Summary
Aims
The aim of this study is to further uncover the neural basis of postural instability gait disorder (PIGD) subtype of Parkinson's disease.
Methods
With F‐18 fluorodeoxyglucose PET (FDG‐PET), brain glucose metabolism of patients with PIGD (n = 15) was compared with healthy controls (n = 17) and tremor‐dominant (TD) patients (n = 15), and the correlation between metabolism and PIGD symptoms was also assessed. Within PIGD symptom‐correlated hypometabolic areas, the relationship of functional connectivity (FC) with motor and cognitive symptoms was examined by using functional MRI.
Results
Compared with controls, patients with PIGD displayed a distributed pattern of brain hypometabolism including striatal, frontal, and parietal areas. Relative to the pattern of TD patients, the pattern of patients with PIGD had additional metabolic decreases in caudate and inferior parietal lobule (IPL, Brodmann area [BA] 40). In PIGD group, the metabolic reductions in IPL (BA 40), middle frontal gyrus (MFG, BA 9) and fusiform gyrus (FG, BA 20) were associated with severe PIGD symptoms. Regions showing such correlation were chosen for further seed‐based FC analysis. Decreased FC within the prefrontal–parietal network (between the MFG and IPL) was associated with severe PIGD symptoms.
Conclusion
The involvement of the caudate, FG, and prefrontal–parietal network may be associated with the prominent gait impairments of PIGD subtype. Our findings expand the pathophysiological knowledge of PIGD subtype and provide valuable information for potential neuromodulation therapies alleviating gait disorders.
Keywords: fMRI, Parkinson's disease, PET, Postural instability gait disorder subtype
Introduction
Relative to the benign tremor‐dominant (TD) subtype of Parkinson's disease (PD), postural instability gait disorder (PIGD) subtype is a much more aggressive subtype, characterized by highly disabling gait disorders such as freezing of gait, as well as increased risks to develop cognitive deterioration 1. These symptoms greatly impact on survival, quality of life, and the demand for nursing home placement. An understanding of the neural basis of PIGD subtype is substantially urgent for the development of therapeutic strategies.
Previous structural and functional imaging investigations have made preliminary attempts. For instance, a voxel‐based‐morphometry (VBM) report of patients with PIGD has identified extensive gray matter atrophy in regions related with motor, cognitive, limbic, and associative functions 2. White matter abnormalities in patients with PIGD were also observed in an MRI study 3. Also, imaging of blood flow has found a frontal and parietal hypoperfusion of PIGD subtype 4. Recently, a functional MRI (fMRI) research of PIGD subtype has reported altered spontaneous neural activities in basal ganglia (BG), cerebellar lobe, temporal, frontal, and parietal regions 5. However, conflicting results across these studies require further efforts. Multimodal imaging such as PET‐fMRI may provide a better understanding of PIGD subtype.
Imaging with PET is more informative and sensitive to pathological processes. Among various radiotracers, F‐18 fluorodeoxyglucose (FDG) is the most commonly used and has been routinely available at university hospitals. Moreover, metabolic imaging with FDG‐PET has been widely employed to assess the regional brain activity. In fact, the regional neuropathological processes can change the functional connectivity (FC) between functionally related areas. Crucially, the resting‐state fMRI (rs‐fMRI) has enabled the examination of FC level within functionally associated regions. It detects spontaneous blood‐oxygen‐level–dependent (BOLD) signal when a subject is at rest. FC is defined as the temporal correlations of these signals or their patterns. We thus aimed to further elucidate the neural basis of PIGD by combining FDG‐PET with BOLD‐fMRI approaches. With FDG‐PET, group comparisons were first made, after which the correlation between hypometabolism and PIGD scores was then examined. Using BOLD‐fMRI, within PIGD score‐associated areas, the relationship of FC level with motor and cognitive symptoms was finally explored. Because gait is no longer viewed as a merely motor task but rather one requiring higher cortical functions 6, we hypothesized that the neural basis of PIGD subtype would go beyond the BG system and extend to cortical areas.
