Summary
Severe spinal cord injury is a devastating condition, tearing apart long white matter tracts and causing paralysis and disability of body functions below the lesion. But caudal to most injuries, the majority of neurons forming the distributed propriospinal system, the localized gray matter spinal interneuronal circuitry, and spinal motoneuron populations are spared. Epidural spinal cord stimulation can gain access to this neural circuitry. This review focuses on the capability of the human lumbar spinal cord to generate stereotyped motor output underlying standing and stepping, as well as full weight‐bearing standing and rhythmic muscle activation during assisted treadmill stepping in paralyzed individuals in response to spinal cord stimulation. By enhancing the excitability state of the spinal circuitry, the stimulation can have an enabling effect upon otherwise “silent” translesional volitional motor control. Strategies for achieving functional movement in patients with severe injuries based on minimal translesional intentional control, task‐specific proprioceptive feedback, and next‐generation spinal cord stimulation systems will be reviewed. The role of spinal cord stimulation can go well beyond the immediate generation of motor output. With recently developed training paradigms, it can become a major rehabilitation approach in spinal cord injury for augmenting and steering trans‐ and sublesional plasticity for lasting therapeutic benefits.
Keywords: Human, Locomotion, Motor control, Neuromodulation, Neuroplasticity, Neurorehabilitation, Spinal cord injury, Spinal cord stimulation
Introduction
Severe spinal cord injury (SCI) locally destroys neural circuitry within the spinal gray matter and tears apart the long white matter tracts, causing paralysis below the lesion 1. Standard‐of‐care rehabilitative options for improving motor function in patients with clinically classified motor‐complete SCI are limited, and independent standing or stepping, or partial recovery of intentional movement control is not achieved 2. The intense efforts exploring experimental strategies for axonal regeneration within the damaged white matter tracts and their translation into a cure for human SCI have remained a long and uncertain process 3, 4. Hence, medical care for patients with severe SCI at present focuses on secondary complications and a large population remains with chronic disabilities and little hope for regaining useful motor function below the injury. Ongoing research is developing strategies that could be introduced into the clinical treatment of SCI in the short term 5. One approach is to move the attention away from the pathology at the injury site and from the attempt to reconstruct the original anatomy that had existed before the injury to harnessing the remaining motor function of the uninjured spinal cord structures 6, 7, 8, 9. Even after severe damage to the white matter tracts, caudal to the injury the majority of neurons of the spinal cord forming the longitudinally distributed propriospinal system 10, 11, localized gray matter interneuronal circuitry, and motoneuron populations 1 are spared. A series of physiological, biochemical, and cellular changes takes place distally in the neurons of the sublesional circuitry, including synaptic reorganization and changes in the expression of immediate early genes and of several subtypes of transmembrane receptors 12, 13, 14. Yet, networks of the spinal cord elements which have survived the injury demonstrate the capacity of propriospinal neural signal transmission 11, 15, 16, sensory‐input processing 17, 18, 19, stereotyped motor‐pattern generation 20, 21, 22, and the ability to exhibit activity‐dependent plasticity 23, 24, 25. With altered or disrupted supraspinal input, task‐specific modulation and sustainable excitability of the spinal circuitry are compromised 2. Experimental animal studies have suggested that the loss of the excitatory drive can be partially compensated pharmacologically and by electrical epidural spinal cord stimulation (SCS) 26, 27, 28, 29, 30. In humans, SCS is available as a US Food and Drug Administration‐approved therapy for chronic diffuse pain in back or limbs 31, 32, 33 and has been independently used for more than 40 years to improve function in various motor disorders 34, 35, 36, including SCI 37, 38, 39. This review focuses on SCS for the activation of lumbar spinal cord circuitry to generate motor output underlying standing and stepping, and on strategies to control these motor functions induced below a severe SCI. The potential of SCS as part of new rehabilitation paradigms to facilitate plasticity across and caudal to the injury zone for lasting therapeutic benefits will be discussed.
The Human Lumbar Spinal Cord can Generate Motor Output Underlying Stepping and Standing in Response to Spinal Cord Stimulation
In vertebrate species, brain structures do not need to specify the complete, detailed set of neural signals to control the repetitive basic spinal motor output as required for locomotion. Locomotor control is facilitated by functional neural networks of the spinal cord that can self‐produce rhythmicity 40. In quadrupedal mammals, such central pattern generators 19, 41, 42, 43 for hindlimb control are situated in the lumbar spinal cord and can be activated by tonic electrical stimulation 44, 45, 46. Indirect evidence has suggested that functional circuitry with the capacity to generate rhythmic motor output also exists within the human lumbar spinal cord 47, 48, 49, 50, 51, 52, 53, 54. Epidural stimulation applied over the posterior structures of the lumbar spinal cord (Figure 1A) at 25–50 Hz was shown to induce rhythmic activity in the legs of paralyzed individuals lying supine, some of which could result in involuntary smooth and coordinated flexion–extension movements at hip, knee, and ankle (Figure 1B) 21. Hence, the human spinal locomotor circuitry can immediately generate rhythmic activity in response to an appropriate excitatory input without requiring therapy‐induced circuit reorganization (cf. 55, 56, 57, 58). These observations may currently present the best indication for the existence of a spinal pattern generator in humans (cf. 54).
Figure 1.
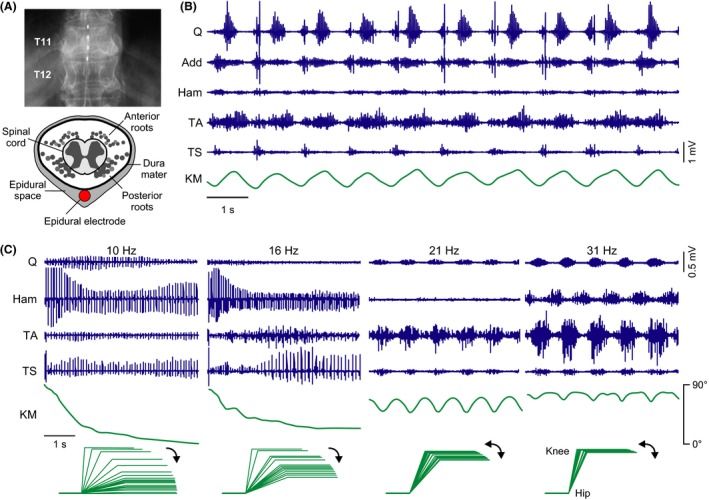
The human lumbar spinal cord circuitry can generate motor output underlying stepping and standing in response to epidural spinal cord stimulation (SCS) in the absence of task‐specific supraspinal or peripheral feedback input. (A) Top: X‐ray of the low thoracic (T) spine and an epidurally placed lead with four electrodes (white rectangles). Bottom: Sketch of a cross section through the 12th thoracic vertebra, showing the position of a dorsally placed epidural electrode (red circle) relative to the neural structures within the vertebral canal. Gray circles represent cross sections of longitudinally oriented posterior and anterior roots (sensory and motor nerve bundles, respectively) surrounding the spinal cord. (B) Generation of rhythmic activity in paralyzed legs by lumbar SCS in supine position. Surface EMG recording from unilateral quadriceps (Q), adductor (Add), hamstrings (Ham), tibialis anterior (TA), and triceps surae (TS) and inclinometer sensor trace from the induced knee movement (KM; deflection up is flexion). Subject with a chronic complete spinal cord injury, neurological level T5; stimulation parameters: 30 Hz, 9 V. Modified with permission from 21. (C) Generation of motor output underlying standing in paralyzed legs by lumbar SCS. Stimulation at 10 and 16 Hz induced leg extension from an initially flexed position, with “sustained” EMG pattern and stereotyped amplitude modulation. Increasing the SCS frequency (21, 31 Hz) changed the EMG pattern from sustained to rhythmic. KM, knee movement derived from goniometric data. Stick figures are constructed based on the knee angle data, with the lower legs manually supported in a horizontal position. Subject with a chronic complete spinal cord injury, neurological level T7; stimulation intensity: 10 V in all cases. Modified with permission from 58.
