Scholar]
- 46. Jordt SE, Bautista DM, Chuang HH, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004;427:260–265. [DOI] [PubMed] [Google Scholar]
- 47. Bautista DM, Jordt SE, Nikai T, et al. Trpa1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006;124:1269–1282. [DOI] [PubMed] [Google Scholar]
- 48. Everaerts W, Gees M, Alpizar YA, et al. The capsaicin receptor trpv1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol 2011;21:316–321. [DOI] [PubMed] [Google Scholar]
- 49. Sjolund KF, Segerdahl M, Sollevi A. Adenosine reduces secondary hyperalgesia in two human models of cutaneous inflammatory pain. Anesth Analg 1999;88:605–610. [DOI] [PubMed] [Google Scholar]
- 50. Haas DA, Nakanishi O, MacMillan RE, Jordan RC, Hu JW. Development of an orofacial model of acute inflammation in the rat. Arch Oral Biol 1992;37:417–422. [DOI] [PubMed] [Google Scholar]
- 51. Han SR, Lee MK, Lim KH, et al. Intramuscular administration of morphine reduces mustard‐oil‐induced craniofacial‐muscle pain behavior in lightly anesthetized rats. Eur J Pain 2008;12:361–370. [DOI] [PubMed] [Google Scholar]
- 52. Dubuisson D, Dennis SG. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977;4:161–174. [DOI] [PubMed] [Google Scholar]
- 53. Oliveira AR, Barros HM. Ultrasonic rat vocalizations during the formalin test: A measure of the affective dimension of pain? Anesth Analg 2006;102:832–839. [DOI] [PubMed] [Google Scholar]
- 54. McNamara CR, Mandel‐Brehm J, Bautista DM, et al. Trpa1 mediates formalin‐induced pain. Proc Natl Acad Sci U S A 2007;104:13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin‐induced activity of rat dorsal horn neurones. Neurosci Lett 1987;83:207–211. [DOI] [PubMed] [Google Scholar]
- 56. Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: An evaluation of the method. Pain 1992;51:5–17. [DOI] [PubMed] [Google Scholar]
- 57. Hunskaar S, Hole K. The formalin test in mice: Dissociation between inflammatory and non‐inflammatory pain. Pain 1987;30:103–114. [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto T, Nozaki‐Taguchi N. The role of cyclooxygenase‐1 and ‐2 in the rat formalin test. Anesth Analg 2002;94:962–967, table of contents. [DOI] [PubMed] [Google Scholar]
- 59. Han P, Zhao J, Liu SB, et al. Interleukin‐33 mediates formalin‐induced inflammatory pain in mice. Neuroscience 2013;241:59–66. [DOI] [PubMed] [Google Scholar]
- 60. Dallel R, Raboisson P, Clavelou P, Saade M, Woda A. Evidence for a peripheral origin of the tonic nociceptive response to subcutaneous formalin. Pain 1995;61:11–16. [DOI] [PubMed] [Google Scholar]
- 61. Puig S, Sorkin LS. Formalin‐evoked activity in identified primary afferent fibers: Systemic lidocaine suppresses phase‐2 activity. Pain 1996;64:345–355. [DOI] [PubMed] [Google Scholar]
- 62. Pitcher GM, Henry JL. Second phase of formalin‐induced excitation of spinal dorsal horn neurons in spinalized rats is reversed by sciatic nerve block. Eur J Neurosci 2002;15:1509–1515. [DOI] [PubMed] [Google Scholar]
- 63. Fu KY, Light AR, Maixner W. Long‐lasting inflammation and long‐term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain 2001;2:2–11. [DOI] [PubMed] [Google Scholar]
- 64. Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: Characteristic biphasic pain response. Pain 1989;38:347–352. [DOI] [PubMed] [Google Scholar]
- 65. Meunier CJ, Burton J, Cumps J, Verbeeck RK. Evaluation of the formalin test to assess the analgesic activity of diflunisal in the rat. Eur J Pharm Sci 1998;6:311–316. [DOI] [PubMed] [Google Scholar]
- 66. Zuniga‐Romero A, Ponce‐Chavez MK, Gauthereau‐Torres MY, Ortega‐Varela LF. Combination of diacerhein and antiepileptic drugs after local peripheral, and oral administration in the rat formalin test. Drug Dev Res 2014;75:510–520. [DOI] [PubMed] [Google Scholar]