Materials and Methods
Participants
All 30 patients with PD (15 PIGD and 15 TD) met both the diagnostic criteria for the Parkinson's UK Brain Bank for idiopathic PD 7 and the specific criteria for PIGD and TD subtypes 8, 9. They had a good response to dopaminergic replacement therapy. Patients were consecutively recruited from the Outpatient Clinics, Department of Neurology, The First Affiliated Hospital of Nanjing Medical University. Exclusion criteria were secondary parkinsonism, parkinsonian plus syndromes, dementia (i.e., Mini Mental State Examination [MMSE] score ≤24 10), clinically relevant depression (17‐item Hamilton Depression Rating Scale, [HDRS] ≥ 14 11) or other major psychiatric illness, antidepressant or other antipsychotic medication, abnormal structural MRI, significant comorbidities affecting gait (e.g., acute illness, visual disturbances, orthopedic disease, musculoskeletal disorders), moderate–severe head tremor, and standard contraindication for MRI examination. Seventeen normal controls, without neurological and psychological disorders or imaging abnormalities, were chosen from healthy individuals from the Health Examination Center of our hospital. All the participants were right‐handed. Patients were examined in two separate occasions, at the similar time each morning after overnight withdrawal from antiparkinsonian medications (72‐h withdrawal periods for prolonged released dopamine agonists), within the space of 2 weeks. The first visit included clinical, neurological, and MRI examinations, and on the second visit, all the patients underwent an FDG‐PET scan. The imaging data were analyzed by two neuroradiologists (Dr. Tian‐nv Li for the PET analysis, and Dr. Min Wang for the fMRI analysis), who were blinded to the clinical profile of subjects. Written informed consent was obtained from each subject, and this study was approved by the ethics committee of our hospital (2014‐SRFA‐097).
Clinical Evaluation
Motor symptoms and disease stages were assessed with the Unified Parkinson's Disease Rating Scale (UPDRS) 12 and Hoehn and Yahr (H‐Y) stage 13, respectively. Motor performances such as balance and gait were evaluated with Tinetti Balance, Tinetti Gait 14, Freezing of Gait Questionnaire (FOGQ) 15, and Timed Up and Go (TUG) 16. The MMSE, Frontal Assessment Battery (FAB) 17, and HDRS were used to assess global cognitive status, executive function, and clinically depressive symptoms, respectively. Levodopa equivalent daily dose (LEDD) of each patient was calculated as previously described 18.
Classification of PIGD and TD Subtypes
Clinically, patients with PD may present a spectrum of motor symptoms such as tremor, bradykinesia, rigidity, as well as postural instability and gait difficulties. However, patients with PD show a clinical heterogeneity and can be classified into different motor subtypes according to the individual predominant motor symptoms. The classification of PIGD and TD subtypes was made based on the ratios between a patient's mean PIGD score, mean tremor score, and mean akinetic‐rigid score. A mean PIGD score was the sum of UPDRS item 13 (falling), 14 (freezing), 15 (walking), 29 (gait), and 30 (postural instability) divided by 5, whereas a mean tremor score was the sum of item 16 (both arms tremor determined by history), 20 (face and all four limbs tremor at rest), and 21 (both arms action or postural tremor) divided by 9. An average akinetic‐rigid score was calculated as the total score of item 22 (rigidity of neck and 4 extremities), 23 (finger tap), 24 (hand movement), 25 (hand rotation), 26 (feet flexibility), and 31 (body bradykinesia) divided by 14. Accordingly, the PIGD subtype was defined as a patient's mean PIGD score/mean tremor score > 1.0 and mean PIGD score/mean akinetic‐rigid score > 1.0, while TD subtype was defined as individual mean tremor score/mean PIGD score ≥ 1.5 and mean tremor score/mean akinetic‐rigid score ≥ 1.5. Patients who fail to meet the above‐mentioned criteria were grouped into akinetic‐rigid or mixed subtypes. The classification criteria we used were modified from previous researches 8, 9. Only patients fulfill the PIGD or TD classification criteria were included in our study for further neuropsychiatric and imaging examination. For patients with PIGD, the ratios of mean PIGD score to mean TD score were 5.16 ± 2.94 (range 2.40–10.80), and the ratios of mean PIGD score to mean akinetic‐rigid score were 3.73 ± 2.73 (range 1.40–14.00). For TD subjects, the ratios of mean TD score to mean PIGD score were 2.31 ± 1.24 (range 1.67–5.56), and the ratios of mean TD score to mean akinetic‐rigid score were 2.74 ± 1.87 (range 1.56–9.33).
MRI Acquisition
All MRI data were acquired with a 3.0 T Siemens MRI scanner (Erlangen, Germany) with an 8‐channel head coil. T1‐weighted anatomical images were first collected with the following parameters: repetition time (TR) = 1900 ms, echo time (TE) = 2.95 ms, flip angle (FA) = 9°, slice thickness = 1 mm, slices = 160, field of view (FOV) = 230 × 230 mm2, and matrix size = 256 × 256. Subsequently, rs‐fMRI was conducted with an echo‐planar‐imaging pulse sequence with the following parameters: TR = 2000 ms, TE = 21 ms, FA = 90°, FOV = 256 × 256 mm2, matrix size = 64 × 64, slices = 35, slice thickness = 3 mm, no slice gap, total volumes = 240.