Computational 59, 60, 61, neurophysiological 61, 62, 63, 64, 65, 66, 67, and pharmacological 61 studies in humans and rats investigated the fibers and circuits recruited by lumbar SCS and the mechanisms underlying pattern generation. The current understanding is that the stimulation activates large‐to‐medium diameter sensory fibers within the posterior roots. The evoked inputs then transsynaptically engage reflex circuits 62, 63, interneuronal circuits involved in the regulation of afferent input and motoneuronal excitability 39, 65, as well as plurisegmentally organized circuitry controlling more complex contraction and relaxation patterns of multiple muscles 21, 58, 62, 64. It is thought that the locomotor circuitry is recruited by the tonic nature of the input, when SCS is applied within an appropriate frequency range, and in turn modifies and coordinates the concomitantly evoked spinal reflex activity at multiple segmental levels 22, 62, 64, 67, 68.
In response to SCS at around 30 Hz, the human lumbar spinal cord can generate a variety of rhythmic lower limb motor patterns in individuals with chronic, (motor‐)complete SCI lying supine, that is, in the absence of locomotor task‐specific proprioceptive feedback or supraspinal input 64. Statistical analysis revealed that the co‐activation, mixed synergy, and locomotor‐like patterns were composed of flexible combinations of few activation timing profiles, each supposedly realized by spinal burst generators with modifiable connection strengths, determining reciprocities and phase lags of the rhythmic drives 43, 64. The rhythmic activity produced by the spinal locomotor circuitry had burst frequencies of 0.27–1.84 Hz, thus covering the physiological range of spinal myoclonus as well as slow and fast gait.
Epidural SCS at 5–16 Hz was shown to evoke “sustained” multimuscle activity with stereotyped amplitude modulation underlying leg extension 58. With the subjects lying supine and their legs manually flexed at hip and knee, strong extension movements were produced with the onset of stimulation, and when full extension was reached, the limbs remained in this position with the muscles strongly contracting under the ongoing stimulation (Figure 1C). With unchanged stimulation site and intensity, and stimulation frequencies increased to 21–50 Hz, limb extension was replaced by rhythmic flexion/extension movement 58. The different motor patterns were suggested to be due to input‐frequency‐dependent differences in the recruitment or configuration of the lumbar circuitry by SCS 22, 58, 64, 69.
Very few studies have addressed the question so far as to whether the SCS‐induced stepping‐ and standing‐like activity in the supine position would translate into actual stepping movements or functional standing in paralyzed individuals. In two chronic, complete SCI individuals, SCS at 20–50 Hz with an intensity close to or above the motor threshold immediately enhanced the gait‐phase synchronized EMG activity 70 as produced by the proprioceptive feedback related to the passive stepping motions alone (cf. 23, 71, 72, 73) (Figure 2A), although stepping movements had to be permanently assisted across conditions 2. Stronger intensities of SCS could occasionally generate irregular flexion–extension movements in the legs, but they did not synchronize to the treadmill‐belt speed and rather impeded the attempts of the therapists to guide continuous stepping motions (unpublished observation; cf. 74). A more recent study applied SCS in a motor‐complete, sensory‐incomplete SCI subject who had undergone extensive locomotor training 75. SCS at 30–40 Hz considerably augmented the rhythmic activity as produced by assisted stepping alone and recruited additional muscles, yet independent functional movements were not produced.
Figure 2.
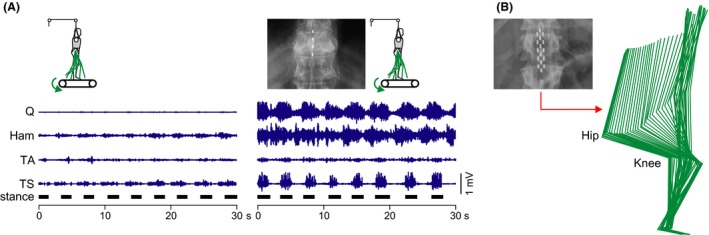
Epidural lumbar spinal cord stimulation (SCS) can generate extensive rhythmic lower limb activity during assisted treadmill stepping and induce full weight‐bearing standing. (A) Lower limb EMG activity induced by therapist‐assisted, partially (50%) body weight‐supported treadmill stepping (0.36 m/s) without (left) and with epidural lumbar SCS. Adding SCS at 30 Hz and supra‐threshold intensity considerably augmented the rhythmic EMG activity and recruited muscles that had not responded to proprioceptive feedback input produced during stepping alone. Leg movements were continuously assisted by therapists. EMG recordings from unilateral quadriceps (Q), hamstrings (Ham), tibialis anterior (TA), and triceps surae (TS); black horizontal bars mark stance phases. Subject with a chronic complete spinal cord injury, neurological level C7. Modified with permission from 9. (B) Kinematic representation of sitting to standing transition induced by epidural SCS applied at 15 Hz and supra‐threshold intensity. After intensive training, the subject could start and maintain full weight‐bearing standing under SCS, with minimal self‐assistance for balance. Subject with a chronic motor‐complete, sensory‐incomplete spinal cord injury, neurological level T2. Modified with permission from 75.
Epidural SCS at 15 Hz and intensities at or above the threshold to produce lower limb activity could generate upright standing in the same subject 75 (Figure 2B). After intensive training, the subject could initiate (by manipulating body position) and maintain full weight‐bearing standing under ongoing SCS, only with minimal self‐assistance for balance. When the subject shifted his center of gravity sagittally while standing, responses resembling corrective postural limb reflexes occurred, suggesting the active involvement of spinal circuitry and the integration of feedback input. Full weight‐bearing standing by SCS was achieved in three additional patients in a succeeding study of the same group 76.
In summary, in response to SCS, the human lumbar spinal cord can produce a variety of rhythmic flexor/extensor activation patterns and physiologically relevant burst frequencies, as well as strong extensor activity for full weight‐bearing standing in paralyzed individuals. However, tonic SCS alone has not been effective in enabling motor‐complete SCI individuals to execute unassisted weight‐bearing stepping. The following sections will discuss strategies for controlling the otherwise stereotyped motor output produced by SCS below a severe SCI.