- 67. Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin‐induced tissue injury. J Neurosci 1992;12:3665–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance p receptor blocked by spinal cyclooxygenase inhibition. Science 1992;257:1276–1279. [DOI] [PubMed] [Google Scholar]
- 69. Clavelou P, Pajot J, Dallel R, Raboisson P. Application of the formalin test to the study of orofacial pain in the rat. Neurosci Lett 1989;103:349–353. [DOI] [PubMed] [Google Scholar]
- 70. Steen KH, Issberner U, Reeh PW. Pain due to experimental acidosis in human skin: Evidence for non‐adapting nociceptor excitation. Neurosci Lett 1995;199:29–32. [DOI] [PubMed] [Google Scholar]
- 71. Steen KH, Steen AE, Reeh PW. A dominant role of acid ph in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J Neurosci 1995;15:3982–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Steen KH, Reeh PW, Kreysel HW. Dose‐dependent competitive block by topical acetylsalicylic and salicylic acid of low ph‐induced cutaneous pain. Pain 1996;64:71–82. [DOI] [PubMed] [Google Scholar]
- 73. Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci 1992;12:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kessler W, Kirchhoff C, Reeh PW, Handwerker HO. Excitation of cutaneous afferent nerve endings in vitro by a combination of inflammatory mediators and conditioning effect of substance p. Exp Brain Res 1992;91:467–476. [DOI] [PubMed] [Google Scholar]
- 75. Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid‐induced pain and its modulation in humans. J Neurosci 2004;24:10974–10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wemmie JA, Taugher RJ, Kreple CJ. Acid‐sensing ion channels in pain and disease. Nat Rev Neurosci 2013;14:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dube GR, Lehto SG, Breese NM, et al. Electrophysiological and in vivo characterization of a‐317567, a novel blocker of acid sensing ion channels. Pain 2005;117:88–96. [DOI] [PubMed] [Google Scholar]
- 78. Mazzuca M, Heurteaux C, Alloui A, et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci 2007;10:943–945. [DOI] [PubMed] [Google Scholar]
- 79. Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue ph and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 2004;101:468–475. [DOI] [PubMed] [Google Scholar]
- 80. Knight B, Katz DR, Isenberg DA, et al. Induction of adjuvant arthritis in mice. Clin Exp Immunol 1992;90:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koo ST, Lee CH, Choi H, et al. The effects of pressure on arthritic knees in a rat model of cfa‐induced arthritis. Pain Physician 2013;16:E95–E102. [PubMed] [Google Scholar]
- 82. Parvathy SS, Masocha W. Gait analysis of c57bl/6 mice with complete Freund's adjuvant‐induced arthritis using the catwalk system. BMC Musculoskelet Disord 2013;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Watanabe M, Endo Y, Kimoto K, Katoh‐Semba R, Arakawa Y. Inhibition of adjuvant‐induced inflammatory hyperalgesia in rats by local injection of neurotrophin‐3. Neurosci Lett 2000;282:61–64. [DOI] [PubMed] [Google Scholar]
- 84. Negri L, Lattanzi R, Giannini E, et al. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: Focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci 2006;26:6716–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li Z, Proud D, Zhang C, Wiehler S, McDougall JJ. Chronic arthritis down‐regulates peripheral mu‐opioid receptor expression with concomitant loss of endomorphin 1 antinociception. Arthritis Rheum 2005;52:3210–3219. [DOI] [PubMed] [Google Scholar]
