Summary
Aims
Spinocerebellar ataxia type 1 (SCA1) is caused by the ataxin‐1 protein (ATXN1) with an abnormally expanded polyglutamine tract and is characterized by progressive neurodegeneration. We previously showed that intrathecal injection of mesenchymal stem cells (MSCs) during the nonsymptomatic stage mitigates the degeneration of the peripheral nervous system (PNS) neurons in SCA1‐knock‐in (SCA1‐KI) mice. We tested in this study whether the therapeutic effects of MSCs in SCA1‐KI mice could be reproduced with MSC‐releasing factor(s).
Methods
To test the effects of MSC‐releasing factor(s), we used MSC‐conditioned medium (MSC‐CM). MSC‐CM was intrathecally and/or intravenously injected into young SCA1‐KI mice, and the therapeutic effects were assessed in the PNS at later ages using immunostaining, electrophysiology, and behavioral tests.
Results
MSC‐CM attenuated the degeneration of axons and myelin of spinal motor neurons. Consequently, the injected SCA1‐KI mice exhibited smaller reductions in nerve conduction velocity in spinal motor neurons and reduced motor incoordination than the untreated mice.
Conclusions
These results suggest that factors released from MSC mitigate the morphological and functional abnormalities in the PNS that are observed in SCA1‐KI mice in a paracrine manner.
Keywords: Axon, Conditioned medium, Mesenchymal stem cell, Myelin, SCA1, Spinal motor neuron
Introduction
Spinocerebellar ataxia type 1 (SCA1) is caused by the expansion of a polyglutamine tract in ataxin‐1 protein (ATXN1) 1 and results in neurodegeneration in numerous areas of the central nervous system (CNS) and peripheral nervous system (PNS) 2. The signs and symptoms include motor incoordination, pyramidal signs, ophthalmoplegia, and cognitive impairment 3.
Mesenchymal stem cells (MSCs) are multipotent progenitor cells that are found in multiple tissues 4, 5, 6 and have clinical potential for use in the treatment of neurodegenerative disorders 7 and stroke 8, 9. Regarding SCA, MSCs proved effective for SCA2 mice because the injection of MSCs into the cerebellar cortex ameliorated motor function deterioration by rescuing cerebellar PCs 10. We have conducted a series of studies involving the treatment of SCA1 mice with MSCs. In the first study, intrathecal injection of MSCs (1 × 103) was found to attenuate the degeneration of cerebellar Purkinje cells (PCs) 11, 12 in SCA1‐transgenic (SCA1‐Tg) mice overexpressing a transgene containing the ATXN1 sequence with an 82‐CAG‐repeat expansion 13, 14. In contrast to the multi‐PC layer alignment, thinner molecular layer and atrophic PC dendrites observed in untreated SCA1‐Tg mice, the MSC‐treated SCA1‐Tg mice exhibited a single layer of PCs and a thicker molecular layer with preserved PC dendrites 11. The morphological improvements observed in the MSC‐treated mice were associated with improved motor coordination performance 11, 12.
We then extended the therapeutic experiment to a study of PNS pathology in SCA1‐knock‐in (SCA1‐KI) mice in which mutant ATXN1 with a 154‐polyglutamine tract was targeted to the endogenous ATXN1 locus in a knock‐in mouse 15. In these animals, intrathecal injection of MSCs during the nonsymptomatic stage mitigated the degeneration of PNS neurons as long as 6 months after injection 16. The MSC‐treated SCA1‐KI mice exhibited larger axon sizes than the untreated SCA1‐KI mice. Thus, MSCs exert therapeutic effects on the pathology of both CNS and PNS neurons in SCA1 model mice.
Despite these findings, the mechanisms by which MSCs successfully ameliorate neurodegeneration of the PNS in SCA1 model mice have not been studied. MSCs might exert their therapeutic potential by differentiating into mesenchymal lineage cells 17, 18, 19. Alternatively, MSCs might provide neurons with multiple trophic factors through paracrine‐mediated mechanisms. Indeed, hepatocyte growth factor (HGF), fibroblast growth factor‐2 (FGF‐2), insulin‐like growth factor‐1 (IGF‐1), and vascular endothelial growth factor (VEGF) have been detected in MSC‐conditioned medium 20. In this study, we tested the hypothesis that unknown factors released from MSCs are effective in preventing neurodegeneration in SCA1 model mice. After injection of MSC‐derived conditioned medium (MSC‐CM) into SCA1‐KI mice, the morphological and functional defects observed in the spinal motor neurons were significantly attenuated. These results indicate the availability of a cell‐free therapeutic approach to prevent SCA1.
Materials and Methods
Mice
Wild‐type (WT) and SCA1‐KI mice on a C57BL/6 background 15 (kindly provided by Dr. Hidehiro Mizusawa of Tokyo Medical and Dental University) were used in all experiments. Animal care and treatment conformed to NIH guidelines and were approved by the Animal Resource Committees of Gunma University. All efforts were made to minimize suffering and to reduce the number of animals used in the experiments. The mice were given either a single intrathecal injection of MSC‐conditioned medium (MSC‐CM) or culture medium (control) at 4 weeks of age or intravenous injections every 2 weeks from the age of 4 weeks to the age of 12 weeks. Some mice were given a single intrathecal injection at 4 weeks of age followed by intravenous injections every 2 weeks between 6 and 24 weeks of age.