Proprioceptive Feedback as a Source of Control
Sensory feedback from the lower limbs regulates activation and coordination of spinal motoneuron pools during stepping through reflex circuits and pattern generating circuitry 19, 77, 78, 79. Adult spinal cats can perform weight‐bearing hindlimb locomotion on a treadmill after task‐specific training 80, 81. Despite the loss of voluntary control, the induced stepping is not entirely stereotypical but immediately adapts to variations in treadmill‐belt speed. Edgerton, Gerasimenko and colleagues have emphasized the role of step‐related feedback input to generate locomotion facilitated by SCS without or with additional pharmacological stimulation in decerebrate or spinal adult cats, and spinal adult rats 28, 29, 82. They concluded that the electrical and pharmacological stimulation at low doses increased the responsiveness of the spinal locomotor circuitry to proprioceptive input that then generated stepping over a range of velocities, loads, and directions. They proposed that proprioceptive information from the hindlimbs could be used to actually control locomotion, when the physiological state of the spinal motor circuitry was enhanced by neuromodulation 29. By providing load‐dependent afferent input on a static treadmill belt, Courtine et al. 30 found that combined pharmacological and electrical SCS facilitated tonic activation of extensor muscles with weaker recruitment of flexor muscles in adult spinal rats, resulting in partially weight‐bearing standing on the hindlimbs. As soon as treadmill‐belt motion was initiated, the tonic motor output immediately changed to rhythmic patterns, and increasing treadmill‐belt speed resulted in adaptation of motoneuron pool recruitment, step frequency, stance‐phase duration, and stride length. With reversed treadmill‐belt rotation, or when placing the rats perpendicular to the treadmill‐belt direction, the spinal animals stepped backwards or sideways. When the treadmill was stopped, the rhythmic hindlimb movements stopped instantly in spite of continuous pharmacological and electrical stimulation.
While in motor‐complete SCI persons the muscle activation produced by assisted treadmill stepping is not sufficient to produce functional leg movement 83, sensory information related to velocity of muscle stretch 18, as well as limb load 17, can be processed by the lumbar spinal circuitry to modulate frequency, duration, and amplitude of the produced rhythmic EMG bursts 73. When lumbar SCS at 20–50 Hz was continuously applied in two complete SCI individuals on a treadmill 70, tonic motor output was generated during supported standing, which became rhythmic as soon as stepping motions were imposed, and turned back to tonic after the treadmill belt was stopped (Figure 3A). Other muscle groups that did not respond to SCS in the supported standing position responded with rhythmic activity when step‐induced feedback was provided. A study utilizing a transcutaneous version of SCS 9, 84, 85, 86, 87 showed adaptations of the stimulation‐induced activities produced in the paralyzed legs during robotic‐guided treadmill stepping to different step‐frequencies and hip‐movement conditions 74. The regulatory impact of proprioceptive feedback was clearly demonstrated in a recent study, where epidural SCS at 25–60 Hz, otherwise appropriate to produce rhythmic activity, together with load‐related sensory feedback enabled full weight‐bearing standing in four motor‐complete SCI individuals 76. Despite unchanged stimulation parameters, the level of EMG activity considerably increased with progressive limb load as the paralyzed individuals actively initiated standing by manipulating body position, thus providing task‐specific afferent information to the spinal cord.
Figure 3.
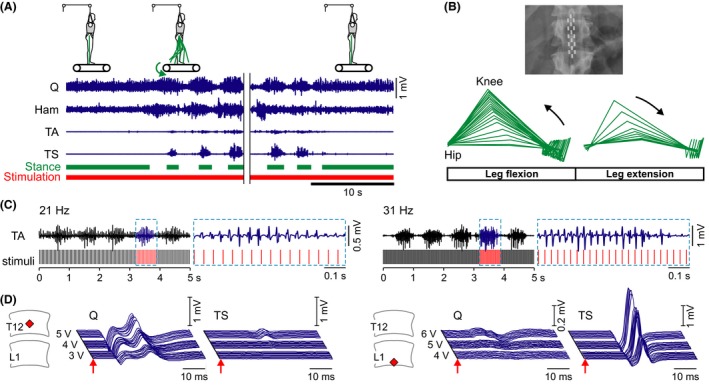
Strategies to control motor output generated by SCS below a paralyzing spinal cord injury. (A) Proprioceptive feedback as a source of control. Lower limb EMG activity of quadriceps (Q), hamstrings (Ham), tibialis anterior (TA), and triceps surae (TS) induced by continuous 30‐Hz SCS during therapist‐assisted standing and treadmill stepping (0.36 m/s) with body weight support (50%). The addition of step‐specific sensory feedback changed continuous EMG patterns to rhythmic ones, and nonresponding muscles in standing responded with rhythmic activity during stepping. Rhythmic activity immediately stopped as soon as the treadmill belt was stopped, despite ongoing stimulation. Same subject as in Fig. 2A. (B) Minimal translesional descending input as a source of control. Kinematic representation of the paralyzed leg when the subject in supine position actively attempted to perform a hip‐flexion movement during ongoing 30‐Hz SCS. Same subject as in Fig. 2B. Modified with permission from 75. (C) SCS frequency entrains the motoneuron pool firing rate and may be used to control the muscle force produced. Continuous sequences of rhythmic EMG activity produced by SCS at 21 Hz and 31 Hz (cf. Fig. 1C) along with detailed EMG data extracted from the time window highlighted by the dashed boxes. Increased numbers of responses per burst were shown to produce increased levels of muscle force in rat 68. (D) Segmental‐selective muscle recruitment by SCS. Stimulus‐triggered EMG responses of the L2–L4 innervated Q and the L5–S2 innervated TS to 2‐Hz stimulation and incremental intensities. Stimulation from the rostral site (12th thoracic vertebra) allowed relatively selective recruitment of upper lumbar posterior roots, stimulation from the caudal site (1st lumbar vertebra) recruited lower lumbar/upper sacral posterior roots at lower intensities. Red diamonds indicate vertebral positions of the active cathode. Left: subject with complete spinal cord injury at C5; right: subject with a motor‐complete, sensory‐incomplete injury at T10. Modified with permission from 62.
In summary, the spinal circuitry under SCS‐provided excitatory drive can use proprioceptive feedback generated below a severe SCI to adapt the produced motor output to standing and different stepping conditions. Another attractive option to be tested is whether electrical stimuli applied to peripheral nerves timed to specific gait phases could control the stereotyped patterns as produced by SCS 88, 89.