- 86. Simjee SU, Jawed H, Quadri J, Saeed SA. Quantitative gait analysis as a method to assess mechanical hyperalgesia modulated by disease‐modifying antirheumatoid drugs in the adjuvant‐induced arthritic rat. Arthritis Res Ther 2007;9:R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact 2001;1:377–385. [PubMed] [Google Scholar]
- 88. McDougall JJ, Karimian SM, Ferrell WR. Prolonged alteration of vasoconstrictor and vasodilator responses in rat knee joints by adjuvant monoarthritis. Exp Physiol 1995;80:349–357. [DOI] [PubMed] [Google Scholar]
- 89. Nisar A, Akhter N, Singh G, et al. Modulation of t‐helper cytokines and inflammatory mediators by Atropa accuminata. Royle in adjuvant induced arthritic tissues. J Ethnopharmacol 2015;162:215–224. [DOI] [PubMed] [Google Scholar]
- 90. Karimian SM, McDougall JJ, Ferrell WR. Neuropeptidergic and autonomic control of the vasculature of the rat knee joint revealed by laser Doppler perfusion imaging. Exp Physiol 1995;80:341–348. [DOI] [PubMed] [Google Scholar]
- 91. Keeble J, Russell F, Curtis B, Starr A, Pinter E, Brain SD. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum 2005;52:3248–3256. [DOI] [PubMed] [Google Scholar]
- 92. Fernandes ES, Russell FA, Spina D, et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha‐induced inflammatory hyperalgesia and Freund's complete adjuvant‐induced monarthritis. Arthritis Rheum 2011;63:819–829. [DOI] [PubMed] [Google Scholar]
- 93. Uematsu T, Sakai A, Ito H, Suzuki H. Intra‐articular administration of tachykinin nk(1) receptor antagonists reduces hyperalgesia and cartilage destruction in the inflammatory joint in rats with adjuvant‐induced arthritis. Eur J Pharmacol 2011;668:163–168. [DOI] [PubMed] [Google Scholar]
- 94. Bendele A, McComb J, Gould T, et al. Animal models of arthritis: Relevance to human disease. Toxicol Pathol 1999;27:134–142. [DOI] [PubMed] [Google Scholar]
- 95. Bendele A, McAbee T, Sennello G, Frazier J, Chlipala E, McCabe D. Efficacy of sustained blood levels of interleukin‐1 receptor antagonist in animal models of arthritis: Comparison of efficacy in animal models with human clinical data. Arthritis Rheum 1999;42:498–506. [DOI] [PubMed] [Google Scholar]
- 96. Schaible HG, Schmidt RF, Willis WD. Enhancement of the responses of ascending tract cells in the cat spinal cord by acute inflammation of the knee joint. Exp Brain Res 1987;66:489–499. [DOI] [PubMed] [Google Scholar]
- 97. Neugebauer V, Schaible HG. Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat's knee. J Neurophysiol 1990;64:299–311. [DOI] [PubMed] [Google Scholar]
- 98. Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and gaba receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol 1995;484(Pt 2):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hanesch U, Pawlak M, McDougall JJ. Gabapentin reduces the mechanosensitivity of fine afferent nerve fibres in normal and inflamed rat knee joints. Pain 2003;104:363–366. [DOI] [PubMed] [Google Scholar]
- 100. Bhattacharyya S, Gill R, Chen ML, et al. Toll‐like receptor 4 mediates induction of the Bcl10‐NFκB‐interleukin‐8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J Biol Chem 2008;283:10550–10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain 2007;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Coulon R, Charlier R, Vandersmissen L. [Action of 2‐mercaptoethylamine on experimental arthritis]. Arch Int Pharmacodyn Ther 1954;99:474–480. [PubMed] [Google Scholar]
- 103. Gardner DL. The experimental production of arthritis: A review. Ann Rheum Dis 1960;19:297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Muirden KD, Peace G. Light and electron microscope studies in carragheenin, adjuvant, and tuberculin‐induced arthris. Ann Rheum Dis 1969;28:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Andruski B, McCafferty DM, Ignacy T, Millen B, McDougall JJ. Leukocyte trafficking and pain behavioral responses to a hydrogen sulfide donor in acute monoarthritis. Am J Physiol Regul Integr Comp Physiol 2008;295:R814–R820. [DOI] [PubMed] [Google Scholar]