PET Acquisition
FDG‐PET was conducted in all subjects. Seventeen controls were chosen from a pool of healthy individuals with normal FDG‐PET data. Before each PET imaging, all subjects fasted over night; the blood glucose level was less than 7.0 mmol/L. PET scans were performed using a Siemens Biograph 16 HR PET/CT in 3D mode. An intravenous injection of 18F‐FDG was administered at the dose of 3.7–5.5 MBq/kg body weight. The CT scan for attenuation correction was first conducted. All subjects underwent a 10‐min 3D scan after 60 min of injection, in a resting state in a dimly lit and quiet room. The PET images were constructed by ordered‐subset expectation maximization.
PET Image Transformation and Analysis
The PET data were analyzed with SPM5 implemented in Matlab7.9 (MathWorks, Sherborn, MA, USA). Scans from participants were first spatially normalized to Montreal Neurological Institute (MNI) standard stereotactic space with linear and nonlinear transformations, and then smoothed using 10‐mm Gaussian filter.
Two sample t‐tests were performed to test group differences in glucose metabolism. Comparisons were made as follows: PIGD versus controls, TD versus controls, and PIGD versus TD. By comparing the metabolism of the two subtypes to that of healthy controls, specific patterns of PIGD and TD subtypes were obtained. Statistical threshold was set at P < 0.01, uncorrected (cluster size ≥ 135 voxels). In PIGD group, we further investigated brain areas whose glucose hypometabolism is associated with higher PIGD scores. A “single subject: Covariates only” general linear model was examined at each voxel, with the age introduced as covariate (P < 0.01, uncorrected, cluster size ≥ 30 voxels).
MRI Image Transformation and FC Analysis
Rs‐fMRI images were preprocessed with DPARSF and SPM5. The preprocessing included conversion, slice timing, head motion correction, normalization, smoothing (4‐mm Gaussian filter), detrending, and temporal filtration (with 0.01–0.08 Hz). Two patients with PIGD were excluded because the head motion exceeded 3 mm or 3°. Nuisance covariates including six head motion parameters, global mean signal, cerebrospinal fluid signal, and white matter signal were regressed out from the image.
Functional connectivity analysis enables detection of functional communication level between spatially remote but functionally related areas. Hence, we investigated FC level between PIGD symptom‐related hypometabolic areas. The above PET analysis revealed that PIGD scores were correlated with hypometabolism in the right fusiform gyrus (FG, Brodmann area [BA] 20), right middle frontal gyrus (MFG, BA 9), and right inferior parietal lobule (IPL, BA 40). These regions were selected and their FC levels between each other were calculated. To define each region‐of‐interest (ROI), we chose a sphere of 4 mm around each MNI coordinate of peak voxel derived from the PET correlation analysis. FC level between the each ROI was presented by z‐value converted from the corresponding correlation coefficient. Further correlation analysis of FC levels with motor and cognitive symptoms was conducted in patients with PIGD. Correlations were tested using Pearson's correlation coefficients or Spearman correlation coefficients (for variables that were not normally distributed). The significance level was set at P < 0.05.
Statistical Analysis of Demographic and Clinical Data
Demographic and clinical data were analyzed using the SPSS version 13 for Windows (SPSS Inc., Chicago, IL, USA). Demographic and clinical differences between groups were compared using λ 2 test, Mann–Whitney U‐test, Kruskal–Wallis test, or analysis of variance as appropriate. Correlations were tested using Spearman correlation coefficients. P < 0.05 was considered as significant.
Results
Clinical Data
Table 1 summarizes the characteristics of all participants. The three groups were similar in their demographic variables including age, gender, and years of education. Disease duration, LEDD, UPDRS‐III score, akinetic‐rigid score, H‐Y stage, MMSE, FAB, and HDRS did not differ between the two subtypes. The PIGD group exhibited more severe balance symptoms than the TD group, as assessed by the Tinetti Balance (P < 0.001). Greater severity of gait impairments (presented by the Tinetti Gait, P < 0.001; the TUG test, P < 0.05; the FOGQ, P < 0.001) was found in patients with PIGD when compared with TD patients.
Table 1.
