Summary
Aims
The aim of this study was to investigate the involvement of the ventral intermediate nucleus of thalamus (Vim) in the tremor‐ and akinetic‐/rigid‐related networks in Parkinson's disease (PD).
Methods
Tremor‐dominant (TD) and akinetic‐/rigid‐dominant (ARD) PD patients were recruited and scanned by resting‐state functional MRI. Functional connectivity from the Vim nucleus was analyzed.
Results
In the TD patients, the Vim nucleus exhibited increased connectivity with the cerebellum/dentate nucleus, primary motor cortex (M1), supplementary motor area (SMA), premotor cortex, thalamus, globus pallidus, putamen, and parietal cortex compared with the controls, while the connections between the Vim nucleus and M1 and cerebellum/dentate nucleus had positive correlations with the tremor scores. In the ARD patients, the Vim nucleus only showed enhanced connectivity with the globus pallidus and limbic lobe compared with the controls, and no connectivity showed correlation against the akinetic–rigidity scores. TD patients had increased connectivity with the Vim nucleus in the cerebellum, M1, SMA, thalamus, globus pallidus, putamen, and parietal cortex compared with ARD patients.
Conclusions
This study demonstrates that the Vim nucleus has an important role in the tremor‐related network, but not in the akinetic‐/rigid‐related network. Our finding is helpful to explain the selective effect of Vim deep brain stimulation in PD.
Keywords: Akinetic–rigidity, Connectivity, Parkinson's disease, Tremor, Vim nucleus
Introduction
Parkinson's disease (PD) is characterized by bradykinesia, tremor, rigidity, and gait disturbance. Based on the predominant motor symptoms, PD can be classified into tremor‐dominant (TD; primary tremor with minimal rigidity, bradykinesia, and other symptoms) and akinetic‐/rigid‐dominant (ARD; primary rigidity and bradykinesia with minimal tremor) subtypes. Pathophysiological mechanisms underlying these two subtypes are different 1. The generation of parkinsonian tremor has been associated with dysfunction of cerebello–thalamo–cortical pathway 2, 3, 4. It suggests that the parkinsonian tremor is triggered from the basal ganglia and driven from the cerebello–thalamo–cortical pathway 5. In contrast, the akinetic/rigid manifestations of PD are closely linked to functionally impaired cortico‐basal ganglia–thalamo–cortical motor circuits 6.
The thalamus is involved in the pathophysiology of both TD and ARD subtypes. However, the role of the thalamus in these two subtypes remains not well understood. For example, it has been recognized that the ventral intermediate nucleus of thalamus (Vim) is involved in the tremor 4, 5. In contrast, whether the Vim has a role in the ARD subtype has not been investigated. The Vim is a cerebellar relay nucleus, but not a basal ganglia relay nucleus 7. It is logical to speculate that the Vim nucleus has a key role in the tremor‐related network, but not in the akinetic‐/rigid‐related network. However, this assumption has not been examined yet.
Resting‐state functional MRI (rsfMRI) is a noninvasive method to investigate brain activity and neural network connectivity. This method is increasingly used in PD and has been proven helpful for our understanding of the pathophysiology of PD 5, 8, 9, 10. In the current study, we applied rsfMRI to investigate the involvement of Vim nucleus in the tremor‐ and akinetic‐/rigid‐related networks. This work will provide new insights on our knowledge of the role of Vim nucleus in PD.
Materials and Methods
Subjects
This study included 84 PD patients (42 TD and 42 ARD patients) and 42 healthy control subjects. The diagnosis of PD was made by an experienced neurologist based on the UK Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria 11. The UPDRS (Unified Parkinson's Disease Rating Scale) 12, the Hoehn and Yahr disability scale 13, and mini‐mental state examination (MMSE) were assessed in PD patients while off their medications. The MMSE was ≥27 in all patients. The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board. All subjects participated in this study had written informed consent.