MSC Culture
KUM10, a mesenchymal stem cell line generated from C57/BL6 mouse bone marrow, was purchased from the RIKEN BioResource Center (Tsukuba, Ibaraki, Japan). The cells were cultured in M061101 medium containing 10% FBS and antibiotics (GP BioSciences, Yokohama, Japan) and maintained in 5% CO2 at 37°C. KUM10 cells were passaged 2–4 times after recovery from frozen stock. The culture medium was changed 5 days prior to collection of the cell culture supernatant.
Injection of MSC‐Conditioned Medium
Deeply anesthetized mice were fixed in a stereotaxic apparatus. For intrathecal injection, the suboccipital skin between the occipital bone and the cervical spine was incised, and the meninges covering the medulla oblongata were exposed. A 30‐G disposable needle (BD, Franklin Lakes, NJ, USA) was inserted into the subarachnoid space. After evacuation of approximately 15 μL of cerebrospinal fluid, 10 μL of MSC‐CM was injected over a period of 1 min using a Hamilton syringe.
For intravenous injections, 100 μL (first and second injections) or 25 μL (third to last injections) of MSC‐CM or of only culture medium was infused into the orbital vein using 30‐G disposable needles.
Electrophysiology
Compound muscle action potential (CMAP) measurements with the MEB9404 Neuropack S1 (Nihon Kohden, Tokyo, Japan) were performed as previously described 21. Forty‐week‐old mice were anesthetized and fixed in the prone position. The stimulating electrodes (cathode and anode) were inserted into the muscle over the fourth lumbar vertebra and positioned on the spinal column with 1 cm between the anode and cathode. The recording electrode and the reference recording plate were inserted into the hind gastrocnemius muscle and the hind gastrocnemius tendon, respectively. The grand electrode was inserted into the tail root. The CMAPs were recorded in response to electrical stimulations of 0.01‐ms duration. The amplitudes of the electrical stimulations were determined such that the stimulations produced the maximum peak amplitude of the muscle action potential. The latency between the electrical stimulation and onset of the CMAP was measured using Neuropack software (Nihon Kohden).
Rotarod Tests
The motor coordination of the mice was examined once at 13 weeks of age. The Rota‐Rod treadmill (Muromachi Kikai, Tokyo, Japan) is composed of a gridded plastic rod (3 cm in diameter, 10 cm in length) flanked by two large round plates (57 cm in diameter). After all the mice to be examined were placed on the rod rotating at 4 rpm, the rod was continuously accelerated from 4 to 40 rpm over a period of 300 second. Between trials, the mice were allowed to rest for 5 min. The time the mice spent on the rod was automatically measured; the averaged time from 4 trials was used for statistical analysis.
Histology
Mice were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The entire vertebral column with the spinal cord was postfixed in the same fixative solution overnight at 4°C. After decalcification, a transverse lumbar vertebral block including L5 was dissected. Five‐micrometer‐thick sections were prepared from paraffin blocks using a microtome. After deparaffinization and treatment with graded alcohol, the sections were processed for immunofluorescence staining as previously described 22. The primary antibodies used for the double immunofluorescence staining were mouse anti‐β‐tubulin (SIGMA, St. Louis, MO, USA) and goat anti‐myelin basic protein (MBP) (DAKO, Glostrup, Denmark) antibodies. After incubation of the sections with the primary antibodies (dilution at 1:200) overnight at 4°C, the sections were treated with biotin‐conjugated anti‐mouse and Cy3‐conjugated anti‐goat secondary antibodies (dilution at 1:200) (Jackson ImmunoResearch, West Grove, PA, USA) for 1 h at room temperature. Then, the sections were treated with streptavidin‐conjugated HRP for 1 h at room temperature. Finally, the sections were incubated in Alexa Fluor 488 tyramide solution (Life Technologies, Grand Island, NY, USA). The fluorescent signals were observed with a confocal microscope (LSM5; Carl Zeiss, Oberkochen, Germany).
To quantify the sizes of the axons, we obtained images from 3–4 fields in sections from 4–5 mice using a 40× objective lens and measured the axonal diameters using LSM510 software (Carl Zeiss, Oberkochen, Germany). The diameters of the short axes of the transverse myelinated axons were measured from β‐tubulin‐stained images. To quantify the myelin, the diameters of the short axes of the myelin inner circles were measured from MBP‐stained images.
Statistical Analysis
The values obtained are expressed as the mean ± SEM or as box plots including the smallest, lower quartile, median, upper quartile, and largest values. Statistical analyses of the differences between the groups were performed using Student's t‐test or ANOVA followed by Tukey's post‐hoc test. P values below 0.05 were considered statistically significant.
