Summary
Aims
We aim to explore whether people with epilepsy have increased white matter hyperintensities (WMHs).
Methods
Eligible patients were categorized into newly diagnosed epilepsy (NE) and chronic epilepsy (CE); the latter were subdivided to those treated with enzyme‐inducing antiepileptic drugs (EIAEDs) with or without non‐enzyme‐inducing antiepileptic drugs (NEIAEDs) and those with NEIAEDs only. WMHs were measured using age‐related white matter changes (ARWMC) scale and compared between patients and healthy control group. Higher scores indicate greater WMH changes. The strengths of associations were estimated as incidence rate ratios (IRRs) with 95% confidence interval (CI).
Results
A total of 217 patients were included in the analysis, of whom 67 had NE, 45 had CE treated with NEIAEDs, and 105 had CE treated with EIAEDs. Age was positively associated with ARWMC score (IRR per year, 1.03; 95%CI, 1.03–1.04, P < 0.001). Compared with the healthy control group (n = 23), all patient groups had higher ARWMC score (P < 0.05). The difference was greatest in patients receiving EIAEDs (IRR, 2.13; 95%CI, 1.22–3.70, P = 0.007).
Conclusions
WMHs tended to be observed in people with epilepsy, especially in those treated with EIAEDs. People with epilepsy with white matter changes should be evaluated for stroke risk, particularly if they are receiving EIAEDs.
Keywords: Antiepileptic drugs, Epilepsy, Magnetic resonance imaging, Stroke, White matter hyperintensities
Introduction
Population studies suggest that people with epilepsy are more likely to have subsequent stroke than the healthy population 1, 2. The reasons for this are unclear. The risk appears to be higher in patients on higher doses of antiepileptic drugs (AEDs) 2. Treatment with AEDs that induce the cytochrome P450 enzyme system (enzyme‐inducing AEDs; EIAEDs) is associated with increased risk markers of atherosclerosis. Hyperlipidemia, increased serum homocysteine and C‐reactive protein, reduced level of folate, vitamin B12, and increased carotid artery intima media thickness 3, 4, 5, all potentially account for the increased stroke risk 6, 7. Conversely, valproate (VPA), an enzyme inhibitor, may decrease adverse vascular events 6, 8.
White matter hyperintensities (WMHs),detected on T2‐weighted Fluid‐Attenuated Inversion Recovery (FLAIR) sequence on brain magnetic resonance imaging (MRI),are presumed to be the result of chronic hypoperfusion of the white matter and disruption of the blood–brain barrier, leading to chronic leakage of plasma into the white matter 9, 10. They are more commonly seen in the elderly and in people with hypertension and other vascular risk factors include smoking, diabetes, and a history of vascular disease 9, 11. WMHs are a recognized biomarker of cerebrovascular disease and are associated with 3‐fold increase in risk of subsequent stroke and 2‐fold increase in mortality 11. Therefore, WMHs may be used as a surrogate marker for stroke risk.
We examined the burden of WMHs on brain MRI in people with epilepsy. We hypothesized that people with epilepsy have increased WMHs, and exposure to EIAEDs is associated with more extensive WMHs.
Materials and Methods
Study Population
This was a retrospective study. The study was approved by the hospital's Human Research Ethics Committee. Subjects were those patients investigated and treated for epileptic seizures at the Royal Melbourne Hospital (RMH) between 1st August 2009 and 31st July 2013. Eligible patients were consecutively identified from electroencephalography (EEG) laboratory and video EEG monitoring unit. Controls were individuals referred from primary care physicians to the neurology clinics for suspected neurological diseases, but in whom a CNS disorder was excluded after thorough assessment. Individuals with documented psychiatric disorders were also excluded. Both epilepsy group and control group had undergone 3T brain MRI using the same protocol 12. Patients were excluded if the seizures were non‐epileptic, for example, psychogenic or other non‐epileptic etiologies (e.g., syncope), or if the patients had a history of stroke or transient ischemic attack, multiple sclerosis, MRI changes of bi‐hemispheric brain injuries (due to trauma, tumor or surgical procedures), or a poor quality MRI (e.g., motion artifact).
Baseline information, including demographics, vascular risk factors (diabetes, smoking, alcohol, hypertension, dyslipidemia, migraine, atrial fibrillation), epilepsy risk factors (head injury, febrile convulsion, head surgery), length of time from the index seizure to MRI, and seizure types (focal onset or generalized onset) 13, was obtained by a thorough review of the hospital medical records. Specifically, age was defined as years from birth to date of eligible MRI. In cases where the hospital records were insufficient, further information was obtained from the primary care physicians.