Clinical characteristics of all subjects
| PIGD | TD | HC | P value | |
|---|---|---|---|---|
| N | 15 | 15 | 17 | NA |
| Gender (M/F) | 8/7 | 9/6 | 9/8 | P = 0.907 |
| Handedness | R | R | R | |
| Age (y) | 68.40 ± 6.07 | 64.27 ± 8.74 | 63.47 ± 5.08 | P = 0.103 |
| Education (y) | 11.80 ± 4.18 | 11.27 ± 2.25 | 10.06 ± 3.01 | P = 0.252 |
| Disease duration (y) | 2.85 ± 2.13 | 3.97 ± 3.54 | NA | P = 0.775 |
| H‐Y | 2.17 ± 0.24 | 1.63 ± 0.74 | NA | P = 0.100 |
| LEDD (mg/d) | 359.00 ± 120.55 | 285.00 ± 194.47 | NA | P = 0.116 |
| Motor signs | ||||
| UPDRS‐III score | 19.80 ± 8.26 | 17.53 ± 11.42 | NA | P = 0.309 |
| Akinetic‐rigid score | 7.47 ± 3.62 | 5.47 ± 3.14 | NA | P = 0.106 |
| PIGD score | 6.47 ± 2.48 | 1.47 ± 1.46 | NA | P < 0.001 |
| Tremor score | 1.33 ± 1.45 | 7.80 ± 3.12 | NA | P < 0.001 |
| Motor performance | ||||
| TUG (s) | 21.07 ± 9.06 | 15.00 ± 5.37 | NA | P = 0.026 |
| Tinetti Balance | 11.27 ± 3.62 | 15.40 ± 1.30 | NA | P < 0.001 |
| Tinetti gait | 7.73 ± 2.82 | 10.93 ± 1.91 | NA | P < 0.001 |
| FOGQ | 12.53 ± 5.82 | 0.93 ± 1.34 | NA | P < 0.001 |
| Nonmotor symptoms | ||||
| MMSE | 28.80 ± 1.37 | 28.87 ± 1.36 | NA | P = 0.870 |
| FAB | 16.33 ± 1.50 | 16.67 ± 1.80 | NA | P = 0.345 |
| HDRS | 4.86 ± 2.75 | 5.73 ± 3.99 | NA | P = 0.490 |
PIGD, postural instability gait difficulty; TD, tremor dominant; HC, healthy controls; NA, not applicable; M, male; F, female; R, right; y, year; H‐Y, Hoehn and Yahr stage; LEDD, Levodopa equivalent daily dose; UPDRS, Unified Parkinson's Disease Rating Scale; TUG, Timed Up & Go; FOGQ, Freezing of Gait Questionnaire; MMSE, Mini Mental State Examination; FAB, Frontal Assessment Battery; HDRS, 17‐item Hamilton Depression Rating Scale.
Data are presented as mean ± SD.
PET Data
Compared with controls, the patients with PIGD had a reduced metabolic activity in subcortical structures involving right lentiform nucleus and bilateral caudate, along with cortical regions including bilateral superior frontal gyrus (SFG, BA 6), right MFG (BA 6), right medial frontal gyrus (MdFG, BA 10), right subcallosal gyrus (BA 25), and left IPL (BA 40). The TD patients exhibited an attenuated hypometabolic pattern when compared to controls, namely the TD subtype had a hypometabolism in subcortical areas such as right lentiform nucleus, and cortical areas including bilateral SFG (BA 6), right MFG (BA 6), right precentral gyrus (BA 6), right inferior frontal gyrus (BA 9), and right subcallosal gyrus (BA 25). When compared directly to the TD, the bilateral posterior cingulate gyrus (BA 31), bilateral precuneus (BA 31), bilateral cuneus (BA 17), bilateral lingual gyrus (BA 17), right parahippocampal gyrus (BA 37), left postcentral gyrus (BA 3), left IPL (BA 40), and bilateral anterior cerebellum lobe were more hypometabolic in patients with PIGD (P < 0.01, uncorrected, cluster size ≥ 135 voxels, as shown in Table 2 and Figure 1).
Table 2.
Brain areas of reduced glucose metabolism**
| Brain region | Side | BA | Cluster size | MNI coordinate | Z score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| PIGD < HC | |||||||
| Superior frontal gyrus | B | 6 | 676 | −8 | 8 | 70 | 3.87 |
| Middle frontal gyrus | R | 6 | 268 | 52 | 2 | 54 | 4.05 |
| Medial frontal gyrus | R | 10 | 283 | 2 | 50 | 16 | 3.02 |
| Lentiform nucleus | R | 25 | 323 | 16 | 12 | 4 | 2.97 |
| Caudate | R | ||||||
| Subcallosal gyrus | R | ||||||
| Inferior parietal lobule | L | 40 | 167 | −58 | −48 | 48 | 2.95 |
| Caudate | L | 149 | −12 | 12 | 8 | 2.75 | |
| TD < HC | |||||||
| Superior frontal gyrus | B | 6 | 667 | −6 | 6 | 68 | 3.55 |
| Middle frontal gyrus | R | 6/9 | 350 | 54 | 8 | 54 | 3.83 |
| Precentral gyrus | R | ||||||
| Inferior frontal gyrus | R | ||||||
| Lentiform nucleus | R | 25 | 138 | 16 | 10 | −2 | 2.63 |
| Subcallosal gyrus | R | ||||||
| PIGD < TD | |||||||
| Posterior cingulate gyrus | B | 31/17/37 | 3183 | 0 | −58 | 26 | 4.11 |
| Precuneus | B | ||||||
| Cuneus | B | ||||||
| Lingual gyrus | B | ||||||
| Anterior cerebellum lobe | |||||||
| Parahippocampal gyrus | R | ||||||
| Postcentral gyrus | L | 3 | 228 | −34 | −28 | 48 | 3.45 |
| Inferior parietal lobule | L | 40/39 | 223 | −58 | −56 | 46 | 3.24 |
PIGD, postural instability gait difficulty; TD, tremor dominant; HC, healthy controls; BA, Brodmann area; MNI, Montreal Neurological Institute; R, right; L, left; B, bilateral;