- 106. Houghton AK, Lu Y, Westlund KN. S‐(+)‐3‐isobutylgaba and its stereoisomer reduces the amount of inflammation and hyperalgesia in an acute arthritis model in the rat. J Pharmacol Exp Ther 1998;285:533–538. [PubMed] [Google Scholar]
- 107. Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain 1993;55:367–377. [DOI] [PubMed] [Google Scholar]
- 108. Han JS, Neugebauer V. mGlur1 and mGlur5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain 2005;113:211–222. [DOI] [PubMed] [Google Scholar]
- 109. Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol 1985;54:1109–1122. [DOI] [PubMed] [Google Scholar]
- 110. Neugebauer V, Schaible HG. Peripheral and spinal components of the sensitization of spinal neurons during an acute experimental arthritis. Agents Actions 1988;25:234–236. [DOI] [PubMed] [Google Scholar]
- 111. Terato K, Hasty KA, Cremer MA, Stuart JM, Townes AS, Kang AH. Collagen‐induced arthritis in mice. Localization of an arthritogenic determinant to a fragment of the type ii collagen molecule. J Exp Med 1985;162:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Brand DD, Latham KA, Rosloniec EF. Collagen‐induced arthritis. Nat Protoc 2007;2:1269–1275. [DOI] [PubMed] [Google Scholar]
- 113. Trentham DE, Townes AS, Kang AH. Autoimmunity to type ii collagen an experimental model of arthritis. J Exp Med 1977;146:857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Inglis JJ, Notley CA, Essex D, et al. Collagen‐induced arthritis as a model of hyperalgesia: Functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheum 2007;56:4015–4023. [DOI] [PubMed] [Google Scholar]
- 115. Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: Review of current evidence with focus on bfgf/jnk pathway. Neuron Glia Biol 2006;2:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dowty ME, Jesson MI, Ghosh S, et al. Preclinical to clinical translation of tofacitinib, a janus kinase inhibitor, in rheumatoid arthritis. J Pharmacol Exp Ther 2014;348:165–173. [DOI] [PubMed] [Google Scholar]
- 117. Vishwakarma S, Iyer LR, Muley M, et al. Tubastatin, a selective histone deacetylase 6 inhibitor shows anti‐inflammatory and anti‐rheumatic effects. Int Immunopharmacol 2013;16:72–78. [DOI] [PubMed] [Google Scholar]
- 118. Thiel MJ, Schaefer CJ, Lesch ME, et al. Central role of the mek/erk map kinase pathway in a mouse model of rheumatoid arthritis: Potential proinflammatory mechanisms. Arthritis Rheum 2007;56:3347–3357. [DOI] [PubMed] [Google Scholar]
- 119. Koch AE. Chemokines and their receptors in rheumatoid arthritis: Future targets? Arthritis Rheum 2005;52:710–721. [DOI] [PubMed] [Google Scholar]
- 120. Olesen AE, Andresen T, Staahl C, Drewes AM. Human experimental pain models for assessing the therapeutic efficacy of analgesic drugs. Pharmacol Rev 2012;64:722–779. [DOI] [PubMed] [Google Scholar]
- 121. Laird JM, Martinez‐Caro L, Garcia‐Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001;92:335–342. [DOI] [PubMed] [Google Scholar]
- 122. Drewes AM, Schipper KP, Dimcevski G, et al. Gut pain and hyperalgesia induced by capsaicin: A human experimental model. Pain 2003;104:333–341. [DOI] [PubMed] [Google Scholar]
- 123. Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 2007;148:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Christoph T, Grunweller A, Mika J, et al. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun 2006;350:238–243. [DOI] [PubMed] [Google Scholar]