AED Treatment
Information on AED, including drug name, initial dosage, duration of each drug, and reason for stopping or switching, was obtained from medical records. AEDs were grouped according to whether they induce the cytochrome P450 (CYP) family of enzymes 14. EIAEDs included carbamazepine (CBZ), phenytoin, oxcarbazepine (OXC), phenobarbital, and primidone; non‐enzyme‐inducing AEDs (NEIAEDs) were clobazam, clonazepam, diazepam, ethosuximide, gabapentin, lacosamide, lamotrigine, levetiracetam, topiramate, valproic acid, and zonisamide. Intermittent time lengths of EIAED and NEIAED treatment were separately accumulated during the period from commencement of AED therapy to date of the MRI.
Subject Categorization
Eligible subjects were categorized into three groups (Figure 1): (1) people with newly diagnosed epilepsy who had been treated with AEDs for <3 months by the date of the MRI; (2) EIAED group, in whom patients with chronic epilepsy, ever receiving either NEIAEDs or not, were treated with EIAEDs for at least 3 months; and (3) NEIAED group, in whom patients received NEIAEDs for at least 3 months, with EIAEDs for <3 months or without EIAEDs. The duration of 3 months was chosen empirically because shorter exposure has been considered as inadequate trial for AEDs 15.
Figure 1.
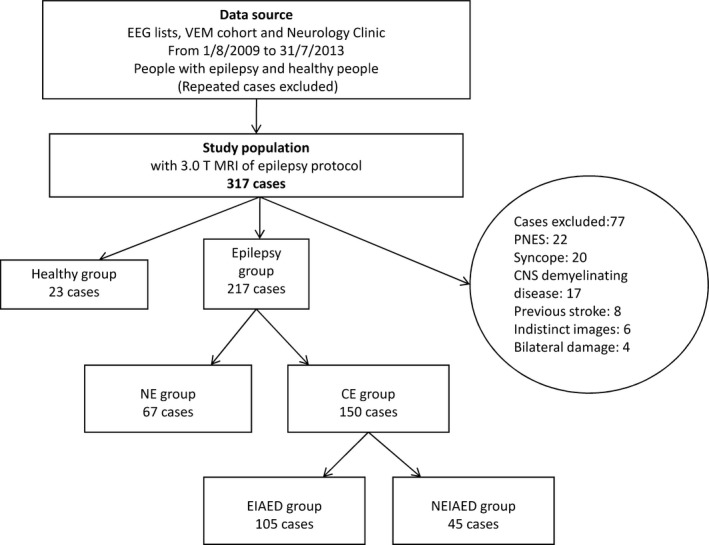
Flowcharts of Patient Inclusion and Exclusion. EEG, electroencephalogram; MRI, magnetic resonance imaging; PNES, psychogenic non‐epileptic seizures; MS, multiple sclerosis; NE, newly diagnosed epilepsy; CE, chronic epilepsy; EIAED, patients with chronic epilepsy receiving enzyme‐inducing antiepileptic drug(s); NEIAED, patients with chronic epilepsy receiving non‐enzyme‐inducing antiepileptic drug(s).
MRI Assessment
Our 3.0 T MRI (Siemens Magnetom Trio, Germany) of epilepsy protocol includes axial FLAIR, T2‐weighted, and T1‐weighted imaging, where slice thickness is 1 mm 12. This protocol fulfills recommended standards and parameters for imaging of small vessel disease 9. WMH was assessed on T2‐weighted FLAIR sequence acquired with the following parameters: repetition time, 5000 ms; echo time, 388 ms; inversion time, 1.8 s; matrix, 258 × 256; flip angle, 120°; scan time, 5 min and 52 s.WMH was defined as high signal intensity in the white matter, basal ganglia, and brainstem depicted on FLAIR without cavitation. WMH of nonvascular origin, such as multiple sclerosis, was excluded based on the combination of clinical syndrome and the pattern of WMH. If a patient had undergone more than one MRI, the most recent set of eligible images was assessed.