Results
MSC‐CM Mitigates the Degeneration of Both Axons and Myelin of Spinal Motor Neurons of SCA1‐KI Mice
To test the effects of MSC‐CM on neuronal degeneration in the spinal cord, we sought to determine the best method of administration of MSC‐CM. We previously showed that a single intrathecal injection of 1 × 103 MSCs reduces Purkinje cell degeneration and motor incoordination in SCA1‐transgenic mice 11. In a study by another group, intravenous injection of MSCs into rats that had received brain injuries resulted in differentiation of the MSCs into neurons and astrocytes and significant improvement in the animals' neurological function 23. These observations indicate that both intrathecal and intravenous injections of MSCs can effectively produce functional recovery of the brain. Furthermore, intrathecal and intravenous injection are relatively noninvasive procedures that can easily be applied to patients. Therefore, we chose to administer MSC‐CM via a single intrathecal injection followed by multiple intravenous injections.
The SCA1‐KI mice were separated into 2 groups. The first group received injections of MSC‐CM, whereas the second group received culture medium as a control. A single intrathecal injection was given at 4 weeks of age (when the animals were nonsymptomatic), followed by intravenous injections every 2 weeks from 6 to 24 weeks of age. For the intravenous injections, 100 μL (for 6‐ and 8‐week‐old mice) or 25 μL (for 10‐ to 24‐week‐old mice) of solution was infused. The analyses were performed beginning at 40 weeks of age (Figure 1A).
Figure 1.
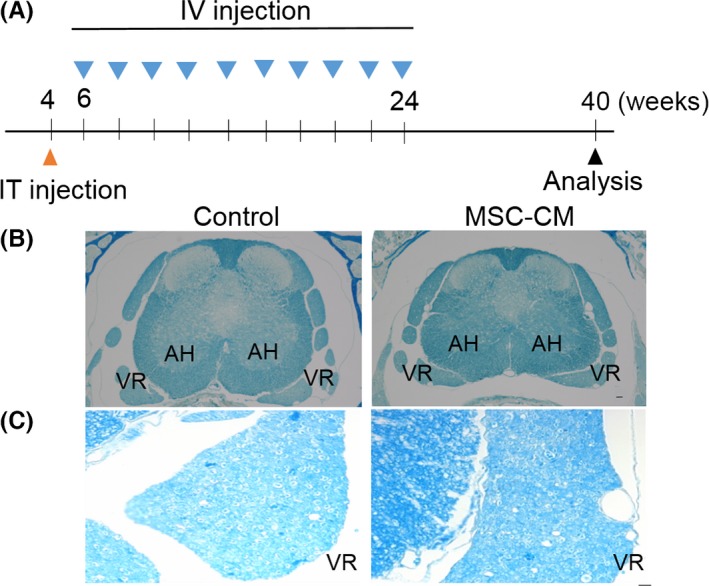
Increased myelin density in the spinal motor neurons of MSC‐CM‐treated SCA1‐KI mice. (A) Schedule of the MSC‐CM injections. The mice were given a single intrathecal (IT) injection of MSC‐CM or only culture medium (control) at 4 weeks of age followed by intravenous (IV) injections every 2 weeks from 6 to 24 weeks of age. For the intravenous injections, 100 μL (for the 6‐ and 8‐week‐old mice) or 25 μL (for the 10‐ to 24‐week‐old mice) of MSC‐CM or only culture medium was infused. Motor nerve conduction velocities were measured at 40 weeks of age. (B and C) Lower (B) and higher (C) magnification images of Luxol fast blue‐stained samples. The region of the ventral root (C) is magnified. The degeneration of myelin was attenuated following injections of MSC‐CM, as evidenced by the increased intensity of the Luxol fast blue signal. AH, anterior horn; VR, ventral root. Scale bars: 50 μm in (B) and 10 μm in (C).
Our previous work revealed a decrease in the amount of myelin in old SCA1‐KI mice compared with WT mice, as revealed by weaker Klüver–Barrera staining signals in the ventral spinal roots of SCA1‐KI animals 21. We tested the effects of MSC‐CM on the density of myelin in highly degenerated SCA1‐KI mice older than 40 weeks using Luxol fast blue staining. As depicted in Figure 1 (B and C), higher‐intensity Luxol fast blue signals were observed in the MSC‐CM‐injected SCA1‐KI mice than in the controls. This result suggests that MSC‐CM suppressed the decrease in the amount of myelin in SCA1‐KI mice.
Both axons and myelin degenerate in old SCA1‐KI mice 21. To evaluate the efficacy of MSC‐CM in preventing such degeneration, we performed double immunofluorescence staining of the ventral roots of the lumbar spinal cords of naïve WT mice, SCA1‐KI mice injected with medium alone, and SCA1‐KI mice injected with MSC‐CM. All the mice were over 40 weeks of age. Anti‐β‐tubulin and anti‐MBP antibodies were used as markers for axons and myelin, respectively. Quantitative analysis revealed that the MSC‐CM‐treated SCA1‐KI mice exhibited larger axons and myelin (3.14 ± 0.06 and 3.90 ± 0.07 μm, axons and myelin, respectively) than the control SCA1‐KI mice (2.44 ± 0.06 and 2.82 ± 0.07 μm, axons and myelin, respectively) (t‐tests, both Ps < 0.001, Figure 2, A–C). These results indicate that injection of the animals with MSC‐CM from the nonsymptomatic stage onward ameliorated the progressive degeneration of both axons and myelin in the spinal neurons of the SCA1‐KI mice.