**P < 0.01, uncorrected, cluster size ≥ 135 voxels.
Figure 1.

Brain areas of reduced glucose metabolism. Brain areas of reduced glucose metabolism are superimposed on representative axial sections of the Montreal Neurological Institute template (P < 0.01, uncorrected, cluster size: ≥135). The left side of the images refers to the right side of the brain. (A): postural instability gait difficulty (PIGD) subjects versus healthy controls (HC); (B): tremor‐dominant (TD) subjects versus HC; and (C): PIGD subjects versus TD subjects.
In PIGD subjects, severe PIGD symptoms were correlated with metabolic reductions in right FG (BA 20), right MFG (BA 9), right IPL (BA 40), and right claustrum (P < 0.01, uncorrected, cluster size ≥ 30 voxels, as shown in Table 3 and Figure 2).
Table 3.
Negative correlations between metabolism and PIGD scores in the PIGD group**
| Brain region | Side | BA | Cluster size | MNI coordinate | Z score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Fusiform gyrus | R | 20 | 300 | 64 | −4 | −32 | 4.18 |
| Claustrum | R | 76 | 36 | −8 | 0 | 2.74 | |
| Middle frontal gyrus | R | 9 | 52 | 58 | 22 | 34 | 2.78 |
| Inferior parietal lobule | R | 40 | 32 | 50 | −62 | 56 | 2.88 |
PIGD, postural instability gait difficulty; BA, Brodmann area; MNI, Montreal Neurological Institute; R, right.
**P < 0.01, uncorrected, cluster size ≥ 30 voxels.
Figure 2.
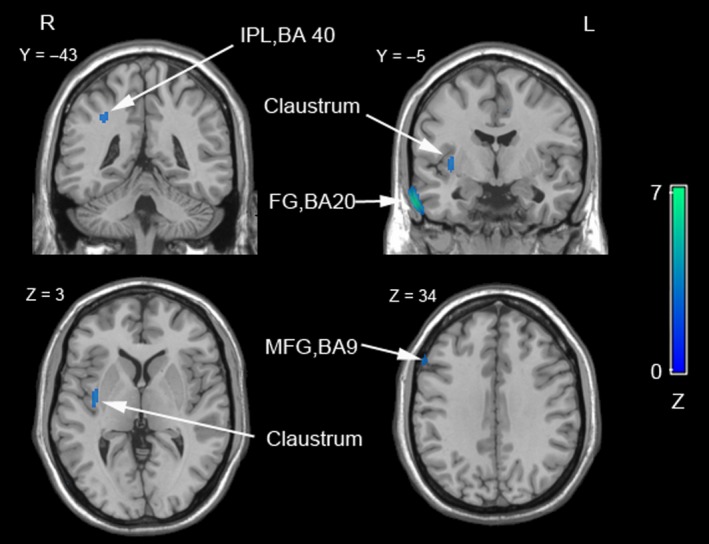
Negative correlations between the PIGD symptoms and metabolism in patients with PIGD. Brain regions showing an association between glucose hypometabolism and the severity of PIGD symptoms in patients with PIGD subtype are shown. Results are superimposed on representative axial sections of the Montreal Neurological Institute template (P < 0.01, uncorrected, cluster size: ≥30). PIGD, postural instability gait difficulty; R, right; L, left; BA, Brodmann area; IPL, inferior parietal lobule; FG, fusiform gyrus; MFG, middle frontal gyrus.
FC Data
In patients with PIGD, we evaluated the FC level between each PIGD symptom‐associated regions. Lower FC level between right MFG (BA 9) and right IPL (BA 40) was associated with more severe PIGD symptoms (rs = −0.593, P = 0.033, shown in Figure 3), but not with other motor or cognitive symptoms (P > 0.05). FC levels of the right FG (BA 20) with right MFG (BA 9) and right IPL (BA 40) were also calculated. However, no statistically significant correlations of the two FC levels with motor or cognitive disturbances were observed (P > 0.05).