White matter hyperintensities were rated semiquantitatively using the age‐related white matter changes (ARWMC) scale 16. The validity of this scale has been demonstrated in clinical practice 16, 17, providing fast and standardized semiquantitative information 18. In this visual rating scale, five regions (frontal, parietal–occipital, temporal, basal ganglia, and infratentorial) of the two hemispheres were rated separately (0: no lesions, 1: focal lesion, 2: beginning confluence of lesions, 3: diffuse changes), followed by summation of scores from each region. Examples of rating score are shown in Figure S1. To evaluate the interrater reliability of the ARWMC scale, all MRI images randomly selected for age‐stratified patients were rated by BY and PK, who are all experienced board‐certified neurologists. The interrater agreement was estimated using interclass correlation coefficients (ICC), with ICC above 0.8 signifying an excellent agreement. All MRI assessment was performed blind to the clinical information.
Statistical Analysis
Continuous variables were summarized as medians and interquartile ranges (IQR) and tested by Mann–Whitney U‐test or Kruskal–Wallis rank test where applicable. Categorical variables were summarized as percentages and were evaluated using Fisher's exact test where applicable. ARWMC score was treated as count variable. The relationships between potential factors and ARWMC score were analyzed using the negative binomial regression model 19, which held the best goodness‐of‐fit in our data among various count models. The strengths of associations were estimated as incidence rate ratios (IRRs) with corresponding 95% confidence interval (CI). For instance, the value of IRRs = 1.5 could be interpreted that per one unit increase in the independent variable, there will be an increase in the ARWMC score by the factor of 1.5. In the multivariate analysis, backwards elimination was used to eliminate variable one by one until all variables achieved a level of conventional significance. Potential factors that were significant at P < 0.20 in the initial univariate analyses were included as candidates into the multivariate model 20. Significance level was set at alpha of 0.05, and all tests were 2‐tailed. Statistical analysis was performed with the Stata/SE 12.0 statistical package (StataCorp LP, College Station, TX, USA).
Results
Subject Characteristics
A total of 317 individuals who had undergone 3T brain MRI of epilepsy protocol were eligible for the study. Of 317, 240 (75.7%) were included for analysis and 77 (24.3%) were excluded (Figure 1). The groups were comparable in demographics, seizure history, and vascular risk factors (Table S1). Of 240, 217 had epilepsy (67 with newly diagnosed epilepsy, 45 with chronic epilepsy receiving NEIAEDs, and 105 with chronic epilepsy receiving EIAEDs); 23 were included in the control group. Their clinical characteristics are summarized in Table 1. The epilepsy group and control group were comparable in demographical and vascular risk factors, except for a higher proportion of patients in the epilepsy group being smokers and alcohol abusers compared with the controls (22.1% vs. 0, P = 0.006; 30.9% vs. 8.7%, P = 0.028; respectively). Not surprisingly higher proportion of patients receiving EIAEDs had focal seizures than generalized seizures compared with other groups, given that CBZ and PHT, both EIAEDs, are first‐line treatments for focal seizures.
Table 1.
Characteristics of patients with epilepsy and controls
| Control (n = 23) | Epilepsy | P a | ||||
|---|---|---|---|---|---|---|
| NE (n = 67) | NEIAED (n = 45) | EIAED (n = 105) | Total (n = 217) | |||
| Age, median (IQR), year | 42 (24–50) | 36 (24–53) | 37 (27–50) | 35 (27–48) | 36 (26–50) | 0.89 |
| Female, n (%) | 12 (52.2) | 35 (52.2) | 23 (51.1) | 53 (50.5) | 111 (51.2) | >0.99 |
| Focal seizures, n (%) | 0 (0) | 38 (56.7) | 34 (75.6) | 90 (85.7) | 162 (74.7) | NA |