Figure 2.
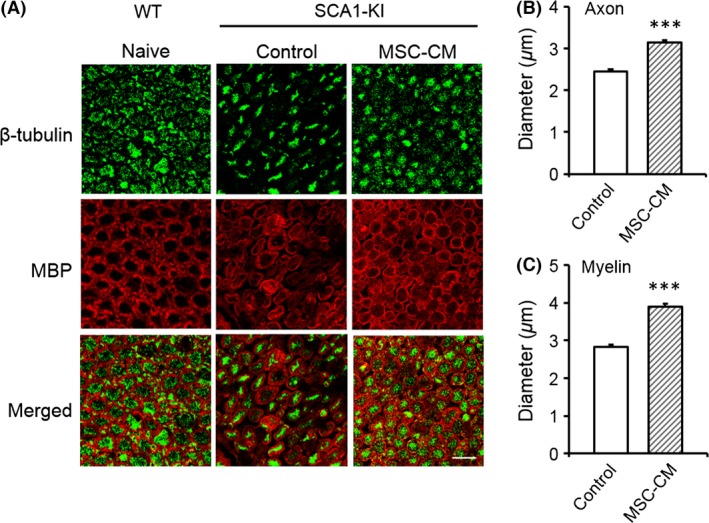
Intrathecal followed by intravenous injection of MSC‐CM attenuates the degeneration of both axons and myelin in spinal motor neurons of SCA1‐KI mice. Double immunofluorescence staining of the ventral roots of the lumbar spinal cords of WT (naive), control SCA1‐KI (medium‐injected, n = 160 cells from 4 mice), and MSC‐CM‐treated SCA1‐KI (n = 160 cells from 4 mice) mice with anti‐β‐tubulin and anti‐MBP antibodies is shown. Representative images are shown in (A); the diameters of the axons (B) and the inner diameters of the myelin (C) were quantified and compared in control and MSC‐CM‐treated SCA1‐KI mice based on images of β‐tubulin and MBP staining, respectively. The error bars represent the SEM. Scale bar: 10 μm. ***P < 0.001.
Faster Neurotransmission in the Axons of Spinal Motor Neurons of MSC‐CM‐Treated SCA1‐KI Mice
Slower neurotransmission has been observed in old SCA1‐KI mice 21 and is probably due to the degeneration of the myelin and the thinner axons of the spinal motor neurons, conditions that reduce the efficiency of saltatory conduction and the spread of electrotonic potential, respectively. Given the therapeutic effects of MSC‐CM on the degeneration of axons and myelin, we conducted functional analyses of nerve conduction in these animals. The velocity of nerve conduction between the lumbar spinal cord and the gastrocnemius muscle in the hindlimb was measured in naïve WT, medium‐injected SCA1‐KI, and MSC‐CM‐treated SCA1‐KI mice at 40 weeks of age. The latencies of the CMAPs in the MSC‐CM‐injected SCA1‐KI mice were shorter (1.78 ± 0.10 ms) than those of the control SCA1‐KI mice (2.25 ± 0.13 ms, t‐test, P = 0.009; Figure 3A,B). Thus, injection of MSC‐CM attenuated the reduction in nerve transmission in the spinal motor neurons of SCA1‐KI animals.
Figure 3.
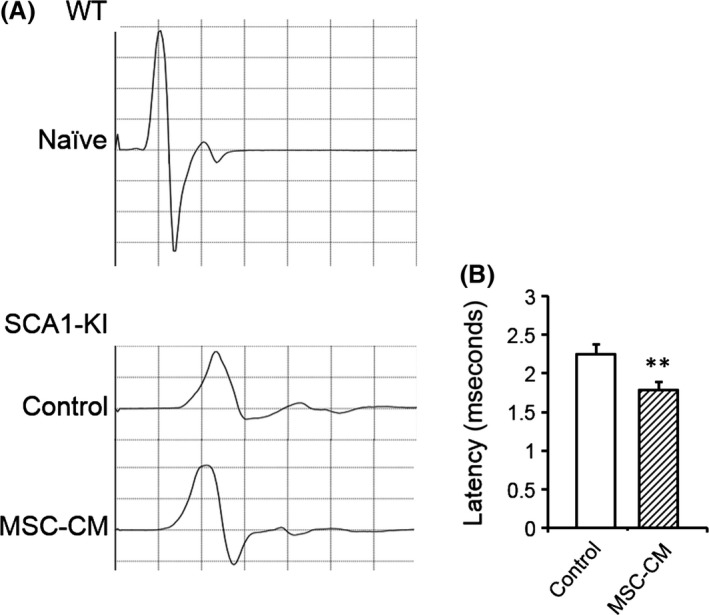
MSC‐CM mitigates the deterioration of neurotransmission in spinal motor neuronal axons of SCA1‐KI mice. Latency to induce the contraction of the hind gastrocnemius muscle following electrical stimulation over the fourth lumbar vertebra was measured. (A) Representative CMAPs recorded in naïve WT (upper) and control and MSC‐CM‐injected SCA1‐KI mice (lower) are shown. (B) The mean latencies of the CMAPs were quantified and compared in control (n = 10 from 5 mice) and MSC‐CM‐treated (n = 12 from 6 mice) SCA1‐KI mice. The error bars represent the SEM. **P < 0.01.