Figure 3.
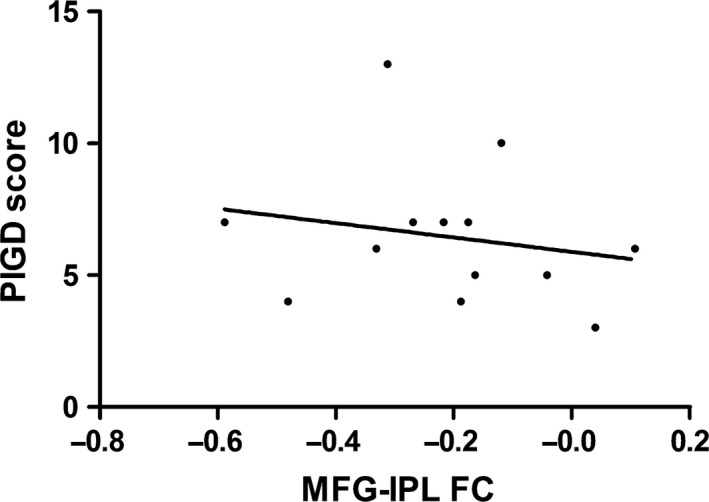
Negative correlation of functional connectivity level within the prefrontal–parietal network with PIGD score in patients with PIGD. rs = −0.593, P = 0.033. PIGD, postural instability gait difficulty; FC, functional connectivity between right middle frontal gyrus (Brodmann area [BA] 9) and right inferior parietal lobule (BA 40).
Discussion
Our study is, to our knowledge, the first demonstration on the neural basis of PIGD subtype by using FDG‐PET and fMRI approaches. Present findings agree with prior hypothesis that both BG system and cortical areas are involved in PIGD subtype. The hypometabolic pattern of the PIGD group mainly included striatal, frontal, and parietal regions. Relative to the pattern of TD subtype, PIGD subtype had additional metabolic reductions in caudate and IPL areas. In patients with PIGD, the metabolic reductions in FG (BA 20), IPL (BA 40), and MFG (BA 9) were associated with severe PIGD symptoms. Among PIGD symptom‐associated hypometabolic areas, lower FC within the prefrontal–parietal network (between MFG and IPL) was correlated with more severe PIGD symptoms. In summary, the specific neural mechanisms of PIGD subtype mainly involved the caudate, FG as well as prefrontal–parietal network. This involvement of BG–cortical areas may be related to the unique gait symptoms in PIGD subtype.
Fundamental Internal Cueing Role of Caudate in Gait Initiation
The caudate contribution to gait is consistent with the correlation between decreased dopamine transporter availability of caudate and reduced step rate of gait in PD 19. Moreover, the association between gait disturbances of PD and caudate tissues loss further supports this assumption 20. Indeed, the initiation of step is usually generated by internal cueing and thus more dependent on the BG than when generated by externally auditory or visual stimuli 21. Additionally, the BG–supplementary motor area (SMA) loop is generally believed to be associated with the self‐initiated step 22. Therefore, damage in caudate and the subsequent disruption of BG‐SMA loop may explain the gait initiation difficulties frequently present in patients with PIGD. Supportably, a VBM study has suggested PIGD subjects had grater gray matter atrophies of caudate and SMA (the rostral part) than TD subjects 2.
External Cueing Role of FG and Prefrontal–Parietal Network in Repetitive Gait Movements
Besides the fundamental internal cueing role played by the caudate in gait initiation, the sensory integration and attention involved in external cueing, with the possible common mechanism of the shunt of BG‐SMA loop 23, have also been linked to the control of repetitive gait movements 23.
Sensory Integration Role of FG and Prefrontal–Parietal Network in Gait
The FG and prefrontal–parietal regions play a cardinal role in sensory process. Malfunctions of sensory process have been found in patients with PD 24 and are more serious in patients with gait problems 25. The observation that passing a narrow door will precipitate the freezing of gait indicates exaggerated responses to visual signals and misperception of space, and implicates the involvement of disrupted visuomotor control of gait in PD. In fact, the FG serves as a visual association area for the perception and categorization of visual stimuli 26. Also, the IPL responses to external stimuli (i.e., visual and auditory cues) 27 and integrates visuospatial information 28. Furthermore, the prefrontal cortex, with projections from the IPL 29, has been hypothesized to compare the input of sensory processing information during perceptual judgment 20. The possible significance of FG and prefrontal–parietal cortices in gait control is supported by our finding that gait difficulties, as primarily presented by the PIGD score, were associated with reduced brain metabolism of these regions. Consistently, a VBM report ascertains that the gray matter atrophy of the FG and prefrontal–parietal areas is associated with gait dysfunctions of PD 20. In specific, the importance of prefrontal–parietal network in gait control is further confirmed by our finding that decreased FC within such network (i.e., MFG [BA 9] and IPL [BA 40]) correlated with greater severity of PIGD symptoms. Collectively, these data suggest that FG and prefrontal–parietal network deficits, with possible subsequently injured visuomotor control of gait, may thus be associated with the gait disturbances in patients with PIGD.