| Generalized seizures, n (%) | 0 (0) | 29 (43.3) | 11 (24.4) | 15 (14.3) | 55 (25.3) | NA |
| Diabetes, n (%) | 0 (0) | 4 (6.0) | 2 (4.4) | 7 (6.7) | 13 (6.0) | 0.62 |
| Smoker, n (%) | 0 (0) | 9 (13.4) | 13 (28.9) | 26 (24.8) | 48 (22.1) | 0.006 |
| Alcohol, n (%) | 2 (8.7) | 25 (37.3) | 13 (28.9) | 29 (27.6) | 67 (30.9) | 0.028 |
| Hypertension, n (%) | 2 (8.7) | 10 (14.9) | 2 (4.4) | 11 (10.5) | 23 (10.6) | >0.99 |
| Dyslipidemia, n (%) | 2 (8.7) | 6 (9.0) | 3(6.7) | 11 (10.5) | 20 (9.2) | >0.99 |
| Migraine, n (%) | 1 (4.4) | 1 (1.5) | 2 (4.4) | 12 (11.4) | 15 (6.9) | >0.99 |
| Atrial fibrillation, n (%) | 1 (4.4) | 2 (3.0) | 0 (0) | 4 (3.8) | 6 (2.8) | 0.51 |
| Head injury, n (%) | 1 (4.4) | 15 (22.4) | 9 (20.0) | 25 (23.8) | 49 (22.6) | 0.055 |
| Febrile convulsion, n (%) | 1 (4.4) | 2 (3.0) | 2 (4.4) | 8 (7.6) | 12 (5.5) | >0.99 |
| Head surgery, n (%) | 0 (0) | 0 (0) | 4 (8.9) | 18 (17.1) | 22 (10.1) | 0.14 |
| Time from first seizure, median (IQR), month | NA | 2.7 (1.3–10.6) | 75.9 (28.7–240.2) | 146.6 (51.3–310.7) | 56.8 (7.2–211.3) | NA |
| AED duration, median (IQR), month | 0 | 0 (0–1.4) | 46.7 (21.0–165.7) | 109.2 (42.2–283.2) | 34.6 (1.8–163.6) | NA |
| EIAED, median (IQR), month | 0 | 0 (0–0) | 0 (0–0) | 94.4 (26.6–275.5) | 2.3(0–80.4) | NA |
| NEIAED, median (IQR), month | 0 | 0 (0–0.2) | 46.7 (21.0–165.7) | 23.9 (0–111.6) | 3.5(0–74.0) | NA |
ARWMC, age‐related white matter changes scale; NE, patients with newly diagnosed epilepsy; AED, antiepileptic drug; EIAED, patients with enzyme‐inducing AEDs; NEIAED, patients with non‐enzyme‐inducing AEDs; IQR, interquartile range; NA, not available. a P value was calculated from the comparison between control group and entire epilepsy group.
Of 150 patients in chronic epilepsy group, CBZ and VPA were the most frequently used EIAED and NEIAED, in 88 (58.7%) and 69 (46.0%) patients, respectively (Table S2); 99 (72.3%) received more than one drug till the time of MRIs. Time from index seizure to MRI and duration of AED treatment were both longer in EIAED group than that in NEIAED group (median time: 146.6 vs. 75.9 months, P = 0.02; median duration: 109.2 vs. 46.7 months, P = 0.009; respectively). Of 67 patients with newly diagnosed epilepsy, 38 (56.7%) had not been commenced on AED therapy and 29 (43.3%) had been treated for <3 months by the time of MRI (median 1.6 [IQR, 1.1–2.2] months).
ARWMC Score
An excellent interrater agreement of the ARWMC score was observed (ICC, 0.94; 95% CI, 0.93–0.95). The median ARWMC score in the chronic epilepsy group was 1 (IQR: 0–3), which was higher than that in control group (0, IQR: 0–2, P = 0.029). Table S3 shows the regional ARWMC scores in the patient subgroups. Compared to the control group, WMHs (ARWMC ≥1) were detected in significantly higher proportions of patients in the chronic epilepsy group (43.5% vs. 66.4%, P = 0.039). Figure 2 shows the distribution of ARWMC scores among the four groups. Univariate analysis showed that age, diabetes, hypertension, dyslipidemia, and atrial fibrillation were associated with ARWMC score (Table 2). Among these covariates, only age remained significant in the multivariate model (IRR per year, 1.03; 95%CI, 1.03–1.04, P < 0.001). There was no significant association between ARWMC score and seizure type, duration of treatment, or time from first seizure.
Figure 2.
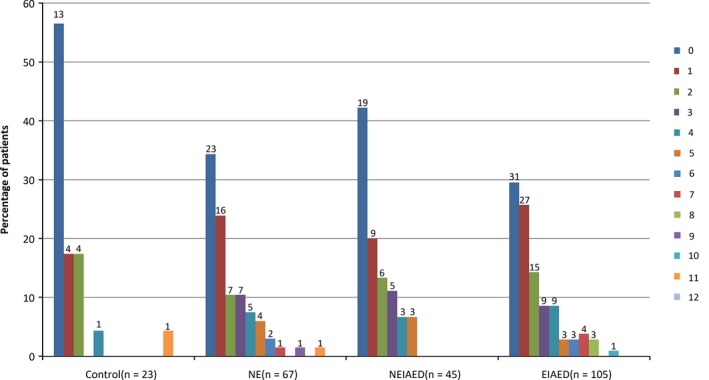
Distribution of ARWMC scores in patient groups. Each column represents the percentage of patients with the specified total ARWMC score, which ranges from 0 to 12. The number above each column indicates the number of patients with the specific ARWMC score. ARWMC, age‐related white matter changes scale; NE, patients with newly diagnosed epilepsy; EIAED, patients with chronic epilepsy receiving enzyme‐inducing antiepileptic drug(s); NEIAED, patients with chronic epilepsy receiving non‐enzyme‐inducing antiepileptic drug(s).