The classification of TD and ARD was based on a numerical ratio which was derived from a patient's mean tremor score and mean akinetic–rigidity score 14, 15, 16. The seven‐item tremor scale was assessed in UPDRS: resting tremor of the head and four limbs (five items), as well as postural tremor of the right and left hands (two items). The akinetic–rigidity scale was composed of 12 items of UPDRS which included passive range of motion rigidity of the neck and each extremity (five items), hand movements (one item), finger taps (one item), arising from a chair (one item), posture (one item), gait (one item), postural instability (one item), and truncal bradykinesia and hypokinesia (one item). Each item was rated from 0 to 4, as the symptoms aggravate gradually. The mean of each scale was computed, and then, the ratio (mean TD/mean ARD score) was determined. Concur with previous studies, TD was defined with a ratio >1.0, whereas ARD was defined with a ratio <0.8. In this study, the average ratio for TD patients was 1.56 ± 0.58 (range 1.03–3.43) and for ARD patients was 0.27 ± 0.26 (range 0–0.76). The demographics and clinical data of the subjects are shown in Table 1. No significant differences were observed among the three groups in age, gender, and MMSE score. In addition, no significant differences in disease duration, UPDRS motor score, the Hoehn and Yahr stage, and levodopa daily dosage were found between the TD and ARD groups.
Table 1.
Demographics and clinical data of the subjects
| TD (n = 42) | ARD (n = 42) | Control (n = 42) | |
|---|---|---|---|
| Age (years) | 60.26 ± 7.78 | 62.35 ± 8.12 | 62.12 ± 8.01 |
| Sex | 25 M, 17 F | 24 M, 18 F | 24 M, 18 F |
| Disease duration (years) | 4.78 ± 3.82 | 5.41 ± 3.36 | – |
| UPDRS III (off medication) | 19.52 ± 7.24 | 20.32 ± 6.89 | – |
| H&Y (off medication) | 1.89 ± 0.64 | 1.95 ± 0.71 | – |
| MMSE | 28.68 ± 1.59 | 28.36 ± 1.73 | 29.02 ± 1.32 |
| Levodopa dosage (mg/day) | 344.76 ± 143.89 | 388.72 ± 161.09 | – |
| Tremor score | 5.93 ± 3.41 | – | – |
| Akinetic–rigidity score | – | 11.92 ± 6.14 | – |
TD, tremor‐dominant; ARD, akinetic‐/rigid‐dominant; M, male; F, female; UPDRS, Unified Parkinson's Disease Rating Scale; H&Y, Hoehn and Yahr Staging; MMSE, mini‐mental state examination.
Data are presented as mean ± SD.
Data Acquisition
Scans were performed on a 3T MR scanner at Xuanwu hospital (Triosystem; Siemens Magnetom scanner, Erlangen, Germany). PD patients were scanned only after their medication had been withdrawn for at least 12 h. A standard head coil was used with foam padding to reduce head motion. Participants would be excluded if the clinically silent lesions were detected in high‐resolution axial T1‐ and T2‐weighted images.
High‐resolution anatomical images were obtained by using 3D‐MPRAGE sequences (TR = 2000 ms, TE = 2.19 ms, 176 sagittal slices, 1 mm slice thickness, field of view [FOV] = 224 mm × 256 mm). fMRI data were acquired with gradient‐echo echo‐planar sequences (TR = 2000 ms, TE = 40 ms, 32 axial slices, 3.5 mm thickness, gap = 0, Flip angle = 90°, FOV = 256 mm × 256 mm, matrix size = 64 × 64). During the fMRI scanning, subjects were instructed to keep awake with eyes closed, and not think of anything. The fMRI scanning session lasted for 8 min.
Data Analysis
Data Preprocessing
Each fMRI time‐series data contained 240 time points. The first 10 time points were discarded owing to the instability of the initial MRI signal and the adaptation of the subjects to the environment. The rest of the images were preprocessed using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) and Data Processing Assistant for Resting‐State fMRI (DPARSF V2.0 Advanced Edition, http://rfmri.org/DPARSF) 17. Time‐series data were first corrected for slice time and aligned to the first image for motion correction. Each subject's head motion parameters were examined. Of all the subjects, no one had more than 1.5 mm maximum translation in x, y, or z, or 1.5° of maximum angular motion in the three axes. After correction, functional images of each subject were coregistered to anatomical images and spatially normalized into the standard Montreal Neurological Institute (MNI) brain space. The nuisance covariates of head motion parameters, cerebrospinal fluid signal, and white matter signal were regressed out. All images were then resampled into 3 × 3×3 mm3 voxels and smoothed with a 4 mm full width at half maximum Gaussian kernel. After linear drift was removed, a temporal filter (0.01–0.1 Hz) was applied to remove very low‐frequency drifts and physiological high‐frequency noise.
