Learning and memory deficit is the characteristic of cognitive impairment, which is often seen in patients with Alzheimer's disease, stroke, depression, chronic fatigue syndrome (CFS), and so on 1. Cognitive impairment has emerged as one of the greatest health threats in the modern society, and drugs including cholinesterase inhibitors, vitamin E, antiinflammatory drugs, or other compounds with possible disease‐modifying effects, have only negative, minor or modest effects. Developing new therapeutic targets for such conditions is still needed.
ORM is an acute‐phase protein, with very low pI of 2.8–3.8 and a very high carbohydrate content of 45%. There are two isoforms of ORM in humans (ORM1 and ORM2) and three isoforms in mice (ORM1, ORM2, and ORM3). Constitutive level of ORM1 is the dominant isoform, and only ORM1 can be induced by acute‐phase stimuli. Many biological activities of ORM have been identified, including modulating immunity, mediating the sphingolipid metabolism, carrying drugs, and also acting as a disease marker 2.
Recently, many reports indicate that ORM may be involved in the regulation of cognitive function. ORM is significantly elevated in the serum of patients with CFS 3, brain tissues of stoke mice 4, sera of the depressed patients 5, and cerebral spinal fluid of the patients with HIV‐associated neurocognitive impairment 6. Especially, ORM is reported to mediate the metabolism of sphingolipid 7, while the disturbance of sphingolipid content in cerebral spinal fluid is associated with memory impairment in patients infected with the HIV 8. Moreover, studies have demonstrated that calorie restriction improved cognitive function at old age and in animal models of Parkinson's and Alzheimer's disease via preventing the sphingolipid turnover dysregulation in the brain 9. All of these studies prompt us to investigate whether ORM could affect cognitive function in addition to acting as an indicative marker.
We first evaluated the physical effect of ORM on the ability to learn and remember. ORM1 knocked‐out mice were generated in our laboratory as previously reported 10. The Morris water maze (MWM) test was applied to investigate spatial learning and memory. Unexpectedly, neither average latency to platform nor mean number of crossing the platform was found to be different between ORM1+/+ and ORM1−/− mice (Figure 1A,B). We further investigated whether exogenous ORM can improve cognitive impairment in a mouse stroke model. Indeed, serum ORM is markedly increased 24h after stroke (Figure 2A). ORM treatment did not improve stroke‐induced impairment in the escape latency (Figure 2B). Also, ORM did not improve the impaired preference for the target quadrant induced by stroke (Figure 2C,D).
Figure 1.
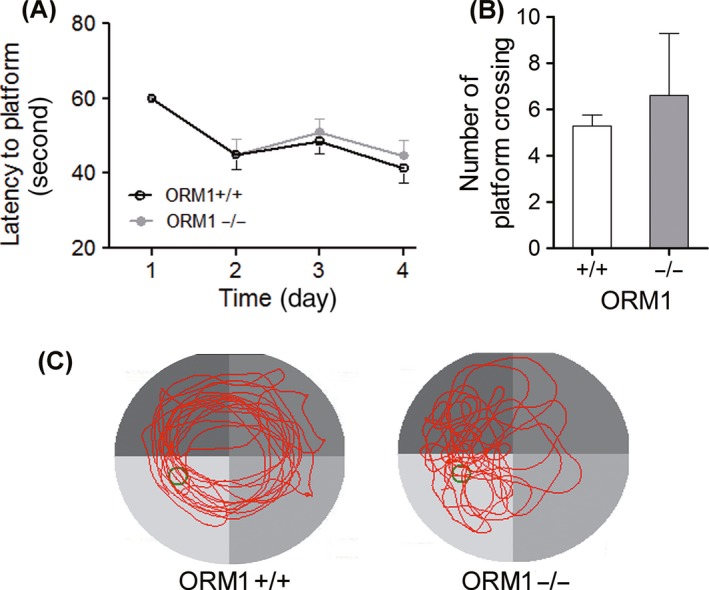
The effect of endogenous ORM on the performance of mice in Morris water maze testing. ORM1+/+ and ORM1−/− mice were used to evaluate the effect of endogenous ORM on the cognitive function. (A) Average latency to platform of 4 trials per day for the consecutive 4 days. (B) Mean number of platform crossing on the 5th day. (C) Representative swim path of mice after platform is removed on the 5th day. Green circle indicates the previous location of platform. Data are presented as mean ± SEM and analyzed by Student's t‐test. N = 5–7 per group. P < 0.05 was considered statistically significant.
Figure 2.