Table 2.
Relationships between risk factors and ARWMC score (univariate analysis)
| Risk factors | ARWMC score | ||
|---|---|---|---|
| IRR | 95%CI | P | |
| Age | 1.03 | 1.02–1.04 | <0.001 |
| Female | 0.96 | 0.70–1.30 | 0.77 |
| Diabetes | 1.53 | 0.89–2.62 | 0.13 |
| Smoker | 1.04 | 0.71–1.53 | 0.83 |
| Alcohol | 1.02 | 0.71–1.47 | 0.91 |
| Hypertension | 2.37 | 1.64–3.43 | <0.001 |
| Dyslipidemia | 2.73 | 1.87–3.99 | <0.001 |
| Migraine | 1.19 | 0.61–2.31 | 0.61 |
| Atrial fibrillation | 2.73 | 1.48–5.05 | 0.001 |
| Head injury | 1.08 | 0.77–1.51 | 0.67 |
| Febrile convulsion | 0.93 | 0.56–1.54 | 0.78 |
| Head surgery | 0.92 | 0.55–1.54 | 0.76 |
| Time from first seizure(month) | 1.00 | 1.00–1.00 | 0.09 |
| AED duration(month) | 1.00 | 1.00–1.00 | 0.17 |
| EIAED duration | 1.00 | 1.00–1.00 | 0.048 |
| NEIAED duration | 1.00 | 1.00–1.00 | 0.39 |
ARWMC, age‐related white matter changes; IRR, incidence rate ratio; CI, confidence interval; AED, antiepileptic drug; EIAED, enzyme‐inducing antiepileptic drug; NEIAED, non‐enzyme‐inducing antiepileptic drug.
After adjusting for age, patients with epilepsy remained to have significantly higher ARWMC score compared to the control group (IRR, 1.93; 95%CI, 1.13–3.30, P = 0.016; Figure 3). Compared to control group, the score was higher in those with chronic epilepsy receiving EIAEDs (IRR, 2.13; 95%CI, 1.22–3.70, P = 0.007) and in NE patients (IRR, 1.79; 95%CI, 1.01–3.16, P = 0.046), and trended higher in those who had received NEIAEDs only (IRR, 1.65; 95%CI, 0.88–3.10, P = 0.12).
Figure 3.
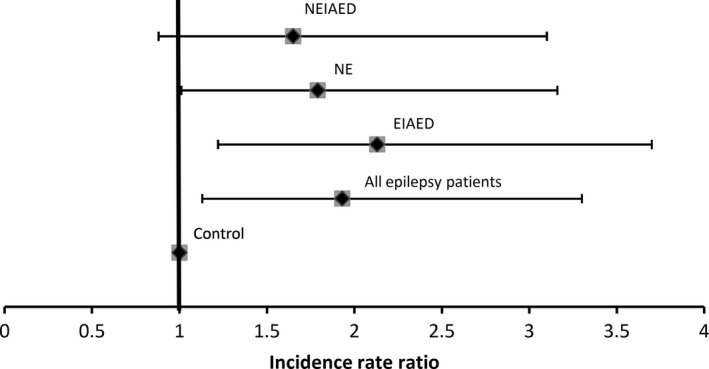
Incidence rate ratio of ARWMC scores in patients with newly diagnosed epilepsy, chronic epilepsy who had received enzyme‐inducing antiepileptic drugs and only non‐enzyme‐inducing antiepileptic drugs, compared to controls. ARWMC, age‐related white matter changes scale; NE, patients with newly diagnosed epilepsy; EIAED, patients with chronic epilepsy receiving enzyme‐inducing antiepileptic drug(s); NEIAED, patients with chronic epilepsy receiving non‐enzyme‐inducing antiepileptic drug(s).