Intrathecal or Intravenous Injection of MSC‐CM Attenuates Impaired Motor Coordination in SCA1‐KI Mice
Given the striking morphological and functional attenuation of PNS degeneration observed after combined intrathecal and intravenous injection of MSC‐CM, we were motivated to test the effects of the administration route, that is, intrathecal injection alone and intravenous injections alone. For intrathecal injection alone, mice received MSC‐CM once at 4 weeks of age, and the morphological amelioration was studied at 13 weeks of age (Figure 4A), when degeneration of axons and myelin normally becomes apparent 16.
Figure 4.
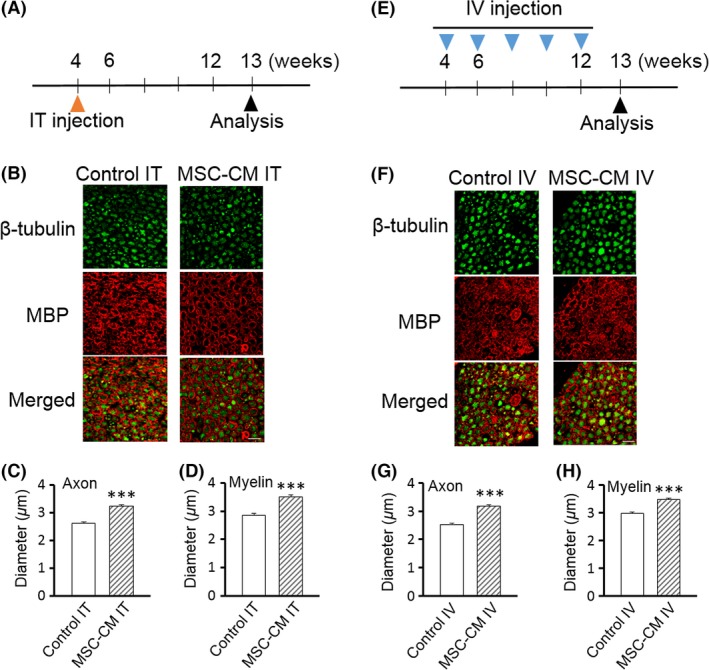
Either intrathecal or intravenous injection of MSC‐CM is sufficient to attenuate the degeneration of both axons and myelin of spinal motor neurons in SCA1‐KI mice. (A, E) Schedule of the MSC‐CM injections.The mice were given a single intrathecal (IT) injection at 4 weeks of age (A) or intravenous (IV) injections every 2 weeks from 4–12 weeks of age (E) of MSC‐CM or only culture medium (control). Morphological and behavioral analyses were performed at 13 weeks of age. (B–D, F–H) Double immunofluorescence staining with anti‐β‐tubulin and anti‐MBP antibodies of the ventral roots of the lumbar spinal cords of intrathecally (IT)‐ (B) and intravenously (IV)‐ (F) injected control (medium‐injected) SCA1‐KI and MSC‐CM‐treated SCA1‐KI mice. Representative images are shown in (B and F); the diameters of the axons (C and G) and the inner diameters of the myelin (D and H) were quantified based on images of β‐tubulin and MBP staining, respectively (n = 200 cells from 5 mice in each group), and were compared in medium‐injected control and MSC‐CM‐treated SCA1‐KI mice. The error bars represent the SEM. Scale bars: 10 μm. ***P < 0.001.
Quantitative analysis showed that the MSC‐CM‐treated SCA1‐KI mice exhibited larger axons and myelin (3.24 ± 0.05 and 3.51 ± 0.06 μm, axons and myelin, respectively) than the medium‐injected control SCA1‐KI mice (2.61 ± 0.05 and 2.86 ± 0.06 μm, axons and myelin, respectively) (t‐tests, both Ps < 0.001; Figure 4, B–D).
Likewise, 5 intravenous injections of SCA1‐KI mice with MSC‐CM from 4–12 weeks of age (Figure 4E) resulted in larger axons and myelin at 13 weeks of age (3.18 ± 0.05 and 3.47 ± 0.05 μm, axons and myelin, respectively) than in the control SCA1‐KI mice (2.52 ± 0.04 and 2.97 ± 0.06 μm, axons and myelin, respectively) (t‐tests, both Ps < 0.001) (Figure 4, F–H).