Attentional Role of Prefrontal–Parietal Network in Gait
Additionally, the prefrontal–parietal network also has a role in attention. Of note, gait in patients with PD is more dependent on attention and worsens when the attention is divided by additional tasks 30. On the other hand, focused attention by external cues such as rhythmic auditory cues and stripes on floor can avoid or overcome the freezing episodes in patients with PD. The prefrontal areas, of which hypometabolism was associated with PIGD symptoms, have shown to allocate and coordinate resources of attention 31. In particular, the dorsolateral prefrontal cortices function as attention control 32. Parietal regions also involve in attention. For example, the IPL is linked to support voluntary attention in the auditory 33 and visual modalities 34. The IPL contribution to attention may also be inferred from the impairments in motor attention task induced by IPL lesions 35. Altogether, these findings suggest that the affected prefrontal–parietal network may also predispose patients with PIGD to disordered attention and thus explain the gait symptoms.
Cognitive Role of Prefrontal–Parietal Network in Gait
Apart from the marked gait impairments, cognitive symptoms are also generally common in PIGD subtype 1. Importantly, a PET study has also corroborated the role of prefrontal–parietal network in cognitive function of PD 36. Although the relationship between gait and cognitive function is complicated, cognitive contribution to gait disturbances has also been suggested, as gait disturbances and fall risks are related to executive dysfunctions 37.
Currently, the idea that BG and cortical areas are affected in PIGD subtype is supported by some functional imaging studies 2, 4, 38 and the present work. However, the interpretations must be made with caution, as specific brain areas reported across studies are not completely same. These discrepancies may result from differences in sample characteristics, inclusion criteria, techniques of functional neuroimaging, and statistical analyses.
This study has some limitations. First, this cross‐sectional study can only reflect the temporary profile of PIGD subtype and further follow‐up studies are needed to evaluate the evolvement over time. Second, given the relatively small sample size, future studies with larger sample size are required to test the generalizability of the pattern. Third, the PIGD subtype was diagnosed according to the clinical examination, but not with posturography. However, the method we employed has been widely used in PD and also regarded as a valid measurement instrument for severity of gait difficulties and balance 8, 9. Finally, we could not exclude the rare but theoretical eventuality of the inclusion of pure akinesia with freezing of gate variant of progressive supranuclear palsy.
In conclusion, our study demonstrates that the hypometabolic pattern of PIGD subtype included striatal, frontal, and parietal regions, with the caudate and IPL being more unique. PIGD symptoms were associated with hypometabolism of the FG and prefrontal–parietal network. Particularly, the FC within the prefrontal–parietal network also correlated with PIGD symptoms. The distinct involvement of caudate, FG, and prefrontal–parietal network may account for the salient gait disorders in PIGD subtype. Our findings expand the pathophysiological knowledge of PIGD subtype and provide valuable information for potential neuromodulation therapies alleviating gait disorders.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Natural science foundation of Jiangsu Province (No. BK20141494), the Jiangsu Provincial Personnel Department “the Great of Six Talented Man Peak” Project (No. 2014‐WSN‐013), the University Natural Science Research Project in Jiangsu Province (No. 13KJB32009), the Opening Project of Jiangsu Key Laboratory of Neurodegeneration (No. SJ11KF01); the project was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Science and Technology Project of Jiangsu Bureau of Traditional Chinese Medicine (No. YB2015163).
The first two authors contributed equally to this work.
References
- 1. Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenberg‐Katz K, Herman T, Jacob Y, Giladi N, Hendler T, Hausdorff JM. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology 2013;80:1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohnen NI, Muller ML, Zarzhevsky N, et al. Leucoaraiosis, nigrostriatal denervation and motor symptoms in Parkinson's disease. Brain 2011;134:2358–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mito Y, Yoshida K, Yabe I, et al. Brain SPECT analysis by 3D‐SSP and phenotype of Parkinson's disease. J Neurol Sci 2006;241:67–72. [DOI] [PubMed] [Google Scholar]
- 5. Chen HM, Wang ZJ, Fang JP, et al. Different patterns of spontaneous brain activity between tremor‐dominant and postural instability/gait difficulty subtypes of Parkinson's disease: a resting‐state fMRI study. CNS Neurosci Ther 2015;21:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yogev‐Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 2008;23:329–342; quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base‐line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990;40:1529–1534. [DOI] [PubMed] [Google Scholar]
- 9. Yuan YS, Zhou XJ, Tong Q, et al. Change in plasma levels of amino acid neurotransmitters and its correlation with clinical heterogeneity in early Parkinson's disease patients. CNS Neurosci Ther 2013;19:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 11. Leentjens AF, Verhey FR, Lousberg R, Spitsbergen H, Wilmink FW. The validity of the Hamilton and Montgomery‐Asberg depression rating scales as screening and diagnostic tools for depression in Parkinson's disease. Int J Geriatr Psychiatry 2000;15:644–649. [DOI] [PubMed] [Google Scholar]
- 12. Fahn S, Elton R. Members of the UPDRS Development Committee Unified Parkinson's disease rating scale.