Limited Translesional Descending Input as a Source of Control and Paradigms to Promote Neuroplasticity
Even after severe SCI resulting in clinically complete paralysis 90, a small amount of white matter crossing the lesion is commonly preserved 91, 92, 93, and propriospinal connections may survive as well 10, 11, 94. Conduction along such preserved fibers and neural circuitry must be clearly compromised, yet they may provide for some inhibitory and excitatory influence over spinal circuits' excitability caudal to the lesion 1. Dimitrijevic and colleagues showed that the majority of individuals with clinically (motor‐)complete SCI can attenuate reflex activity below the lesion 95, 96 or, by performing forceful, voluntary activation of nonparalyzed muscles above the lesion, can translesionally increase the excitability of the lumbar spinal circuitry 16, 97. Such reinforcement maneuvers may result in a delayed generalized recruitment of multiple muscles of the paralyzed limbs through a slowly conducting residual descending system 16 and produce multijoint movements 16, 98. Thirty years ago, these residual translesional connections were recognized as potential basis for future recovery strategies 16, 92. Clinical studies in multiple sclerosis 99, 100, 101 and SCI 38, 102, 103 revealed many cases of motor function augmented and voluntary movement enabled by SCS. Edgerton, Harkema and colleagues recently revisited this therapeutic potential of SCS in four individuals with (motor‐)complete SCI 75, 104. Under 25‐ or 30‐Hz SCS, all subjects could intentionally induce hip and knee flexion (Figure 3B), dorsiflexion, and toe extension timed to command cues in the supine position. Two of these subjects could increase the rhythmic muscle activity produced during therapist‐assisted treadmill stepping with SCS, when consciously thinking about moving the legs through the step cycles 104. The immediate enabling effect of SCS within the first experimental sessions indicated that residual descending connections had existed in these subjects since the time of their injury and could utilize the increased excitability of the lumbar circuitry under SCS to generate motor output. Further improvement of supraspinal control over leg movement with ongoing active training combined with SCS suggested changes of local spinal circuitry and presumably of translesional connections 4, 94, 104. Activity‐dependent neuroplasticity in humans was described with task‐specific training 23, 25, 105, 106, 107. With SCS, progressive motor improvements were reported related to the duration of stimulation, and cases of temporary carryover effects after discontinuing SCS were observed 24, 34, 99, 103. An important aspect of the training as employed in 75 and 104 was to encourage the patients to actively attempt to perform a purposeful movement despite their paralysis. In a rat model with two staggered lateral hemisections with a spared tissue bridge, leading to complete paralysis, automated treadmill training with SCS and pharmacological facilitation promoted spinal plasticity caudal to the lesion, but failed to promote translesional plasticity and recovery of hindlimb overground locomotion with trunk support 108. Rats trained overground and encouraged to voluntarily use the paralyzed hindlimbs to step toward a food reward could initiate and sustain full weight‐bearing bipedal locomotion during the electrochemical stimulation after a few weeks of training 108. This volitional task‐based training 4 resulted in sprouting of descending fibers into the gray matter between the two hemisections and increased projections from interneurons in this area to the lumbar spinal cord 108. The relevance of the interaction of residual descending volitional input with electrical stimulation of spinal circuits for improving motor recovery was clearly shown in rats with a cervical hemicontusion injury of the spinal cord stimulated through intraspinal microwire implants 109. Intraspinal microstimulation was applied to target specific cervical motor circuits below the lesion, similar to the method used by Mushahwar and colleagues 110, 111, 112, 113 to activate lumbar spinal cord circuitry in cats to induce functional hindlimb movements. During training sessions involving a forelimb reach and grasp task, stimulus application was synchronized with the arrival of functionally related volitional motor commands. Rats receiving this activity‐dependent stimulation exhibited significantly enhanced motor recovery compared with animals receiving intraspinal microstimulation applied temporarily unrelated to the movement attempts and rats receiving physical training alone. The therapeutic benefit persisted for weeks after discontinuation of stimulation, and it was suggested that the activity‐dependent intervention had strengthened synapses along spared spinal motor pathways in a manner consistent with spike timing‐dependent plasticity.
In summary, subclinical supraspinal input via limited translesional connections can exert generalized inhibition or excitation below the injury and provide for a rudimentary level of intentional motor control. SCS can increase the excitability of the spinal circuitry and its responsiveness to minimal translesional neural input, enabling some voluntary movements in otherwise paralyzed legs. The combined activation of spinal circuitry by volitional effort and SCS‐provided excitatory drive may trigger and guide the formation of new translesional connections via networks of neurons and induce lasting therapeutic improvements.
Neuro‐Engineering Strategies to Control Movement Generated by SCS below a Paralyzing SCI
All SCS systems used so far in studies to enable or generate movement in SCI individuals were originally developed for the treatment of chronic intractable pain. The stimulation parameters were programmed manually and remained fixed regardless of the subject's intention to rest or move, or the actual leg position. With invariant stimulation, some of the excitatory input presumably comes with incorrect timing relative to the natural spatiotemporal dynamics of spinal circuit activity for functional movement. Further, tonic stimulation from a fixed site is most likely inadequate to control frequency or pattern of rhythmic activity across multiple muscles 64. Another major drawback of current SCS systems is the brief interruption of stimulus delivery with each change of parameter settings.
Experimental animal studies have explored whether control of SCS parameters could improve stepping performance 68, 114. SCS at 40–50 Hz induced a rapid flexion of the hindlimb during partially weight‐supported standing when applied to the L2 spinal cord level in adult spinal rats, and promoted whole‐limb extension when applied to the S1‐level 30. During bipedal treadmill stepping, increasing SCS intensity at L2 progressively increased hip flexion and step height during swing, while increasing SCS‐intensity at S1 primarily resulted in stronger extension. Capogrosso et al. 61 showed that 40‐Hz SCS applied over the spinal cord midline enabled bilateral hindlimb stepping movements in adult spinal rats on a treadmill, while stimulation applied approx. 750 μm laterally facilitated the movement of the ipsilateral hindlimb only, with contralateral foot drag. Wenger et al. 68 monitored kinematics, ground reaction forces, and muscle activity of stepping in spinal rats to interpret hindlimb endpoint trajectory and gait phases in real time for adjusting SCS frequency based on the current stepping behavior and according to the requirements of the next step (using feedforward models). A linear correlation between SCS frequency and step height allowed for the predictive adjustment of leg movements during locomotion. This was possible, because each burst under SCS was composed of series of stimulus‐triggered spinal reflexes, and graded increase in SCS frequency led to a progressive increase in the number of these evoked responses within each burst (cf. Figure 3C) and hence influenced the muscle force produced. Closed‐loop controlled increase in SCS frequency prevented early exhaustion of locomotor output during continuous treadmill stepping by counteracting the decrease in the amplitude of muscle activity. Furthermore, with the rats positioned overground using an overhead support robot that moved the rats forward along a runway, automated tuning of the SCS frequency allowed the rats to climb combinations of staircases. Yet, this paradigm remained completely computer assisted, without any voluntary contribution to the movement by the rats. The same group implanted epidural electrode arrays in two intact rhesus macaques, connected to pulse generators approved for human use, but with a modified firmware allowing real‐time control of stimulation parameters and stimulation site along the array 115. Recording the neural ensemble activity in the motor cortex and EMG activity and kinematics of the legs while the primates were actively walking on a treadmill allowed for decoding their intention to rest or walk as well as to perform swing or stance during gait. These motor predictions were transmitted wirelessly to the SCS system in real time to tune the site, timing, and frequency of stimulation, leading to task‐ and phase‐appropriate reinforcement of the voluntary stepping movements.