Definition of Regions of Interest (ROIs)
The bilateral Vim nuclei were chosen as the regions of interest (ROIs) for functional connectivity analysis. The centers of the ROIs were located at x = −11.9, y = −15.2, z = 3.96 (left) and x = 12.4, y = −14.1, z = 3.61 (right) according to a recent study 18, which defined the coordinates of the Vim nucleus from a high‐resolution histological atlas 19 transformed using nonlinear deformation into Colin27 MRI space. This atlas follows neurosurgical labeling conventions as defined by Schaltenbrand and Wahren 20 and Hirai and Jones 7 and has been validated interoperatively 21. The radius of the ROIs was chosen as 1 mm.
Network Connectivity Analysis
Functional connectivity was analyzed using the REST Toolkit (http://restfmri.net/forum/REST) 22. Firstly, by averaging the time courses within each ROI, we could obtain the seed reference time course. Subsequently, correlation analysis was performed in a voxel‐wise manner between the seed reference and the whole brain. The individual correlation coefficients were further transformed to z‐values using the Fisher r‐to‐z transformation. One‐sample t‐test was applied to investigate functional connectivity within each group. A one‐way analysis of covariance (ANCOVA) was performed to examine differences of connectivity in each ROI among the three groups, with age and gender taken as covariant factors. A subsequent post hoc analysis was applied to explore the differences between the groups. In addition, a correlation analysis of functional connectivity results against the tremor scores in the TD group, or against the akinetic–rigidity scores in the ARD group was performed to explore whether the interactions of brain networks correlate with the severity of clinical symptoms.
As functional connectivity analysis is sensitive to gross head motion 23, we further evaluated effect of head motion on our results. Imaging data were motion‐corrected with FSL (http://www.fmrib.ox.ac.uk/fsl), and then the differences among the three groups were analyzed with the same method described above.
Results
In all groups, the Vim nucleus had functional connectivity with the primary motor cortex (M1), premotor cortex (PMC), supplementary motor area (SMA), parietal cortex, temporal lobe, thalamus, globus pallidus, putamen, cerebellum, midbrain, and pons (one‐sample t‐test, P < 1 × 10−5, false discovery rate (FDR) corrected; Figure 1). There were significant differences in connectivity among the TD, ARD, and control groups in the M1, PMC, SMA, parietal cortex, temporal lobe, putamen, globus pallidus, thalamus, and cerebellum (ANCOVA, P < 0.05, FDR corrected; Figure 2). Compared with the controls, TD patients exhibited increased connectivity with the Vim nucleus in several areas, including the cerebellum, M1, SMA, PMC, thalamus, globus pallidus, putamen, and parietal cortex (post hoc analysis, P < 0.05, FDR corrected; Figure 3A and Table 2). In contrast, ARD patients only showed enhanced connectivity with the Vim nucleus in the globus pallidus and limbic lobe compared with the controls (post hoc analysis, P < 0.05, FDR corrected; Figure 3B and Table 2). TD patients had increased connectivity with the Vim nucleus in the cerebellum, M1, SMA, thalamus, globus pallidus, putamen, and parietal cortex compared with ARD patients (post hoc analysis, P < 0.05, FDR corrected; Figure 3C).
Figure 1.