The morphological improvement observed in the animals that received MSC‐CM might lead to behavioral recovery. We conducted a rotarod test to examine possible attenuation of the motor incoordination seen in SCA1‐KI mice. A single intrathecal injection of MSC‐CM at 4 weeks of age resulted in significantly better performance than that of control SCA1‐KI mice at 13 weeks of age; naïve and medium‐injected control SCA1‐KI mice remained on the rod for approximately 120 second, whereas MSC‐CM‐injected SCA1‐KI mice remained on the rod for approximately 160 second (ANOVA followed by Tukey's post‐hoc test, *P < 0.05) (Figure 5A). The performance of the medium‐injected control mice did not differ from that of naïve mice. Essentially the same result was obtained with mice given intravenous injections of MSC‐CM every 2 weeks between 4 and 12 weeks of age. However, this repetitive procedure aggravated the rotarod performance at 13 weeks of age, as shown by the fact that the time spent on the rod by the medium‐injected control group was significantly shorter than that of untreated naïve SCA1‐KI mice (ANOVA followed by Tukey's post‐hoc test, *P < 0.05) (Figure 5B). Under this condition, MSC‐CM injection markedly prolonged the time for which the animals remained on the rod; medium‐injected control SCA1‐KI mice stayed on the rod for approximately 60 second, whereas MSC‐CM‐injected SCA1‐KI mice stayed on the rod for approximately 170 second (ANOVA followed by Tukey's post‐hoc test, ***P < 0.001) (Figure 5B).
Figure 5.
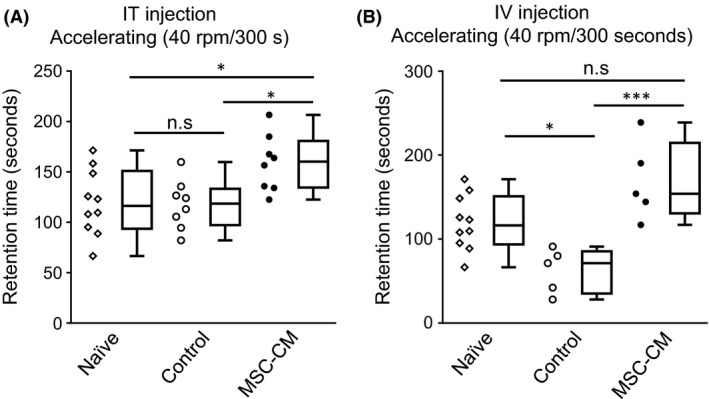
Intrathecal or intravenous injection of MSC‐CM attenuates motor incoordination in SCA1‐KI mice. Performance on the accelerating rotarod test at 13 weeks of age of naïve mice (n = 10) and mice given intrathecal (IT) (n = 8, each) (A) or intravenous (IV) (n = 5, each) (B) injections of medium (control) or MSC‐CM is shown. The schedule of injections was the same as in Figure 4, A and E. Individual values are plotted; box plots including smallest, lower quartile, median, upper quartile, and largest values are also shown. *P < 0.05; ***P < 0.001. n.s., not significant.
Taken together, the results show that both intrathecal and intravenous injection of MSC‐CM attenuated degeneration and motor incoordination in SCA1‐KI mice, although repetitive intravenous injections of MSC‐CM compromised the animals' rotarod performance.
Discussion
The causative molecule of SCA1 is ATXN1 with an expanded polyglutamine tract 1, which forms nuclear aggregates that result in neurodegeneration. The use of MSCs in SCA patients has been reported in 2 previous studies. A 2011 study described 14 SCA patients who were treated with at least 4 weekly intrathecal injections of umbilical cord MSCs (UCMSCs) 24. In a 2013 study in which SCA patients were treated with UCMSCs, sixteen genomically diagnosed SCA patients, including 5 cases of SCA1, received combined intravenous and intrathecal infusion of UCMSCs 25. Each patient received four consecutive UCMSC treatments at 1‐week intervals; the first treatment involved an intravenous injection, whereas the following 3 treatments comprised both intravenous and intrathecal injections. These two studies demonstrated that UCMSCs improved the patients' ability to move and the quality of their daily lives for at least several months, and serious side effects were not observed. However, because introduction of MSCs into the medullary cavity might induce unpredicted adverse effects, it would be better if the therapeutic effects could be achieved without infusing MSCs. Therefore, in this study, we administered MSC‐CM to SCA1‐KI mice. The animals that received MSC‐CM displayed marked attenuation of neuronal degeneration and of motor incoordination. This result indicates the potential of an MSC‐based cell‐free therapeutic approach to the treatment of SCA1.
With respect to the route of administration, the use of intrathecal injections of MSCs in the SCA patients in the aforementioned studies justified our strategy of utilizing intrathecal injection of MSC‐CM as the first method of administration. In the present study, we performed single intrathecal injections of MSC‐CM into 4‐week‐old SCA1‐KI mice, a time at which no apparent neurodegenerative signs or symptoms were observed. In addition to the intrathecal injection, we also performed multiple intravenous injections of MSC‐CM as accessory administrations after the appearance of neurodegenerative signs and symptoms. Specifically, the first intrathecal injection was followed by multiple intravenous injections every 2 weeks from 6 to 24 weeks of age. For the 6‐ and 8‐week‐old mice, an intravenous injection volume of 100 μL was used. However, we used a smaller volume (25 μL) of MSC‐CM in the 10‐ to 24‐week‐old mice because some mice did not survive after multiple intravenous injections of 100 μL in our preliminary experiment, probably due to fluid overload in their circulatory systems. With the injection protocol detailed above, all mice survived during the injection period, and the morphological and functional defects normally observed in the spinal motor neurons of SCA1‐KI mice were attenuated. We also compared the therapeutic effect of intrathecal injection alone at 4 weeks of age with that of multiple intravenous injections from 4–12 weeks of age. Both routes of administration proved effective; however, multiple intravenous injections of even control medium resulted in significantly worse rotarod performance in SCA1‐KI mice. Thus, less‐invasive single intrathecal injection might be safer than multiple intravenous injections and should be considered a possible therapeutic intervention for SCA patients in future.