- 125. Robinson DR, Gebhart GF. Inside information: The unique features of visceral sensation. Mol Interv 2008;8:242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Laird JM, Olivar T, Roza C, De Felipe C, Hunt SP, Cervero F. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin nk1 receptor gene. Neuroscience 2000;98:345–352. [DOI] [PubMed] [Google Scholar]
- 127. Shin JW, Hwang KS, Kim YK, Leem JG, Lee C. Nonsteroidal antiinflammatory drugs suppress pain‐related behaviors, but not referred hyperalgesia of visceral pain in mice. Anesth Analg 2006;102:195–200. [DOI] [PubMed] [Google Scholar]
- 128. Maia JL, Lima‐Junior RC, David JP, David JM, Santos FA, Rao VS. Oleanolic acid, a pentacyclic triterpene attenuates the mustard oil‐induced colonic nociception in mice. Biol Pharm Bull 2006;29:82–85. [DOI] [PubMed] [Google Scholar]
- 129. Kimball ES, Prouty SP, Pavlick KP, Wallace NH, Schneider CR, Hornby PJ. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol Motil 2007;19:390–400. [DOI] [PubMed] [Google Scholar]
- 130. Al‐Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 2000;119:1276–1285. [DOI] [PubMed] [Google Scholar]
- 131. Larauche M, Mulak A, Tache Y. Stress and visceral pain: From animal models to clinical therapies. Exp Neurol 2012;233:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Targan SR, Hanauer SB, van Deventer SJ, et al. A short‐term study of chimeric monoclonal antibody ca2 to tumor necrosis factor alpha for Crohn's disease. Crohn's disease ca2 study group. N Engl J Med 1997;337:1029–1035. [DOI] [PubMed] [Google Scholar]
- 133. Siegmund E, Cadmus R, Lu G. A method for evaluating both non‐narcotic and narcotic analgesics. Proc Soc Exp Biol Med 1957;95:729–731. [DOI] [PubMed] [Google Scholar]
- 134. Eckhardt ET, Cheplovitz F, Lipo M, Govier WM. Etiology of chemically induced writhing in mouse and rat. Proc Soc Exp Biol Med 1958;98:186–188. [DOI] [PubMed] [Google Scholar]
- 135. Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev 2001;53:597–652. [PubMed] [Google Scholar]
- 136. Schwartz ES, Gebhart GF. Visceral pain. Curr Top . Behav Neurosci 2014;20:171–197. [DOI] [PubMed] [Google Scholar]
- 137. Ribeiro RA, Vale ML, Thomazzi SM, et al. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 2000;387:111–118. [DOI] [PubMed] [Google Scholar]
- 138. Berkenkopf JW, Weichman BM. Production of prostacyclin in mice following intraperitoneal injection of acetic acid, phenylbenzoquinone and zymosan: Its role in the writhing response. Prostaglandins 1988;36:693–709. [DOI] [PubMed] [Google Scholar]
- 139. Heapy CG, Shaw JS, Farmer SC. Differential sensitivity of antinociceptive assays to the bradykinin antagonist hoe 140. Br J Pharmacol 1993;108:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gadotti VM, Martins DF, Pinto HF, et al. Diacerein decreases visceral pain through inhibition of glutamatergic neurotransmission and cytokine signaling in mice. Pharmacol Biochem Behav 2012;102:549–554. [DOI] [PubMed] [Google Scholar]
- 141. Vale ML, Marques JB, Moreira CA, et al. Antinociceptive effects of interleukin‐4, ‐10, and ‐13 on the writhing response in mice and zymosan‐induced knee joint incapacitation in rats. J Pharmacol Exp Ther 2003;304:102–108. [DOI] [PubMed] [Google Scholar]
- 142. Pavao‐de‐Souza GF, Zarpelon AC, Tedeschi GC, et al. Acetic acid‐ and phenyl‐p‐benzoquinone‐induced overt pain‐like behavior depends on spinal activation of map kinases, pi(3)k and microglia in mice. Pharmacol Biochem Behav 2012;101:320–328. [DOI] [PubMed] [Google Scholar]
- 143. Porreca F, Mosberg HI, Omnaas JR, Burks TF, Cowan A. Supraspinal and spinal potency of selective opioid agonists in the mouse writhing test. J Pharmacol Exp Ther 1987;240:890–894. [PubMed] [Google Scholar]