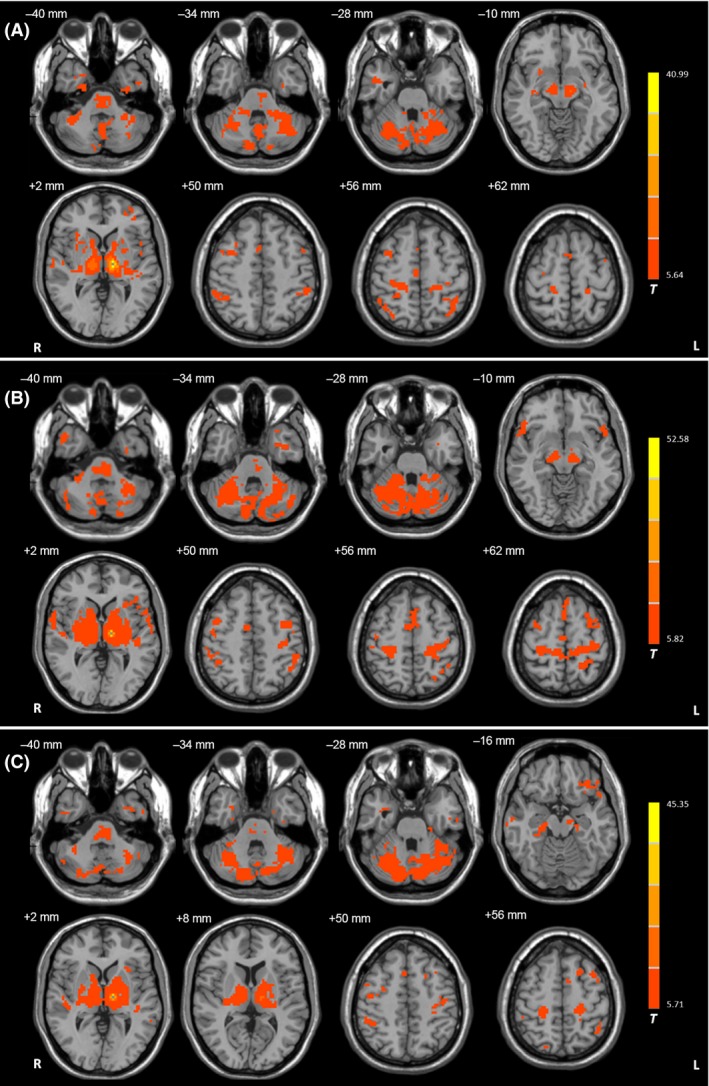
Functional connectivity in the left Vim nucleus. Brain regions showing significant connectivity with the left Vim nucleus in the resting state in (A) healthy control subjects, (B) tremor‐dominant (TD), and (C) akinetic‐/rigid‐dominant (ARD) patients (one‐sample t‐test, P < 1 × 10−5, FDR corrected). T value bar is shown on the right. Abbreviations: L, left; R, right.
Figure 2.
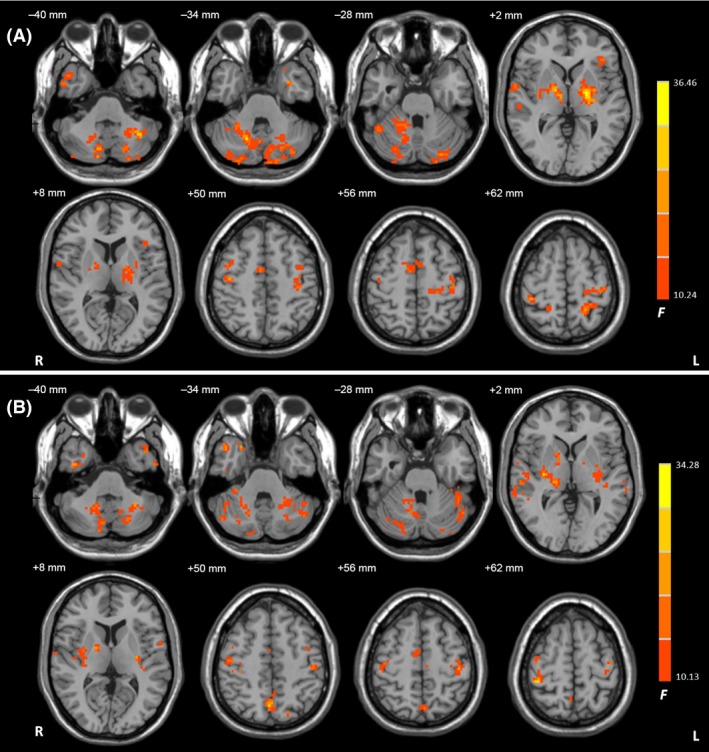
The results of ANCOVA. Brain regions showing significant differences in connectivity among the TD, ARD, and control groups in the left (A) and right (B) Vim nucleus (ANCOVA, P < 0.05, FDR corrected). F value bar is shown on the right. Abbreviations: L, left; R, right.