In humans, site‐specific stimulation of a limited number of posterior roots is possible in principle, thus allowing for some specificity of the neural inputs provided to spinal circuitry and motoneuron pools 59. In (motor‐)complete SCI individuals lying supine, 2‐Hz SCS with active cathodes at T11 or T12 vertebral levels predominantly evoked posterior root‐muscle reflexes in quadriceps and adductors, while cathodes at the L1 vertebral level produced stronger activation of tibialis anterior and triceps surae (Figure 3D) 62, 116. Further, selective recruitment of quadriceps was shown to be possible with the cathode located over the L1–L4 spinal cord segments, while triceps surae had lower response thresholds than quadriceps with cathodes caudal to the S2 spinal cord segment 22, 59, 60. A recent study employing a multi‐electrode array placed epidurally over the lumbosacral spinal cord showed that stimulation delivered from the rostral sites along the array predominantly activated the iliopsoas muscle, whereas caudal stimulation more selectively recruited tibialis anterior, medial gastrocnemius, and gluteus maximus 63. Danner et al. 64 retrospectively found that in 84% of the cases, where SCS at around 30 Hz had evoked rhythmic multimuscle EMG patterns in (motor‐)complete SCI individuals lying supine, the active cathode was located over the L2–L4 spinal cord segments. Lower limb extension in response to 5–16 Hz SCS as reported by Jilge et al. 58 was produced with cathode positions ranging from L2–S2 spinal cord segments, with lower stimulation intensities from the more caudal sites. Similarly, Rejc et al. 76 found that cathodes in the caudal portion of the epidural arrays generally promoted a more effective EMG pattern for standing. Left–right specificity of epidural stimulation has not been directly addressed in human studies. However, with slight asymmetrical electrode positions relative to the spinal cord midline 117, SCS from locations closer to the posterior roots of one side normally produced rhythmic EMG activity in the ipsilateral leg only, with the contralateral leg displaying tonic motor output 21, 64.
In summary, the dynamic control of SCS parameters for harnessing spinal “motor programs” and principles of muscle force generation would likely improve the control of timing and degree of extensor versus flexor activity for stabilizing extension across the lower limb joints or trigger and facilitate swing in the attempt to produce stepping movements in individuals after a paralyzing SCI. Closed‐loop, real‐time control over the stimulation parameters could be realized during treadmill therapy or overground gait training with an overhead support robot 118 through the assessment of step kinematics, ground reaction forces, and EMG activity (cf. 68). For the use outside of laboratory environments, the necessary control signals for a closed‐loop system could be derived from foot switches or tilt sensors 9, 24 or inertial sensor technology 119, used for the temporal detection of toe‐off and heel‐strike, and for the estimation of foot clearance. Brain–machine interface technology may develop into an option to decode motor intentions from cortical signals in humans and incorporate them into control algorithms for SCS systems 24, 115, 120, 121.
Conclusion
In response to SCS, the relatively isolated human lumbar spinal cord circuitry can generate motor output underlying full weight‐bearing standing and rhythmic activity during assisted stepping. Limited volitional translesional input and task‐specific proprioceptive feedback to the spinal circuitry can provide for some degree of feedforward and feedback control of movement below the injury level. With new volitional task‐based training paradigms, SCS may become a major approach for triggering and steering trans‐ and sublesional plasticity for long‐term therapeutic benefit. Overhead support robots 118 providing for a safe environment for clinically (motor‐)complete SCI patients to try to actively engage their legs during overground gait training and next‐generation SCS systems with real‐time control of stimulation parameters over multiple electrode sites 115 could be rapidly introduced into clinical treatment of SCI 5 and reveal an unprecedented level of rehabilitation. The recent finding of the return of supraspinal control over previously paralyzed muscles outlasting the time period of SCS 104, if proven to be reproducible, may indeed be evidence for the activity‐dependent reorganization of descending and propriospinal connections in clinically (motor‐)complete SCI individuals. By adding pharmacological stimulation 27, the physiological state of the spinal circuitry could be modulated toward one that can more readily generate movement in response to SCS, residual volitional input, and task‐specific proprioceptive feedback 29, 30. A recently completed clinical trial in SCI individuals (cf. clinicaltrials.gov, NCT01484184) provided preliminary evidence for the efficacy of a drug combination specifically designed to activate the spinal locomotor circuitry 48, 122. Finally, SCS can have beneficial effects upon bladder, bowel, and sexual function below the lesion level 5, 37, 75, 103 as recently rediscovered (cf. nibib.nih.gov/2015‐nibib‐consortium‐report). SCS may finally become a major physiological intervention in neurorehabilitation and a practical application for the concept of plasticity in the spinal cord structures which have survived a severe injury.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors are grateful to W. Barry McKay (Hulse SCI Research Lab, Crawford Research Institute, Shepherd Center, Atlanta, GA, USA) for his continuous support and for critical reading of the manuscript.