- 144. Jett MF, Ramesha CS, Brown CD, et al. Characterization of the analgesic and anti‐inflammatory activities of ketorolac and its enantiomers in the rat. J Pharmacol Exp Ther 1999;288:1288–1297. [PubMed] [Google Scholar]
- 145. Satyanarayana PS, Jain NK, Singh A, Kulkarni SK. Isobolographic analysis of interaction between cyclooxygenase inhibitors and tramadol in acetic acid‐induced writhing in mice. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:641–649. [DOI] [PubMed] [Google Scholar]
- 146. Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Methods 1999;87:185–193. [DOI] [PubMed] [Google Scholar]
- 147. Luo ZD, Chaplan SR, Higuera ES, et al. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve‐injured rats. J Neurosci 2001;21:1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Menetrey D, Besson JM. Electrophysiological characteristics of dorsal horn cells in rats with cutaneous inflammation resulting from chronic arthritis. Pain 1982;13:343–364. [DOI] [PubMed] [Google Scholar]
- 149. Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of c‐fiber evoked pain. Pain 2007;129:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Vaccarino AL, Chorney DA. Descending modulation of central neural plasticity in the formalin pain test. Brain Res 1994;666:104–108. [DOI] [PubMed] [Google Scholar]
- 151. Urban MO, Jiang MC, Gebhart GF. Participation of central descending nociceptive facilitatory systems in secondary hyperalgesia produced by mustard oil. Brain Res 1996;737:83–91. [DOI] [PubMed] [Google Scholar]
- 152. Zhuo M. A synaptic model for pain: Long‐term potentiation in the anterior cingulate cortex. Mol Cells 2007;23:259–271. [PubMed] [Google Scholar]
- 153. Campana G, Rimondini R. Mechanical nociception measurement in mice and rats with automated von frey equipment. Methods Mol Biol 2015;1230:229–231. [DOI] [PubMed] [Google Scholar]
- 154. Schott E, Berge OG, Angeby‐Moller K, Hammarstrom G, Dalsgaard CJ, Brodin E. Weight bearing as an objective measure of arthritic pain in the rat. J Pharmacol Toxicol Methods 1994;31:79–83. [DOI] [PubMed] [Google Scholar]
- 155. Tetreault P, Dansereau MA, Dore‐Savard L, Beaudet N, Sarret P. Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiol Behav 2011;104:495–502. [DOI] [PubMed] [Google Scholar]
- 156. Kim HT, Uchimoto K, Duellman T, Yang J. Automated assessment of pain in rats using a voluntarily accessed static weight‐bearing test. Physiol Behav 2015;151:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ekman P, Friesen W. Facial action coding system. Palo Alto, CA: Consulting Psychologists Press, 1978. [Google Scholar]
- 158. Williams AC. Facial expression of pain: An evolutionary account. Behav Brain Sci 2002;25:439–455; discussion 455‐488. [DOI] [PubMed] [Google Scholar]
- 159. Langford DJ, Bailey AL, Chanda ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 2010;7:447–449. [DOI] [PubMed] [Google Scholar]
- 160. Darwin C. The expression of the emotions in man and animals. London: Albemarle, 1872. [Google Scholar]
- 161. Sotocinal SG, Sorge RE, Zaloum A, et al. The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain 2011;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988;32:77–88. [DOI] [PubMed] [Google Scholar]
- 163. Kocevski D, Tvrdeic A. The effect of repeated daily measurements on paw withdrawal latencies in Hargreaves test. Coll Antropol 2008;32(Suppl 1):93–97. [PubMed] [Google Scholar]
- 164. Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 1957;111:409–419. [PubMed] [Google Scholar]
- 165. Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res 1996;742:352–354. [DOI] [PubMed] [Google Scholar]
- 166. Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: Activation by stress. Science 1984;226:1270–1277. [DOI] [PubMed] [Google Scholar]