Figure 3.
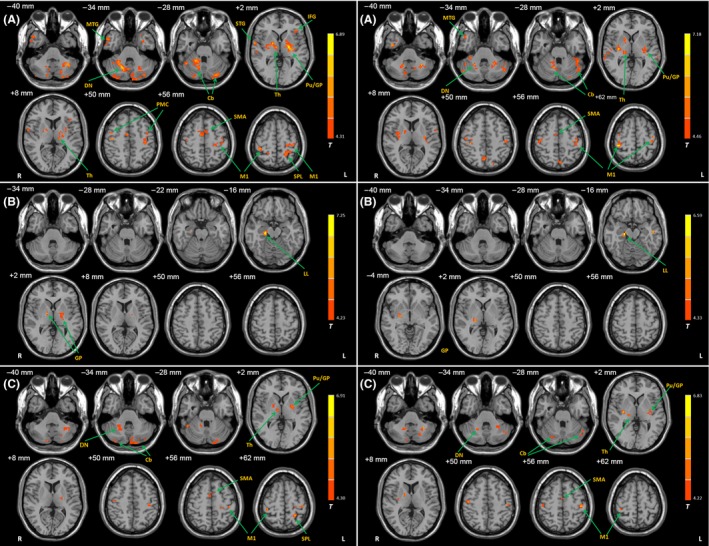
Differences of functional connectivity with the Vim nucleus between the groups. Brain regions showing significant increased connectivity with the left (left column) and right (right column) Vim nucleus in the TD patients compared with healthy controls (A), in the ARD patients compared with healthy controls (B), and in the TD patients compared with ARD patients (C). post hoc analysis, P < 0.05, FDR corrected. T value bars are shown on the right. Abbreviations: L, left; R, right; Cb, cerebellum; DN, dentate nucleus; GP, globus pallidus; IFG, inferior frontal gyrus; LL, limbic lobe; M1, primary motor cortex; MTG, middle temporal gyrus; PMC, premotor cortex; Pu, putamen; SMA, supplementary motor area; SPL, superior parietal lobule; Th, thalamus.
Table 2.
The difference of functional connectivity between PD patients and controls
| Group | Brain region | Brodmann area | MNI coordinates | t Value | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| TD > Control | R Precentral Gyrus | 4 | 41 | −14 | 52 | 5.03 | 486 |
| L M1 | 4 | −35 | −26 | 62 | 5.59 | 2808 | |
| R M1 | 4 | 37 | −31 | 60 | 5.82 | 945 | |
| L Superior Parietal Lobule | 5 | −22 | −47 | 64 | 5.31 | 2376 | |
| R Superior Parietal Lobule | 5 | 21 | −46 | 62 | 4.64 | 324 | |
| L PMC | 6 | −39 | −2 | 51 | 4.50 | 432 | |
| R PMC | 6 | 43 | 0 | 48 | 4.67 | 351 | |
| R SMA | 6 | 7 | −4 | 56 | 5.27 | 1944 | |
| R Middle Temporal Gyrus | 21 | 53 | 7 | −32 | 5.04 | 1134 | |
| R Superior Temporal Gyrus | 21 | 56 | −23 | −2 | 4.59 | 432 | |
| R Superior Temporal Gyrus | 22 | 62 | −2 | 4 | 5.57 | 918 | |
| L Limbic Lobe | 28 | −25 | 3 | −30 | 4.47 | 486 | |
| L Inferior Frontal Gyrus | 47 | −40 | 29 | 4 | 4.87 | 702 | |
| L Thalamus, Pulvinar | – | −15 | −23 | 8 | 5.23 | 513 | |
| R Thalamus, Ventral Lateral Nucleus | – | 9 | −11 | 3 | 5.85 | 756 | |
| L Globus Pallidus | – | −20 | −11 | 3 | 6.89 | 2727 | |
| R Globus Pallidus | – | 18 | −2 | 2 | 6.06 | 918 | |
| L Putamen | – | −30 | −2 | 9 | 4.79 | 378 | |
| R Putamen | – | 31 | −5 | 4 | 5.11 | 567 | |
| L Cerebellum, Posterior Lobe, Declive | – | −30 | −84 | −24 | 6.49 | 5859 | |
| L Cerebellum, Posterior Lobe, Tonsil | – | −27 | −54 | −42 | 5.74 | 3132 | |
| R Cerebellum, Anterior Lobe, Culmen | – | 21 | −47 | −28 | 6.03 | 2511 | |
| R Cerebellum, Anterior Lobe, Dentate | – | 18 | −62 | −34 | 6.41 | 6345 | |
| R Cerebellum, Posterior Lobe, Uvula | – | 30 | −88 | −32 | 5.23 | 1485 | |