Discussion
We found significant increase in WMHs among people with epilepsy, especially in those treated with EIAEDs. To our best knowledge, this is the first study demonstrating an association between EIAEDs and WMHs on MRI brain in people with epilepsy. This finding is consistent with the accumulating evidence suggesting that EIAEDs may pose deleterious effects on arterial vasculature 27. Several studies demonstrated elevated levels of total cholesterol, low‐density lipoprotein cholesterol and triglycerides among patients treated with EIAEDs 5, 21, 22. It has been proposed that CYP induction may reduce the levels of oxysterol intermediates, leading to reduced feedback inhibition of hydroxymethylglutaryl‐coenzyme A reductase on cholesterol production 14, 23. In addition, elevation of lipoprotein(a) 24, C‐reactive protein 25 and homocysteine 4, and increased intima media thickness(IMT) of the common carotid artery(CCA) have been observed in EIAEDs users 5, 25. Conversion of carbamazepine or phenytoin (both EIAEDs) to NEIAEDs, such as lamotrigine and levetiracetam, has been shown to reduce homocysteine levels 3.
The risk of stroke associated with exposure to valproic acid (an enzyme inhibitor) remains controversial. While valproic acid may inhibit atherosclerosis by increasing resistance of the endoplasmic reticulum 26, 27, it also increases insulin resistance and the risk of metabolic syndrome 28, 29, possibly leading to accelerated atherosclerosis 25. In our study, we found a trend of higher prevalence of WMHs in people with epilepsy receiving NEIAEDs, of which valproic acid was the most common agent. This supports the notion that valproic acid may also increase atherosclerotic burden.
We also found increased WMHs in newly diagnosed patients, implying that seizures or the epileptogenic process per se may also play a role. It may be postulated that white matter changes on MRI represents cerebrovascular endothelial dysfunction due to increased blood–brain barrier permeability after epileptic discharges 30. The existence of white matter tract injuries has also been demonstrated by MRI studies in these patients 31, 32. In addition, several animal models have demonstrated increased oxidative stress in neural cells after seizures, especially in mitochondria, leading to subsequent cell damage 33, 34, 35. Oxidative stress is recognized to play an important role in atherosclerosis development 36. Therefore, recurrent seizures may also contribute to atherosclerosis in patients with epilepsy 37.
White matter hyperintensities is recognized as a reliable predictor of stroke events, cognitive impairment, and mortality. A systematic review including 22 longitudinal studies demonstrated that WMHs were associated with substantially increased risks of stroke (hazard ratio 3.3, 95% CI 2.6–4.4), dementia (hazard ratio 1.9), and premature death (hazard ratio 2.0, 1.6–2.7) 11. The observed increase of WMH among patients with epilepsy might, therefore, explain their increased prevalence of stroke risk, dementia, and mortality. WMHs might be considered as a useful tool to predict risk of comorbidities or outcomes in people with epilepsy.
Previous studies showed that duration of AED treatment (PHT, CBZ, and VPA) was associated with increased CCA IMT, which is a marker of atherosclerosis 25. Our study did not find any relationship between AED duration and WMHs, which may be due to the heterogeneous treatment regimens. This study reinforced the previous finding that age is a major predictor of WMHs 11, 38. The prevalence of WMH in the general population has been reported to be 11–21% in people aged 64, rising to 94% in 82‐year‐olds using 1.5 T or lower MRIs 11. The median age of the patients included in our study was 36 years (IQR, 26–50). So far, no study has reported WMHs on 3.0 T MRI in the healthy population aged younger than 50. Abnormal ARWMC score was found in 43.5% of control group. This was higher than the prevalence (5.3%) of white matter lesions previously reported in young healthy population (age, 16–65 years; mean, 37 years) 39, possibly due to the increased sensitivity accorded by the high‐field MRI performed in our study. Another possible explanation for the observation is that the controls were not truly “healthy controls” as they were selected from people seen in neurology clinics rather than randomly recruited from the “normal population”.
Our study has limitations. As this was a cross‐sectional analysis of MRI, demonstration of association does not necessarily imply causation. While recurrent seizures and use of EIAEDs might have contributed to WMHs, an alternative interpretation of the data might be that WMHs are a causative factor of epilepsy. For practical purpose, controls were recruited from neurology clinics rather than the general population. However, any selection bias would likely lead to underestimation of the burden of WMHs in the epilepsy patients. Underreporting of smoking and alcohol drinking cannot be ruled out. Classifying AEDs is controversial in the context of vascular risks. OXC, for instance, has intermediate effects on enzyme induction although it does not affect lipids metabolism as CBZ does 40. However, including patients receiving OXC in either EIAED group or NEIAED group is unlikely to affect the results given the small number of patients treated with this drug. In addition, our study lacks information about clinical outcomes, such as stroke events, seizure frequency, and seizure severity postdiagnosis, which should be investigated in future studies.