Although it is not easy to explore the mechanisms by which MSCs exert their therapeutic effects in humans, studies using animal models of neuronal disorders provide insight into these mechanisms. In a study using mice, MSC‐CM was found to reduce the functional deficits in a mouse model of multiple sclerosis and to promote the development of oligodendrocytes and neurons 26. Moreover, intrathecal administration of CM from MSCs obtained from human deciduous dental pulp (SHEDs) into injured rat spinal cords during the acute postinjury period produced remarkable functional recovery 27. These two studies suggest that factors that are released from MSCs act on neurons via a paracrine‐mediated mechanism to exert therapeutic effects that improve symptoms of multiple sclerosis and spinal cord injury, at least in mouse models. Therefore, we tested whether this was also the case for the degeneration of the spinal neurons and myelin that occurs in SCA1‐KI mice.
Our current study extends the list of diseases for which MSCs can effectively ameliorate neuronal abnormalities. Both axons and myelin degenerate in old SCA1‐KI mice 23. We recently showed that intrathecal injection of MSCs relieves the degeneration of axons and myelin in SCA1‐Tg mice 16. In the current study, we reproduced these morphological improvements using MSC‐CM rather than MSCs themselves. The reduced nerve conduction velocities of the spinal motor neurons and motor incoordination were also ameliorated by MSC‐CM. Thus, factor(s) released from the MSCs suppressed the degeneration of the spinal neurons and myelin through a paracrine‐mediated mechanism in SCA1‐KI mice. Because MSC‐CM produces therapeutic effects in multiple disease models, it does not appear likely that CM acts directly on ATXN1; rather, it appears to nonspecifically prevent neuronal degeneration by providing trophic factors.
Regarding the specific factors that are released from MSCs and have been shown to reduce the symptoms of disease in previous animal studies, hepatocyte growth factor (HGF) and its primary receptor cMet have been found to be critical to MSC‐stimulated recovery in a mouse model of multiple sclerosis, neural cell development, and re‐myelination 26. We detected a significant amount of HGF in KUM10 MSC‐CM by ELISA (N. Suto, K. Nakamura & H. Hirai, unpublished data), and intrathecal injection of SCA1‐KI mice with HGF at 4 weeks of age attenuated degeneration of both axons and myelin and motor incoordination at 13 weeks of age compared to naïve SCA1‐KI mice (N. Suto, K. Nakamura & H. Hirai,unpublished data). Interestingly, the PC degeneration in the cerebellum and poor rotarod performance of SCA7‐knock‐in (SCA7‐KI) mice were attenuated in the SCA7‐KI/HGF‐transgenic mice. In SCA7‐KI mice, expression levels of glutamate/aspartate transporter (GLAST) and glutamate transporter‐1 (GLT‐1) in the cerebellar cortex were significantly decreased, resulting in PC excitotoxicity 28. The expression levels of GLAST and GLT‐1 were maintained in the SCA7‐KI/HGF‐transgenic mice, suggesting suppression of the excitotoxicity and consequent degeneration of PCs 28. GLAST and GLT‐1 expressions were also reduced in the cerebellum of SCA1‐KI mice 29. As GLAST and GLT‐1 are also found in the spinal cord 30, HGF may increase the glutamate transporter levels also in the spinal cord of SCA1‐KI mice, thereby removing glutamate from the extracellular fluid and eventually attenuating degeneration of the spinal cord. Alternatively, HGF could exert beneficial influence on the spinal motor neurons by enhancing the angiogenesis as infusion of recombinant vascular endothelial growth factor (VEGF) ameliorated the ataxia and PC degeneration in SCA1‐KI mice 31, and HGF was shown to promote angiogenesis in various disease models 32. We are currently testing whether neutralizing antibody to HGF abolishes the therapeutic effects of MSC‐CM in SCA1‐KI mice.
Conflict of Interest
There are no potential conflict of interests related to the content of this paper.
Acknowledgments
We thank Junko Sugiyama for maintaining and genotyping the SCA1‐KI mice. This work was supported by MEXT KAKENHI Grant Number 26111701 and 15H04354, grants from Research on Measures for Intractable Diseases (Ataxic Diseases and Neurodegenerative Diseases) from the Ministry of Health, Labour and Welfare (to H. Hirai), and grants‐in‐aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to K. Nakamura).