| ARD > Control | R Limbic Lobe | 35 | 21 | −21 | 15 | 7.25 | 729 |
| L Globus Pallidus | – | −20 | −9 | 3 | 4.95 | 1107 | |
| R Globus Pallidus | – | 17 | −6 | 4 | 5.71 | 324 | |
L, left; R, right; ARD, akinetic‐/rigid‐dominant; TD, tremor‐dominant; M1, primary motor cortex; PMC, premotor cortex; SMA, supplementary motor area.
The results are the comparison of functional connectivity in the left Vim nucleus between subtypes of PD patients and controls (post hoc analysis, P < 0.05, FDR corrected).
The connections between the Vim nucleus and M1 and cerebellum had positive correlations with the tremor scores in the TD group (correlation analysis, P < 0.05, FDR corrected; Figure 4 and Table 3). In contrast, no connectivity showed significant correlation against the akinetic–rigidity scores in the ARD group.
Figure 4.

The results of correlation analysis. Brain regions showing significant correlation between connectivity results in the left (A) and right (B) Vim nucleus and tremor scores in TD patients (correlation analysis, P < 0.05, FDR corrected). T value bar is shown on the right. Abbreviations: L, left; R, right; Cb, cerebellum; DN, dentate nucleus; M1, primary motor cortex.
Table 3.
Correlation between connectivity and tremor scores in the TD group
| Brain region | Brodmann area | MNI coordinates | t Value | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L Postcentral Gyrus | 3 | −37 | −35 | 53 | 0.65 | 621 |
| L M1 | 4 | −38 | −19 | 51 | 0.74 | 675 |
| R Cerebellum, Anterior Lobe, Dentate | – | 15 | −45 | −33 | 0.70 | 1863 |
| L Cerebellum, Posterior Lobe, Tonsil | – | −52 | −50 | −38 | 0.61 | 1998 |
| R Cerebellum, Anterior Lobe, Uvula | – | 30 | −83 | −35 | 0.58 | 324 |
L, left; R, right; M1, primary motor cortex.
Brain regions showed significant correlation between connectivity results in the left Vim nucleus and tremor scores in the TD group (correlation analysis, P < 0.05, FDR corrected).
After motion‐corrected with FSL, compared with controls, TD patients had increased connectivity with the cerebellum, M1, SMA, PMC, thalamus, globus pallidus, putamen, and parietal cortex; ARD patients showed enhanced connectivity with the globus pallidus and limbic lobe. In addition, TD patients had increased connectivity with the cerebellum, M1, SMA, thalamus, globus pallidus, putamen, and parietal cortex compared with ARD patients (post hoc analysis, P < 0.05, FDR corrected; Figure 5).
Figure 5.