- 167. Matson DJ, Broom DC, Cortright DN. Locomotor activity in a novel environment as a test of inflammatory pain in rats. Methods Mol Biol 2010;617:67–78. [DOI] [PubMed] [Google Scholar]
- 168. Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation‐induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther 2007;320:194–201. [DOI] [PubMed] [Google Scholar]
- 169. Cho H, Jang Y, Lee B, et al. Voluntary movements as a possible non‐reflexive pain assay. Mol Pain 2013;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Van de Weerd HA, Bulthuis RJ, Bergman AF, et al. Validation of a new system for the automatic registration of behaviour in mice and rats. Behav Processes 2001;53:11–20. [DOI] [PubMed] [Google Scholar]
- 171. Amaya F, Wang H, Costigan M, et al. The voltage‐gated sodium channel na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci 2006;26:12852–12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Deacon RM. Burrowing in rodents: A sensitive method for detecting behavioral dysfunction. Nat Protoc 2006;1:118–121. [DOI] [PubMed] [Google Scholar]
- 173. Deacon RM. Burrowing: A sensitive behavioural assay, tested in five species of laboratory rodents. Behav Brain Res 2009;200:128–133. [DOI] [PubMed] [Google Scholar]
- 174. Rutten K, Robens A, Read SJ, Christoph T. Pharmacological validation of a refined burrowing paradigm for prediction of analgesic efficacy in a rat model of sub‐chronic knee joint inflammation. Eur J Pain 2014;18:213–222. [DOI] [PubMed] [Google Scholar]
- 175. Rutten K, Schiene K, Robens A, et al. Burrowing as a non‐reflex behavioural readout for analgesic action in a rat model of sub‐chronic knee joint inflammation. Eur J Pain 2014;18:204–212. [DOI] [PubMed] [Google Scholar]
- 176. Bryden LA, Nicholson JR, Doods H, Pekcec A. Deficits in spontaneous burrowing behavior in the rat bilateral monosodium iodoacetate model of osteoarthritis: An objective measure of pain‐related behavior and analgesic efficacy. Osteoarthritis Cartilage 2015;23:1605–1612. [DOI] [PubMed] [Google Scholar]
- 177. Jirkof P, Leucht K, Cesarovic N, et al. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Lab Anim 2013;47:274–283. [DOI] [PubMed] [Google Scholar]
- 178. Andrews N, Legg E, Lisak D, et al. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain 2012;16:485–495. [DOI] [PubMed] [Google Scholar]
- 179. Schaible HG, Schmidt RF. Responses of fine medial articular nerve afferents to passive movements of knee joints. J Neurophysiol 1983;49:1118–1126. [DOI] [PubMed] [Google Scholar]
- 180. McDougall JJ, Pawlak M, Hanesch U, Schmidt RF. Peripheral modulation of rat knee joint afferent mechanosensitivity by nociceptin/orphanin FQ. Neurosci Lett 2000;288:123–126. [DOI] [PubMed] [Google Scholar]
- 181. McDougall JJ, Andruski B, Schuelert N, Hallgrimsson B, Matyas JR. Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain 2009;141:222–232. [DOI] [PubMed] [Google Scholar]
- 182. Russell FA, Schuelert N, Veldhoen VE, Hollenberg MD, McDougall JJ. Activation of PAR2 receptors sensitizes primary afferents and causes leukocyte rolling and adherence in the rat knee joint. Br J Pharmacol 2012;167:1665–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. McDougall JJ. Peripheral analgesia: Hitting pain where it hurts. Biochim Biophys Acta 2011;1812:459–467. [DOI] [PubMed] [Google Scholar]
- 184. Calvino B, Villanueva L, Le Bars D. The heterotopic effects of visceral pain: Behavioural and electrophysiological approaches in the rat. Pain 1984;20:261–271. [DOI] [PubMed] [Google Scholar]