Conclusion
Compared to controls, WMHs were more prevalent in people with epilepsy, especially in those treated with EIAEDs. These findings shed new light on the potential mechanisms for the increased risk of stroke observed in people with epilepsy. Our findings suggest that people with epilepsy whose MRI showed WMHs may benefit from evaluation of stroke risk, particularly if they are receiving EIAEDs.
Conflicts of Interest
The authors declare no conflict of interest.
Supporting information
Fig S1. Examples of Age‐Related White Matter Changes (ARWMC) scale.
Table S1. Baseline characteristics of study population.
Table S2. Distribution of AEDs in chronic epilepsy cohort (n = 150).
Table S3 ARWMC scores in regions.
Acknowledgments
We would like to thank Dr. Francesco Gaillard for his advice on MRIs of control group.
Dr. Kwan has received research grants from the National Health and Medical Research Council of Australia, Australian Research Council, Hong Kong Research Grants Council, and Health and Medical Research Fund. He/his institution also received speaker or consultancy fees and/or research grants from Eisai, GlaxoSmithKline, and UCB Pharma.
References
- 1. Cleary P, Shorvon S, Tallis R. Late‐onset seizures as a predictor of subsequent stroke. Lancet 2004;363:1184–1186. [DOI] [PubMed] [Google Scholar]
- 2. Chang CS, Liao CH, Lin CC, et al. Patients with epilepsy are at an increased risk of subsequent stroke: a population‐based cohort study. Seizure 2014;23:377–381. [DOI] [PubMed] [Google Scholar]
- 3. Mintzer S, Skidmore CT, Abidin CJ, et al. Effects of antiepileptic drugs on lipids, homocysteine, and C‐reactive protein. Ann Neurol 2009;65:448–456. [DOI] [PubMed] [Google Scholar]
- 4. Linnebank M, Moskau S, Semmler A, et al. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol 2011;69:352–359. [DOI] [PubMed] [Google Scholar]
- 5. Chuang YC, Chuang HY, Lin TK, et al. Effects of long‐term antiepileptic drug monotherapy on vascular risk factors and atherosclerosis. Epilepsia 2012;53:120–128. [DOI] [PubMed] [Google Scholar]
- 6. Olesen JB, Abildstrom SZ, Erdal J, et al. Effects of epilepsy and selected antiepileptic drugs on risk of myocardial infarction, stroke, and death in patients with or without previous stroke: a nationwide cohort study. Pharmacoepidemiol Drug Saf 2011;20:964–971. [DOI] [PubMed] [Google Scholar]
- 7. Hsieh CY, Lai EC, Yang YH, et al. Comparative stroke risk of antiepileptic drugs in patients with epilepsy. Epilepsia 2013;54:172–180. [DOI] [PubMed] [Google Scholar]
- 8. Olesen JB, Hansen PR, Abildstrom SZ, et al. Valproate attenuates the risk of myocardial infarction in patients with epilepsy: a nationwide cohort study. Pharmacoepidemiol Drug Saf 2011;20:146–153. [DOI] [PubMed] [Google Scholar]
- 9. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Heerden J, Desmond PM, Tress BM, et al. Magnetic resonance imaging in adults with epilepsy: a pictorial essay. J Med Imaging Radiat Oncol 2014;58:312–319. [DOI] [PubMed] [Google Scholar]
- 13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–685. [DOI] [PubMed] [Google Scholar]
- 14. Brodie MJ, Mintzer S, Pack AM, et al. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 2013;54:11–27. [DOI] [PubMed] [Google Scholar]
- 15. Hao X, Goldberg D, Kelly K, et al. Uncontrolled epilepsy is not necessarily the same as drug‐resistant epilepsy: differences between populations with newly diagnosed epilepsy and chronic epilepsy. Epilepsy Behav 2013;29:4–6. [DOI] [PubMed] [Google Scholar]
- 16. Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 17. Li L, Simoni M, Kuker W, et al. Population‐based case‐control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke 2013;44:3063–3070. [DOI] [PubMed] [Google Scholar]
- 18. Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis 2002;13(Suppl 2):31–36. [DOI] [PubMed] [Google Scholar]
- 19. Lu HX, Wong MC, Lo EC, et al. Risk indicators of oral health status among young adults aged 18 years analyzed by negative binomial regression. BMC Oral Health 2013;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Budtz‐Jorgensen E, Keiding N, Grandjean P, et al. Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol 2007;17:27–35. [DOI] [PubMed] [Google Scholar]