References
- 1. Kakulas BA, Kaelan C. The neuropathological foundations for the restorative neurology of spinal cord injury. Clin Neurol Neurosurg 2015;129:S1–S7. [DOI] [PubMed] [Google Scholar]
- 2. Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain 2014;137:654–667. [DOI] [PubMed] [Google Scholar]
- 3. Hug A, Weidner N. From bench to beside to cure spinal cord injury: Lost in translation? Int Rev Neurobiol 2012;106:173–196. [DOI] [PubMed] [Google Scholar]
- 4. van den Brand R, Mignardot J‐B, von Zitzewitz J, et al. Neuroprosthetic technologies to augment the impact of neurorehabilitation after spinal cord injury. Ann Phys Rehabil Med 2015;58:232–237. [DOI] [PubMed] [Google Scholar]
- 5. Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol 2014;13:1241–1256. [DOI] [PubMed] [Google Scholar]
- 6. Dimitrijevic M, Kakulas B, McKay W, Vrbova G. Restorative neurology of spinal cord injury. New York: Oxford University Press, 2011. [Google Scholar]
- 7. Brown JM, Deriso DM, Tansey KE. From contemporary rehabilitation to restorative neurology. Clin Neurol Neurosurg 2012;114:471–474. [DOI] [PubMed] [Google Scholar]
- 8. Illis LS. Central nervous system regeneration does not occur. Spinal Cord 2012;50:259–263. [DOI] [PubMed] [Google Scholar]
- 9. Minassian K, Hofstoetter U, Tansey K, Mayr W. Neuromodulation of lower limb motor control in restorative neurology. Clin Neurol Neurosurg 2012;114:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nathan PW, Smith MC. Fasciculi proprii of the spinal cord in man. Brain 1959;82:610–668. [DOI] [PubMed] [Google Scholar]
- 11. Faganel J, Dimitrijevic MR. Study of propriospinal interneuron system in man. Cutaneous exteroceptive conditioning of stretch reflexes. J Neurol Sci 1982;56:155–172. [DOI] [PubMed] [Google Scholar]
- 12. Edgerton VR, Leon RD, Harkema SJ, et al. Retraining the injured spinal cord. J Physiol 2001;533:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landry ES, Rouillard C, Levesque D, Guertin PA. Profile of immediate early gene expression in the lumbar spinal cord of low‐thoracic paraplegic mice. Behav Neurosci 2006;120:1384–1388. [DOI] [PubMed] [Google Scholar]
- 14. Lapointe NP, Ung R‐V, Guertin PA. Plasticity in sublesionally located neurons following spinal cord injury. J Neurophysiol 2007;98:2497–2500. [DOI] [PubMed] [Google Scholar]
- 15. Dimitrijevíc MR, Nathan PW. Studies of spasticity in man. 2. Analysis of stretch reflexes in spasticity. Brain 1967;90:333–358. [DOI] [PubMed] [Google Scholar]
- 16. Dimitrijevic MR, Dimitrijevic MM, Faganel J, Sherwood AM. Suprasegmentally induced motor unit activity in paralyzed muscles of patients with established spinal cord injury. Ann Neurol 1984;16:216–221. [DOI] [PubMed] [Google Scholar]
- 17. Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 1997;77:797–811. [DOI] [PubMed] [Google Scholar]
- 18. Beres‐Jones JA, Harkema SJ. The human spinal cord interprets velocity‐dependent afferent input during stepping. Brain 2004;127:2232–2246. [DOI] [PubMed] [Google Scholar]
- 19. Rossignol S, Dubuc R, Gossard J‐P. Dynamic sensorimotor interactions in locomotion. Physiol Rev 2006;86:89–154. [DOI] [PubMed] [Google Scholar]
- 20. Grillner S. Control of locomotion in bipeds, tetrapods, and fish In: Brooks V, editor. Handbook of physiology. Section 1: The nervous system, vol. II. Motor control. Bethesda, MD: American Physiological Society, 1981;1179–1236. [Google Scholar]
- 21. Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci 1998;860:360–376. [DOI] [PubMed] [Google Scholar]
- 22. Minassian K, Persy I, Rattay F, Pinter MM, Kern H, Dimitrijevic MR. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor‐like activity. Hum Mov Sci 2007;26:275–295. [DOI] [PubMed] [Google Scholar]
- 23. Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev 2008;57:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jackson A, Zimmermann JB. Neural interfaces for the brain and spinal cord–restoring motor function. Nat Rev Neurol 2012;8:690–699. [DOI] [PubMed] [Google Scholar]
- 25. Knikou M. Functional reorganization of soleus H‐reflex modulation during stepping after robotic‐assisted step training in people with complete and incomplete spinal cord injury. Exp Brain Res 2013;228:279–296. [DOI] [PubMed] [Google Scholar]
- 26. Rossignol S, Chau C, Brustein E, et al. Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann N Y Acad Sci 1998;860:346–359. [DOI] [PubMed] [Google Scholar]
- 27. Guertin PA. Synergistic activation of the central pattern generator for locomotion by l‐beta‐3,4‐dihydroxyphenylalanine and quipazine in adult paraplegic mice. Neurosci Lett 2004;358:71–74. [DOI] [PubMed] [Google Scholar]
- 28. Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett 2005;383:339–344. [DOI] [PubMed] [Google Scholar]
- 29. Edgerton VR, Courtine G, Gerasimenko YP, et al. Training locomotor networks. Brain Res Rev 2008;57:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Courtine G, Gerasimenko Y, van den Brand R, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 2009;12:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg 1967;46:489–491. [PubMed] [Google Scholar]
- 32. Krames E, Rezai A, Peckham P, Aboelsaad F. What is neuromodulation? In: Krames E, Peckham P, Rezai A, editors. Neuromodulation. London: Elsevier‐Academic Press, 2009;3–8. [Google Scholar]
- 33. Gildenberg P. Neuromodulation: A historical perspective In: Krames E, Peckham P, Rezai A, editors. Neuromodulation. London: Elsevier‐Academic Press, 2009;9–20. [Google Scholar]
- 34. Cook AW, Weinstein SP. Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. N Y State J Med 1973;73:2868–2872. [PubMed] [Google Scholar]
- 35. Waltz JM, Reynolds LO, Riklan M. Multi‐lead spinal cord stimulation for control of motor disorders. Appl Neurophysiol 1981;44:244–257. [DOI] [PubMed] [Google Scholar]
- 36. Gybels J, Van Roost D. Spinal cord stimulation for the modification of dystonic and hyperkinetic conditions: A critical Review In: Eccles J, Dimitrijevic MR, editors. Upper motor neuron functions and dysfunctions. Recent achievements in restorative neurology. Basel: S Karger AG, 1985;58–70. [Google Scholar]
- 37. Richardson RR, McLone DG. Percutaneous epidural neurostimulation for paraplegic spasticity. Surg Neurol 1978;9:153–155. [PubMed] [Google Scholar]
- 38. Dimitrijevic MM, Dimitrijevic MR, Illis LS, Nakajima K, Sharkey PC, Sherwood AM. Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical Observations. Cent Nerv Syst Trauma 1986;3:129–144. [DOI] [PubMed] [Google Scholar]
- 39. Pinter MM, Gerstenbrand F, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord 2000;38:524–531. [DOI] [PubMed] [Google Scholar]
- 40. Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science 1985;228:143–149. [DOI] [PubMed] [Google Scholar]
- 41. Brown T. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B 1911;84:308–319. [Google Scholar]
- 42. Fedirchuk B, Nielsen J, Petersen N, Hultborn H. Pharmacologically evoked fictive motor patterns in the acutely spinalized marmoset monkey (Callithrix jacchus). Exp Brain Res 1998;122:351–361. [DOI] [PubMed] [Google Scholar]
- 43. Grillner S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron 2006;52:751–766. [DOI] [PubMed] [Google Scholar]