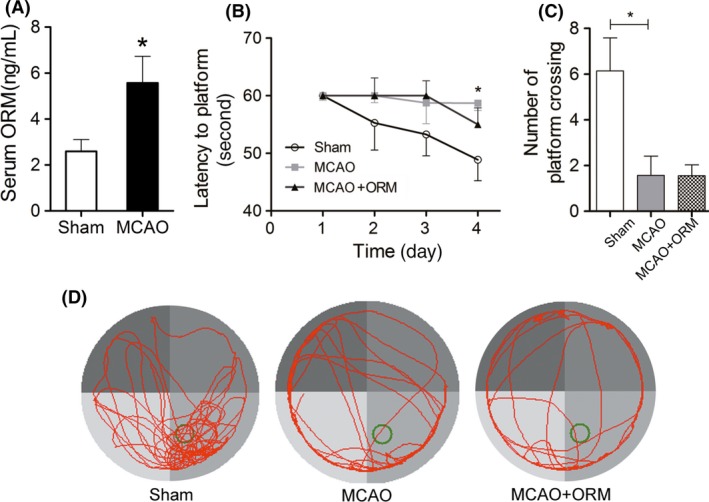
The effect of exogenous ORM on the performance of stroke mice in Morris water maze testing. (A) Serum ORM level in mice 24 h after sham operation or MCAO. N = 6 per group. (B–D) Mice underwent sham operation, MCAO with or without ORM treatment (50 mg/kg via tail vein 30 min ahead of each Morris water maze [MWM] testing), and MWM testing was started on the 7th day post‐MCAO. (B) Average latency to platform of 4 trials per day for the consecutive 4 days. (C) Mean number of platform crossing on the 5th day. (D) Representative swim path of mice after platform is removed on the 5th day. Green circle indicates the previous location of platform. N = 9 per group for B–D. Data are presented as mean ± SEM and analyzed by Student's t‐test for A and one‐way analysis of variance for B and C. P < 0.05 was considered statistically significant.
Our present studies showed that ORM did not exhibit the protective role in cognitive function. ORM elevation is just an accompanying phenomenon, not acting as a feedback mechanism, in response to cognitive impairment. ORM seems not a target for the treatment of cognitive deficit.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (No. 81273606, No. 81473259), and National Science and Technology Major Project (2014ZX09J14103‐08C).
References
- 1. Joubert S, Gour N, Guedj E, et al. Early‐onset and late‐onset Alzheimer's disease are associated with distinct patterns of memory impairment. Cortex 2016;74:217–232. [DOI] [PubMed] [Google Scholar]
- 2. Luo Z, Lei H, Sun Y, et al. Orosomucoid, an acute response protein with multiple modulating activities. J Physiol Biochem 2015;71:329–340. [DOI] [PubMed] [Google Scholar]
- 3. Sun Y, Zhang ZX, Liu X, et al. Orosomucoid (ORM) as a potential biomarker for the diagnosis of Chronic Fatigue Syndrome (CFS). CNS Neurosci Ther 2016;22:251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu L, Jiang Y, Zhu J, et al. Orosomucoid 1: Involved in vascular endothelial growth factor‐induced blood‐brain barrier leakage after ischemic stroke in mouse. Brain Res Bull 2014;109:88–98. [DOI] [PubMed] [Google Scholar]
- 5. Adeoye O, Ferrell R, Kirshner M, et al. alpha1‐acid glycoprotein in late‐life depression: relationship to medical burden and genetics. J Geriatr Psychiatry Neurol 2003;16:235–239. [DOI] [PubMed] [Google Scholar]
- 6. Bora A, Mohien C, Chaerkady R, et al. Identification of putative biomarkers for HIV‐associated neurocognitive impairment in the CSF of HIV‐infected patients under cART therapy determined by mass spectrometry. J Neurovirol 2014;20:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breslow D, Collins S, Bodenmiller B, et al. Orm family proteins mediate sphingolipid homeostasis. Nature 2010;463:1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mielke M, Bandaru V, Mcarthur J, et al. Disturbance in cerebral spinal fluid sphingolipid content is associated with memory impairment in subjects infected with the human immunodeficiency virus. J Neurovirol 2010;16:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Babenko NA, Shakhova EG. Long‐term food restriction prevents aging‐associated sphingolipid turnover dysregulation in the brain. Arch Gerontol Geriatr 2014;58:420–426. [DOI] [PubMed] [Google Scholar]
- 10. Lei H, Sun Y, Luo Z, et al. Fatigue‐induced orosomucoid 1 Acts on C‐C chemokine receptor type 5 to enhance muscle endurance. Sci Rep 2016;6:18839. [DOI] [PMC free article] [PubMed] [Google Scholar]