- 21. Sonmez FM, Demir E, Orem A, et al. Effect of antiepileptic drugs on plasma lipids, lipoprotein (a), and liver enzymes. J Child Neurol 2006;21:70–74. [DOI] [PubMed] [Google Scholar]
- 22. Tomoum HY, Awadallah MM, Fouad DA, et al. Lipid profile, apolipoproteins A and B in children with epilepsy. J Child Neurol 2008;23:1275–1281. [DOI] [PubMed] [Google Scholar]
- 23. Gibbons GF. The role of cytochrome P450 in the regulation of cholesterol biosynthesis. Lipids 2002;37:1163–1170. [DOI] [PubMed] [Google Scholar]
- 24. Bramswig S, Sudhop T, Luers C, et al. Lipoprotein(a) concentration increases during treatment with carbamazepine. Epilepsia 2003;44:457–460. [DOI] [PubMed] [Google Scholar]
- 25. Tan TY, Lu CH, Chuang HY, et al. Long‐term antiepileptic drug therapy contributes to the acceleration of atherosclerosis. Epilepsia 2009;50:1579–1586. [DOI] [PubMed] [Google Scholar]
- 26. Bowes AJ, Khan MI, Shi Y, et al. Valproate attenuates accelerated atherosclerosis in hyperglycemic apoE‐deficient mice: evidence in support of a role for endoplasmic reticulum stress and glycogen synthase kinase‐3 in lesion development and hepatic steatosis. Am J Pathol 2009;174:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khan MI, Pichna BA, Shi Y, et al. Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid Redox Signal 2009;11:2289–2298. [DOI] [PubMed] [Google Scholar]
- 28. Chengappa KN, Chalasani L, Brar JS, et al. Changes in body weight and body mass index among psychiatric patients receiving lithium, valproate, or topiramate: an open‐label, nonrandomized chart review. Clin Ther 2002;24:1576–1584. [DOI] [PubMed] [Google Scholar]
- 29. Martin CK, Han H, Anton SD, et al. Effect of valproic acid on body weight, food intake, physical activity and hormones: results of a randomized controlled trial. J Psychopharmacol 2009;23:814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marchi N, Lerner‐Natoli M. Cerebrovascular remodeling and epilepsy. Neuroscientist 2013;19:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woermann FG, Sisodiya SM, Free SL, et al. Quantitative MRI in patients with idiopathic generalized epilepsy. Evidence of widespread cerebral structural changes. Brain 1998;121(Pt 9):1661–1667. [DOI] [PubMed] [Google Scholar]
- 32. Kori P, Garg RK, Malhotra HS, et al. Evaluation of cerebral white‐matter micro‐structural alterations in patients with medically refractory epilepsy using diffusion tensor tractography. Epilepsy Res 2013;107:82–90. [DOI] [PubMed] [Google Scholar]
- 33. Gluck MR, Jayatilleke E, Shaw S, et al. CNS oxidative stress associated with the kainic acid rodent model of experimental epilepsy. Epilepsy Res 2000;39:63–71. [DOI] [PubMed] [Google Scholar]
- 34. Cock HR. The role of mitochondria and oxidative stress in neuronal damage after brief and prolonged seizures. Prog Brain Res 2002;135:187–196. [DOI] [PubMed] [Google Scholar]
- 35. Liang LP, Patel M. Seizure‐induced changes in mitochondrial redox status. Free Radic Biol Med 2006;40:316–322. [DOI] [PubMed] [Google Scholar]
- 36. Peluso I, Morabito G, Urban L, et al. Oxidative stress in atherosclerosis development: the central role of LDL and oxidative burst. Endocr Metab Immune Disord Drug Targets 2012;12:351–360. [DOI] [PubMed] [Google Scholar]
- 37. Hamed SA, Hamed EA, Hamdy R, et al. Vascular risk factors and oxidative stress as independent predictors of asymptomatic atherosclerosis in adult patients with epilepsy. Epilepsy Res 2007;74:183–192. [DOI] [PubMed] [Google Scholar]
- 38. Longstreth WT Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 39. Hopkins RO, Beck CJ, Burnett DL, et al. Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging 2006;16:243–251. [DOI] [PubMed] [Google Scholar]
- 40. Isojarvi JI, Pakarinen AJ, Rautio A, et al. Liver enzyme induction and serum lipid levels after replacement of carbamazepine with oxcarbazepine. Epilepsia 1994;35:1217–1220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Examples of Age‐Related White Matter Changes (ARWMC) scale.
Table S1. Baseline characteristics of study population.
Table S2. Distribution of AEDs in chronic epilepsy cohort (n = 150).
Table S3 ARWMC scores in regions.