- 13. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 14. Kegelmeyer DA, Kloos AD, Thomas KM, Kostyk SK. Reliability and validity of the Tinetti Mobility Test for individuals with Parkinson disease. Phys Ther 2007;87:1369–1378. [DOI] [PubMed] [Google Scholar]
- 15. Hanakawa T, Katsumi Y, Fukuyama H, et al. Mechanisms underlying gait disturbance in Parkinson's disease: a single photon emission computed tomography study. Brain 1999;122(Pt 7):1271–1282. [DOI] [PubMed] [Google Scholar]
- 16. Shumway‐Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community‐dwelling older adults using the Timed Up & Go Test. Phys Ther 2000;80:896–903. [PubMed] [Google Scholar]
- 17. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology 2000;55:1621–1626. [DOI] [PubMed] [Google Scholar]
- 18. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 19. Ouchi Y, Kanno T, Okada H, et al. Changes in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in Parkinson's disease. Brain 2001;124:784–792. [DOI] [PubMed] [Google Scholar]
- 20. Kostic VS, Agosta F, Pievani M, et al. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology 2012;78:409–416. [DOI] [PubMed] [Google Scholar]
- 21. Hallett M. The intrinsic and extrinsic aspects of freezing of gait. Mov Disord 2008;23(Suppl 2):S439–S443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011;10:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Azulay JP, Mesure S, Blin O. Influence of visual cues on gait in Parkinson's disease: contribution to attention or sensory dependence? J Neurol Sci 2006;248:192–195. [DOI] [PubMed] [Google Scholar]
- 24. Johnson AM, Almeida QJ, Stough C, Thompson JC, Singarayer R, Jog MS. Visual inspection time in Parkinson's disease: deficits in early stages of cognitive processing. Neuropsychologia 2004;42:577–583. [DOI] [PubMed] [Google Scholar]
- 25. Almeida QJ, Lebold CA. Freezing of gait in Parkinson's disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry 2010;81:513–518. [DOI] [PubMed] [Google Scholar]
- 26. Rouw R, Scholte HS. Increased structural connectivity in grapheme‐color synesthesia. Nat Neurosci 2007;10:792–797. [DOI] [PubMed] [Google Scholar]
- 27. Macaluso E, George N, Dolan R, Spence C, Driver J. Spatial and temporal factors during processing of audiovisual speech: a PET study. NeuroImage 2004;21:725–732. [DOI] [PubMed] [Google Scholar]
- 28. Friedman HR, Goldman‐Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci 1994;14:2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones EG, Powell TP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain 1970;93:793–820. [DOI] [PubMed] [Google Scholar]
- 30. Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson's disease. J Neurol Sci 2006;248:173–176. [DOI] [PubMed] [Google Scholar]
- 31. Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 2000;97:13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Osaka M, Komori M, Morishita M, Osaka N. Neural bases of focusing attention in working memory: an fMRI study based on group differences. Cogn Affect Behav Neurosci 2007;7:130–139. [DOI] [PubMed] [Google Scholar]
- 33. Brungart DS, Simpson BD. Cocktail party listening in a dynamic multitalker environment. Percept Psychophys 2007;69:79–91. [DOI] [PubMed] [Google Scholar]
- 34. Marois R, Leung HC, Gore JC. A stimulus‐driven approach to object identity and location processing in the human brain. Neuron 2000;25:717–728. [DOI] [PubMed] [Google Scholar]
- 35. Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci 2001;4:656–661. [DOI] [PubMed] [Google Scholar]
- 36. Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. NeuroImage 2007;34:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2007;62:844–850. [DOI] [PubMed] [Google Scholar]
- 38. Hou Y, Zhang J, Chen B, Wu T. [Local brain activity in different motor subtypes of Parkinson's disease with fMRI]. Zhonghua Yi Xue Za Zhi 2015;95:483–488. [PubMed] [Google Scholar]