- 185. Ibeakanma C, Miranda‐Morales M, Richards M, et al. Citrobacter rodentium colitis evokes post‐infectious hyperexcitability of mouse nociceptive colonic dorsal root ganglion neurons. J Physiol 2009;587:3505–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sanoja R, Tortorici V, Fernandez C, Price TJ, Cervero F. Role of RVM neurons in capsaicin‐evoked visceral nociception and referred hyperalgesia. Eur J Pain 2010;14:120 e121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Guilbaud G, Benoist JM, Condes‐Lara M, Gautron M. Further evidence for the involvement of SmI cortical neurons in nociception: Their responsiveness at 24 hr after carrageenin‐induced hyperalgesic inflammation in the rat. Somatosens Mot Res 1993;10:229–244. [DOI] [PubMed] [Google Scholar]
- 188. Neugebauer V, Li W, Bird GC, Bhave G, Gereau R. Synaptic plasticity in the amygdala in a model of arthritic pain: Differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 2003;23:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bresnihan B, Alvaro‐Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin‐1 receptor antagonist. Arthritis Rheum 1998;41:2196–2204. [DOI] [PubMed] [Google Scholar]
- 190. Seymour HE, Worsley A, Smith JM, Thomas SH. Anti‐TNF agents for rheumatoid arthritis. Br J Clin Pharmacol 2001;51:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kalso E, Smith L, McQuay HJ, Andrew Moore R. No pain, no gain: Clinical excellence and scientific rigour–lessons learned from ia morphine. Pain 2002;98:269–275. [DOI] [PubMed] [Google Scholar]
- 192. Rovensky J, Svik K, Istok R, Stancikova M. Quantitative assessment of markers of inflammation, arthritis and pain in rats with adjuvant arthritis following the treatment with ibuprofen cream and placebo. Drugs Exp Clin Res 2003;29:85–90. [PubMed] [Google Scholar]
- 193. Ong CK, Lirk P, Tan CH, Seymour RA. An evidence‐based update on nonsteroidal anti‐inflammatory drugs. Clin Med Res 2007;5:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Beirne OR. Corticosteroids decrease pain, swelling and trismus. Evid Based Dent 2013;14:111. [DOI] [PubMed] [Google Scholar]
- 195. Varadi G, Zhu Z, Blattler T, et al. Randomized clinical trial evaluating transdermal ibuprofen for moderate to severe knee osteoarthritis. Pain Physician 2013;16:E749–E762. [PubMed] [Google Scholar]
- 196. Hill R. Nk1 (substance p) receptor antagonists–why are they not analgesic in humans? Trends Pharmacol Sci 2000;21:244–246. [DOI] [PubMed] [Google Scholar]
- 197. Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo‐controlled clinical trial with the irreversible fatty acid amide hydrolase‐1 inhibitor pf‐04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012;153:1837–1846. [DOI] [PubMed] [Google Scholar]
- 198. Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol 2011;164:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Mao J. Current challenges in translational pain research. Trends Pharmacol Sci 2012;33:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Williamson A, Hoggart B. Pain: A review of three commonly used pain rating scales. J Clin Nurs 2005;14:798–804. [DOI] [PubMed] [Google Scholar]
- 201. Meibohm B, Derendorf H. Basic concepts of pharmacokinetic/pharmacodynamic (pk/pd) modelling. Int J Clin Pharmacol Ther 1997;35:401–413. [PubMed] [Google Scholar]
- 202. Lin JH. Species similarities and differences in pharmacokinetics. Drug Metab Dispos 1995;23:1008–1021. [PubMed] [Google Scholar]
- 203. Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human cyp‐mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2006;2:875–894. [DOI] [PubMed] [Google Scholar]
- 204. Whiteside GT, Adedoyin A, Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic‐pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 2008;54:767–775. [DOI] [PubMed] [Google Scholar]