- 44. Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res 1979;34:241–261. [DOI] [PubMed] [Google Scholar]
- 45. Iwahara T, Atsuta Y, Garcia‐Rill E, Skinner RD. Spinal cord stimulation‐induced locomotion in the adult cat. Brain Res Bull 1992;28:99–105. [DOI] [PubMed] [Google Scholar]
- 46. Gerasimenko Y, Roy RR, Edgerton VR. Epidural stimulation: Comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp Neurol 2008;209:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grillner S. Neuroscience. Human locomotor circuits conform. Science 2011;334:912–913. [DOI] [PubMed] [Google Scholar]
- 48. Guertin PA. Preclinical evidence supporting the clinical development of central pattern generator‐modulating therapies for chronic spinal cord‐injured patients. Front Hum Neurosci 2014;8:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bussel B, Roby‐Brami A, Azouvi P, Biraben A, Yakovleff A, Held JP. Myoclonus in a patient with spinal cord transection. Possible involvement of the spinal stepping generator. Brain 1988;111:1235–1245. [DOI] [PubMed] [Google Scholar]
- 50. Calancie B, Needham‐Shropshire B, Jacobs P, Willer K, Zych G, Green BA. Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain 1994;117:1143–1159. [DOI] [PubMed] [Google Scholar]
- 51. Duysens J, Van de Crommert HW. Neural control of locomotion; the central pattern generator from cats to humans. Gait Posture 1998; 7: 131–141. [DOI] [PubMed] [Google Scholar]
- 52. Calancie B. Spinal myoclonus after spinal cord injury. J Spinal Cord Med 2006;29:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nadeau S, Jacquemin G, Fournier C, Lamarre Y, Rossignol S. Spontaneous motor rhythms of the back and legs in a patient with a complete spinal cord transection. Neurorehabil Neural Repair 2010;24:377–383. [DOI] [PubMed] [Google Scholar]
- 54. Guertin PA. Central pattern generator for locomotion: Anatomical, physiological, and pathophysiological considerations. Front Neurol 2012;3:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shapkova EY, Schomburg ED. Two types of motor modulation underlying human stepping evoked by spinal cord electrical stimulation (SCES). Acta Physiol Pharmacol Bulg 2001;26:155–157. [PubMed] [Google Scholar]
- 56. Gerasimenko Y, Daniel O, Regnaux J, Combeaud M, Bussel B. Mechanisms of locomotor activity generation under epidural spinal cord stimulation In: Dengler R, Kossev A, editors. Sensorimotor control. Washington, DC: IOS Press, 2001;164–171. [Google Scholar]
- 57. Gerasimenko YP, Makarovskii AN, Nikitin OA. Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neurosci Behav Physiol 2002;32:417–423. [DOI] [PubMed] [Google Scholar]
- 58. Jilge B, Minassian K, Rattay F, et al. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res 2004;154:308–326. [DOI] [PubMed] [Google Scholar]
- 59. Rattay F, Minassian K, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord 2000;38:473–489. [DOI] [PubMed] [Google Scholar]
- 60. Ladenbauer J, Minassian K, Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: A computer simulation study. IEEE Trans Neural Syst Rehabil Eng 2010;18:637–645. [DOI] [PubMed] [Google Scholar]
- 61. Capogrosso M, Wenger N, Raspopovic S, et al. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 2013;33:19326–19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Minassian K, Jilge B, Rattay F, et al. Stepping‐like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: Electromyographic study of compound muscle action potentials. Spinal Cord 2004;42:401–416. [DOI] [PubMed] [Google Scholar]
- 63. Sayenko DG, Angeli C, Harkema SJ, Edgerton VR, Gerasimenko YP. Neuromodulation of evoked muscle potentials induced by epidural spinal‐cord stimulation in paralyzed individuals. J Neurophysiol 2014;111:1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Danner SM, Hofstoetter US, Freundl B, et al. Human spinal locomotor control is based on flexibly organized burst generators. Brain 2015;138:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hofstoetter US, Danner SM, Freundl B, et al. Periodic modulation of repetitively elicited monosynaptic reflexes of the human lumbosacral spinal cord. J Neurophysiol 2015;114:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gerasimenko YP, Lavrov IA, Courtine G, et al. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods 2006;157:253–263. [DOI] [PubMed] [Google Scholar]
- 67. Lavrov I, Dy CJ, Fong AJ, et al. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J Neurosci 2008;28:6022–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wenger N, Moraud EM, Raspopovic S, et al. Closed‐loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci Transl Med 2014;6:255ra133. [DOI] [PubMed] [Google Scholar]
- 69. Tator CH, Minassian K, Mushahwar VK. Spinal cord stimulation: Therapeutic benefits and movement generation after spinal cord injury. Handb Clin Neurol 2012;109:283–296. [DOI] [PubMed] [Google Scholar]
- 70. Minassian K, Persy I, Rattay F, Dimitrijevic MR. Effect of peripheral afferent and central afferent input to the human lumbar spinal cord isolated from brain control. Biocybern Biomed Eng 2005;25:11–29. [Google Scholar]
- 71. Wernig A, Müller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia 1992;30:229–238. [DOI] [PubMed] [Google Scholar]
- 72. Dobkin BH, Harkema S, Requejo P, Edgerton VR. Modulation of locomotor‐like EMG activity in subjects with complete and incomplete spinal cord injury. J Neurol Rehabil 1995;9:183–190. [PubMed] [Google Scholar]
- 73. Dietz V, Müller R, Colombo G. Locomotor activity in spinal man: Significance of afferent input from joint and load receptors. Brain 2002;125:2626–2634. [DOI] [PubMed] [Google Scholar]
- 74. Minassian K, Hofstoetter US, Danner SM, et al. Spinal rhythm generation by step‐induced feedback and transcutaneous posterior root stimulation in complete spinal cord‐injured individuals. Neurorehabil Neural Repair 2015. pii: 1545968315591706. [DOI] [PubMed] [Google Scholar]
- 75. Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011;377:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rejc E, Angeli C, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS ONE 2015;10:e0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 1980;187:321–332. [DOI] [PubMed] [Google Scholar]
- 78. Andersson O, Grillner S. Peripheral control of the cat's step cycle. II. Entrainment of the central pattern generators for locomotion by sinusoidal hip movements during “fictive locomotion”. Acta Physiol Scand 1983;118:229–239. [DOI] [PubMed] [Google Scholar]
- 79. Hultborn H, Nielsen JB. Spinal control of locomotion–from cat to man. Acta Physiol (Oxf) 2007;189:111–121. [DOI] [PubMed] [Google Scholar]
- 80. Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full‐weight‐bearing stepping in the adult spinal cat. Exp Neurol 1986;92:421–435. [DOI] [PubMed] [Google Scholar]
- 81. Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 1987;412:84–95. [DOI] [PubMed] [Google Scholar]
- 82. Gerasimenko YP, Lavrov IA, Bogacheva IN, Shcherbakova NA, Kucher VI, Musienko PE. Formation of locomotor patterns in decerebrate cats in conditions of epidural stimulation of the spinal cord. Neurosci Behav Physiol 2005;35:291–298. [PubMed] [Google Scholar]
- 83. MacKay‐Lyons M. Central pattern generation of locomotion: A review of the evidence. Phys Ther 2002;82:69–83. [DOI] [PubMed] [Google Scholar]
