Chronic fatigue syndrome (CFS) is an illness characterized by unexplained fatigue lasting at least 6 months that is not relieved by rest, which affects different body systems and results in cognitive problems, muscle pains, sleep problems, and so on. Physical or mental activity could worsen this illness. CFS is a widespread problem, and estimate for the prevalence of CFS varies widely from 0.007% to 2.8% 1. At present, diagnosis of CFS usually depends on subjective self‐reported feeling and can be made only when other etiologies of fatigue have been excluded. For example, the commonly used criteria for diagnosing CFS include headache, pain in multiple joints or muscles, impaired memory, concentration, and sleep. However, these conditions are also frequently associated with other psychosocial factors, such as sleep disorder, depression, and anxiety 2, 3. Therefore, discovering associated characteristic biomarkers are essential for improving the diagnosis and treatment of CFS.
Orosomucoid (ORM) is an acute phage protein which has many biological activities including modulating immunity, carrying drugs, maintaining the barrier function of capillary, mediating the sphingolipid metabolism, and also acting as an disease marker 4. In our previous study, we found that ORM was significantly upregulated in serum of various forms of fatigued rodents, especially dominantly in sleep‐deprivation rats which have been used in the study of CFS 5, 6. To explore whether ORM could be utilized as a biomarker for CFS, we examined the serum level of ORM in 46 patients diagnosed with CFS according to CDC diagnosis criteria 7 and a group of 38 healthy volunteers. A significantly elevated level of ORM was found in sera from CFS patients (2.78 mg/mL) compared with that from healthy volunteers (0.44 mg/mL) (Figure 1A). The levels of ORM in sera from the volunteers are all lower than 1 mg/mL, whereas sera from 45 of the 46 CFS individuals contain ORM level higher than 1 mg/mL (Figure 1B). Glucocorticoids are known as a major regulator of ORM expression. However, the serum cortisol level in these CFS patients was moderately decreased (Figure 2),which is in accordance previous studies which reported mild hypocortisolism in CFS patients indicating clinically relevant hypothalamic–pituitary–adrenal (HPA) axis dysfunction 8. These findings indicated that the ORM increase is not a direct result of stress response.
Figure 1.
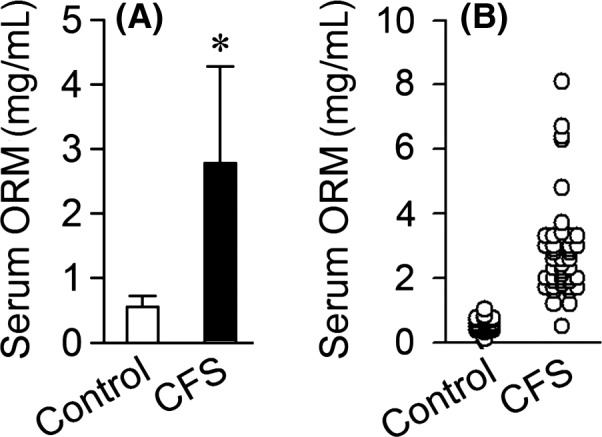
Serum ORM level in individuals with chronic fatigue syndrome. ORM level in sera from 38 healthy volunteers (control) and 46 individuals diagnosed with CFS. Data are mean ± SD. *P < 0.05, by Student's t‐test.
Figure 2.
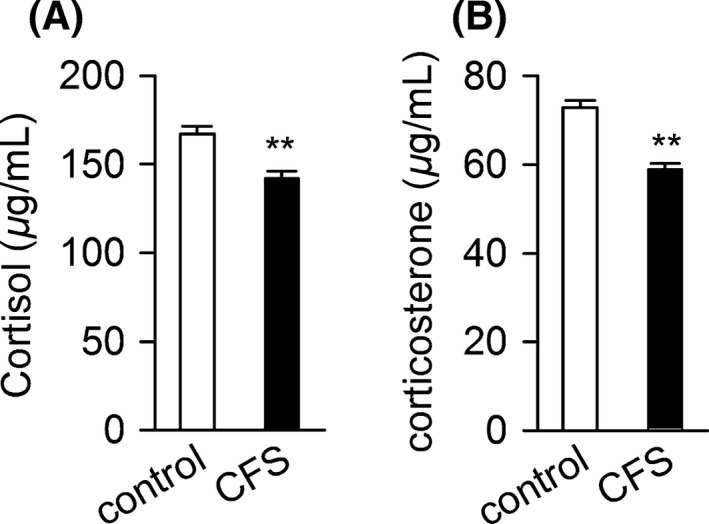
Serological changes in individuals with chronic fatigue syndrome. Serum levels of cortisol (A) and corticosterone (B) in 38 healthy volunteers (control) and 46 individuals diagnosed with CFS. Data are mean ± SD. **P < 0.01 by Student's t‐test.
Fatigue is a commonly experienced, but nonspecific and highly subjective symptom. Detecting and evaluating fatigue is a common task both in various society activities and in clinical practice. At present, there are still no specific factors that have been consistently associated with CFS, and there are no official or semi‐official recommendations for the treatment of CFS 9. We found in this study that ORM is significantly elevated in sera from individuals with CFS. The ORM protein contains genetic polymorphisms. In human, there are two isoforms of ORM 4. Interestingly, it has been shown through proteomics analysis that increased ORM2 is a characteristic change in cerebrospinal fluid from patients diagnosed with CFS 10. These results suggest that it is worth to further explore whether ORM family protein could be used as a biomarker for CFS in the future.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (81273606 and 81473259 to XL, 81230083 to DFS), and National Science and Technology Major Project (2014ZX09J14103‐08C to XL). The authors declare no competing financial interests.
The first two authors contributed equally to this work.
References
- 1. Prins JB, van der Meer JW, Bleijenberg G. Chronic fatigue syndrome. Lancet 2006;367:346–355. [DOI] [PubMed] [Google Scholar]
- 2. Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology (Oxford) 2011;50:1009–1018. [DOI] [PubMed] [Google Scholar]
- 3. Pan J, Zhao P, Cai H, et al. Hypoxemia, sleep disturbances, and depression correlated with fatigue in neuromyelitis optica spectrum disorder. CNS Neurosci Ther 2015;21:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo Z, Lei H, Sun Y, Liu X, Su DF. Orosomucoid, an acute response protein with multiple modulating activities. J Physiol Biochem 2015;71:329–340. [DOI] [PubMed] [Google Scholar]
- 5. Yasui M, Yoshimura T, Takeuchi S, et al. A chronic fatigue syndrome model demonstrates mechanical allodynia and muscular hyperalgesia via spinal microglial activation. Glia 2014;62:1407–1417. [DOI] [PubMed] [Google Scholar]
- 6. Lei H, Sun Y, Luo ZM, et al. Fatigue‐induced Orosomucoid 1 Acts on C‐C Chemokine receptor Type 5 to enhance muscle endurance. Sci Rep 2016;6:18839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994;121:953–959. [DOI] [PubMed] [Google Scholar]
- 8. Klimas NG, Broderick G, Fletcher MA. Biomarkers for chronic fatigue. Brain Behav Immun 2012;26:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischer DB, William AH, Strauss AC, et al. Chronic fatigue syndrome: The current status and future potentials of emerging biomarkers. Fatigue 2014;2:93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baraniuk JN, Casado B, Maibach H, Clauw DJ, Pannell LK, Hess SS. A Chronic Fatigue Syndrome ‐ related proteome in human cerebrospinal fluid. BMC Neurol 2005;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]