References
- 1. Matilla‐Duenas A, Goold R, Giunti P. Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum 2008;7:106–114. [DOI] [PubMed] [Google Scholar]
- 2. Robitaille Y, Schut L, Kish SJ. Structural and immunocytochemical features of olivopontocerebellar atrophy caused by the spinocerebellar ataxia type 1 (SCA‐1) mutation define a unique phenotype. Acta Neuropathol 1995;90:572–581. [DOI] [PubMed] [Google Scholar]
- 3. Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet 1983;1:1151–1155. [DOI] [PubMed] [Google Scholar]
- 4. Baddoo M, Hill K, Wilkinson R, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 2003;89:1235–1249. [DOI] [PubMed] [Google Scholar]
- 5. Gimble J, Guilak F. Adipose‐derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003;5:362–369. [DOI] [PubMed] [Google Scholar]
- 6. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004;103:1669–1675. [DOI] [PubMed] [Google Scholar]
- 7. Mazzini L, Ferrero I, Luparello V, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol 2010;223:229–237. [DOI] [PubMed] [Google Scholar]
- 8. Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res 2003;73:778–786. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 2005;49:407–417. [DOI] [PubMed] [Google Scholar]
- 10. Chang YK, Chen MH, Chiang YH, et al. Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells. J Biomed Sci 2011;18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsuura S, Shuvaev AN, Iizuka A, Nakamura K, Hirai H. Mesenchymal stem cells ameliorate cerebellar pathology in a mouse model of spinocerebellar ataxia type 1. Cerebellum 2014;13:323–330. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura K, Mieda T, Suto N, Matsuura S, Hirai H. Mesenchymal stem cells as a potential therapeutic tool for spinocerebellar ataxia. Cerebellum 2015;14:165–170. [DOI] [PubMed] [Google Scholar]
- 13. Burright EN, Clark HB, Servadio A, et al. SCA1 transgenic mice: A model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 1995;82:937–948. [DOI] [PubMed] [Google Scholar]
- 14. Clark HB, Burright EN, Yunis WS, et al. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci 1997;17:7385–7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watase K, Weeber EJ, Xu B, et al. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron 2002;34:905–919. [DOI] [PubMed] [Google Scholar]
- 16. Mieda T, Suto N, Iizuka A, et al. Mesenchymal stem cells attenuate peripheral neuronal degeneration in spinocerebellar ataxia type 1 knockin mice. J Neurosci Res 2016;94:246–252. [DOI] [PubMed] [Google Scholar]
- 17. Lagasse E, Connors H, Al‐Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000;6:1229–1234. [DOI] [PubMed] [Google Scholar]
- 18. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 19. Woodbury D, Reynolds K, Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res 2002;69:908–917. [DOI] [PubMed] [Google Scholar]
- 20. Olson SD, Pollock K, Kambal A, et al. Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington's disease. Mol Neurobiol 2012;45:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takechi Y, Mieda T, Iizuka A, et al. Impairment of spinal motor neurons in spinocerebellar ataxia type 1‐knock‐in mice. Neurosci Lett 2013;535:67‐72. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura K, Kosugi I, Lee DY, et al. Prolyl isomerase Pin1 regulates neuronal differentiation via beta‐catenin. Mol Cell Biol 2012;32:2966–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anbari F, Khalili MA, Bahrami AR, et al. Intravenous transplantation of bone marrow mesenchymal stem cells promotes neural regeneration after traumatic brain injury. Neural Regen Res 2014;9:919‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dongmei H, Jing L, Mei X, et al. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy‐cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy 2011;13:913–917. [DOI] [PubMed] [Google Scholar]
- 25. Jin JL, Liu Z, Lu ZJ, et al. Safety and efficacy of umbilical cord mesenchymal stem cell therapy in hereditary spinocerebellar ataxia. Curr Neurovasc Res 2013;10:11–20. [DOI] [PubMed] [Google Scholar]
- 26. Bai L, Lennon DP, Caplan AI, et al. Hepatocyte growth factor mediates mesenchymal stem cell‐induced recovery in multiple sclerosis models. Nat Neurosci 2012;15:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsubara K, Matsushita Y, Sakai K, et al. Secreted ectodomain of sialic acid‐binding Ig‐like lectin‐9 and monocyte chemoattractant protein‐1 promote recovery after rat spinal cord injury by altering macrophage polarity. J Neurosci 2015;35:2452–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noma S, Ohya‐Shimada W, Kanai M, Ueda K, Nakamura T, Funakoshi H. Overexpression of HGF attenuates the degeneration of Purkinje cells and Bergmann glia in a knockin mouse model of spinocerebellar ataxia type 7. Neurosci Res 2012;73:115–121. [DOI] [PubMed] [Google Scholar]
- 29. Shiwaku H, Yoshimura N, Tamura T, et al. Suppression of the novel ER protein Maxer by mutant ataxin‐1 in Bergman glia contributes to non‐cell‐autonomous toxicity. EMBO J 2010;29:2446–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeng J, Cui LY, Feng Y, Ding MX. Electroacupuncture relieves neuropathic pain via upregulation of glutamate transporters in the spinal cord of rats. Neurosci Lett 2016;620:38–42. [DOI] [PubMed] [Google Scholar]
- 31. Cvetanovic M, Patel JM, Marti HH, Kini AR, Opal P. Vascular endothelial growth factor ameliorates the ataxic phenotype in a mouse model of spinocerebellar ataxia type 1. Nat Med 2011;17:1445–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Funakoshi H, Nakamura T. Hepatocyte growth factor (HGF): Neurotrophic functions and therapeutic implications for neuronal injury/diseases. Curr Signal Transduct Ther 2011;6:156–167. [Google Scholar]