- 84. Minassian K, Persy I, Rattay F, Dimitrijevic MR, Hofer C, Kern H. Posterior root‐muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 2007;35:327–336. [DOI] [PubMed] [Google Scholar]
- 85. Minassian K, Hofstoetter U, Tansey K, Rattay F, Mayr W, Dimitrijevic M. Transcutaneous stimulation of the human lumbar spinal cord: Facilitating locomotor output in spinal cord injury. In: Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2010; Program No. 286.19. [Google Scholar]
- 86. Hofstoetter US, Minassian K, Hofer C, Mayr W, Rattay F, Dimitrijevic MR. Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif Organs 2008;32:644–648. [DOI] [PubMed] [Google Scholar]
- 87. Hofstoetter U, Krenn M, Danner S, et al. Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor‐incomplete spinal cord injured individuals. Artif Organs 2015;39:E176–E186. [DOI] [PubMed] [Google Scholar]
- 88. Rybak IA, Stecina K, Shevtsova NA, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: Insights from the effects of afferent stimulation. J Physiol 2006;577:641–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 2008;57:134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kirshblum SC, Burns SP, Biering‐Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kakulas BA. Pathology of spinal injuries. Cent Nerv Syst Trauma 1984;1:117–129. [DOI] [PubMed] [Google Scholar]
- 92. Kakulas A. The applied neurobiology of human spinal cord injury: A review. Paraplegia 1988;26:371–379. [DOI] [PubMed] [Google Scholar]
- 93. Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 1993;59:75–89. [PubMed] [Google Scholar]
- 94. Pohland M, Glumm J. Propriospinal interneurons in the spotlight for anatomical and functional recovery after spinal cord injury. Neural Regen Res 2015;10:1737–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cioni B, Dimitrijevic MR, McKay WB, Sherwood AM. Voluntary supraspinal suppression of spinal reflex activity in paralyzed muscles of spinal cord injury patients. Exp Neurol 1986;93:574–583. [DOI] [PubMed] [Google Scholar]
- 96. Sherwood AM, Dimitrijevic MR, McKay WB. Evidence of subclinical brain influence in clinically complete spinal cord injury: Discomplete SCI. J Neurol Sci 1992;110:90–98. [DOI] [PubMed] [Google Scholar]
- 97. McKay WB, Lim HK, Priebe MM, Stokic DS, Sherwood AM. Clinical neurophysiological assessment of residual motor control in post‐spinal cord injury paralysis. Neurorehabil Neural Repair 2004;18:144–153. [DOI] [PubMed] [Google Scholar]
- 98. Wernig A. No dawn yet of a new age in spinal cord rehabilitation. Brain 2015;138:e362. [DOI] [PubMed] [Google Scholar]
- 99. Illis LS, Oygar AE, Sedgwick EM, Awadalla MA. Dorsal‐column stimulation in the rehabilitation of patients with multiple sclerosis. Lancet 1976;1:1383–1386. [DOI] [PubMed] [Google Scholar]
- 100. Davis R, Gray E, Kudzma J. Beneficial augmentation following dorsal column stimulation in some neurological diseases. Appl Neurophysiol 1981;44:37–49. [DOI] [PubMed] [Google Scholar]
- 101. Berg V, Bergmann S, Hovdal H, et al. The value of dorsal column stimulation in multiple sclerosis. Scand J Rehabil Med 1982;14:183–191. [PubMed] [Google Scholar]
- 102. Barolat G, Myklebust JB, Wenninger W. Enhancement of voluntary motor function following spinal cord stimulation–case study. Appl Neurophysiol 1986;49:307–314. [DOI] [PubMed] [Google Scholar]
- 103. Barolat G, Myklebust JB, Wenninger W. Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. Appl Neurophysiol 1988;51:29–44. [DOI] [PubMed] [Google Scholar]
- 104. Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014;137:1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wernig A, Müller S, Nanassy A, Cagol E. Laufband therapy based on “rules of spinal locomotion” is effective in spinal cord injured persons. Eur J Neurosci 1995;7:823–829. [DOI] [PubMed] [Google Scholar]
- 106. Dietz V, Wirz M, Curt A, Colombo G. Locomotor pattern in paraplegic patients: Training effects and recovery of spinal cord function. Spinal Cord 1998;36:380–390. [DOI] [PubMed] [Google Scholar]
- 107. Knikou M, Mummidisetty CK. Locomotor training improves premotoneuronal control after chronic spinal cord injury. J Neurophysiol 2014;111:2264–2275. [DOI] [PubMed] [Google Scholar]
- 108. van den Brand R, Heutschi J, Barraud Q, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 2012;336:1182–1185. [DOI] [PubMed] [Google Scholar]
- 109. McPherson JG, Miller RR, Perlmutter SI. Targeted, activity‐dependent spinal stimulation produces long‐lasting motor recovery in chronic cervical spinal cord injury. Proc Natl Acad Sci USA 2015;112:12193–12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mushahwar VK, Horch KW. Selective activation and graded recruitment of functional muscle groups through spinal cord stimulation. Ann N Y Acad Sci 1998;860:531–535. [DOI] [PubMed] [Google Scholar]
- 111. Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal‐cord injury. IEEE Trans Neural Syst Rehabil Eng 2004;12:430–440. [DOI] [PubMed] [Google Scholar]
- 112. Guevremont L, Norton JA, Mushahwar VK. Physiologically based controller for generating overground locomotion using functional electrical stimulation. J Neurophysiol 2007;97:2499–2510. [DOI] [PubMed] [Google Scholar]
- 113. Bamford JA, Mushahwar VK. Intraspinal microstimulation for the recovery of function following spinal cord injury. Prog Brain Res 2011;194:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Capogrosso M, Formento E, Martin Moraud E, Courtine G, Micera S. Mechanisms underlying the modulation of motor patterns during epidural electrical stimulation of the lumbar spinal cord. In: Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2015; Program No. 95.12. [Google Scholar]
- 115. Capogrosso M, Milekovic T, Borton D, et al. A brain spinal interface to alleviate lower limb deficits after neuromotor disorders. In: Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2015; Program No. 428.08. [Google Scholar]
- 116. Murg M, Binder H, Dimitrijevic MR. Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. muscle twitches ‐ a functional method to define the site of stimulation. Spinal Cord 2000;38:394–402. [DOI] [PubMed] [Google Scholar]
- 117. Holsheimer J, den Boer JA, Struijk JJ, Rozeboom AR. MR assessment of the normal position of the spinal cord in the spinal canal. AJNR Am J Neuroradiol 1994;15:951–959. [PMC free article] [PubMed] [Google Scholar]
- 118. Vallery H, Lutz P, von Zitzewitz J, et al. Multidirectional transparent support for overground gait training. IEEE Int Conf Rehabil Robot 2013; 6650512. [DOI] [PubMed]
- 119. Mariani B, Rochat S, Büla CJ, Aminian K. Heel and toe clearance estimation for gait analysis using wireless inertial sensors. IEEE Trans Biomed Eng 2012;59:3162–3168. [DOI] [PubMed] [Google Scholar]
- 120. Courtine G, Bloch J. Defining ecological strategies in neuroprosthetics. Neuron 2015;86:29–33. [DOI] [PubMed] [Google Scholar]
- 121. Rigosa J, Panarese A, Dominici N, et al. Decoding bipedal locomotion from the rat sensorimotor cortex. J Neural Eng 2015;12:056014. [DOI] [PubMed] [Google Scholar]
- 122. Steuer I, Rouleau P, Guertin PA. Pharmacological approaches to chronic spinal cord injury. Curr Pharm Des 2013;19:4423–4436. [DOI] [PubMed] [Google Scholar]