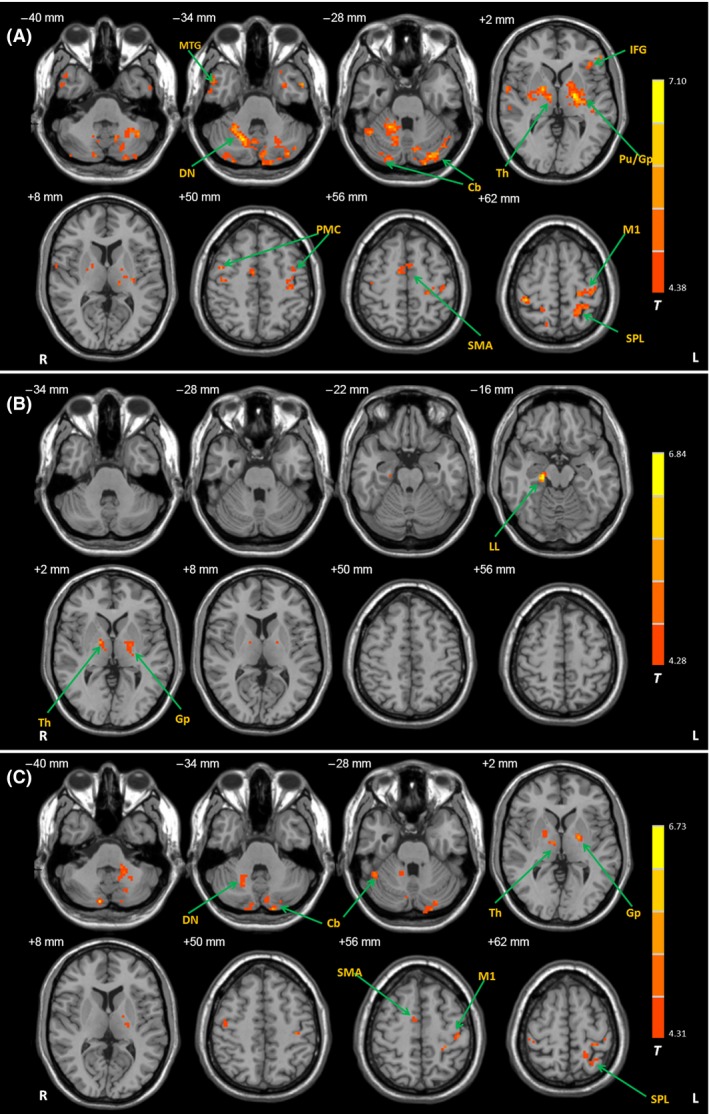
Differences of functional connectivity with the Vim nucleus between the groups after motion correction. Brain regions showing significant increased connectivity with the left Vim nucleus in the TD patients compared with healthy controls (A), in the ARD patients compared with healthy controls (B), and in the TD patients compared with ARD patients (C). Imaging data were motion‐corrected with FSL. post hoc analysis, P < 0.05, FDR corrected. T value bars are shown on the right. Abbreviations: L, left; R, right; Cb, cerebellum; DN, dentate nucleus; GP, globus pallidus; IFG, inferior frontal gyrus; LL, limbic lobe; M1, primary motor cortex; MTG, middle temporal gyrus; PMC, premotor cortex; Pu, putamen; SMA, supplementary motor area; SPL, superior parietal lobule; Th, thalamus.
Discussion
In the current study, we investigated functional connectivity in the Vim nucleus in TD and ARD subtypes of PD. We have shown that the involvement of Vim nucleus in the tremor‐ and akinetic‐/rigid‐related networks is different. In the TD patients, the Vim nucleus had enhanced connectivity with the cerebellum, M1, SMA, PMC, thalamus, globus pallidus, putamen, and parietal cortex compared with healthy controls, while the connections between the Vim nucleus and M1 and cerebellum positively correlated with the tremor scores. In contrast, the Vim nucleus only had increased connectivity with the globus pallidus and limbic lobe in the ARD patients. Our findings demonstrate that the Vim nucleus is important in the tremor‐related network, but not in the akinetic‐/rigid‐related network in PD.
The Vim Nucleus in the Tremor‐Related Network
The Vim nucleus functionally connected with extensive regions, including the M1, SMA, PMC, parietal cortex, globus pallidus, putamen, cerebellum, midbrain, and pons in all groups (Figure 1), which is largely consistent with a recent report 18. The Vim nucleus had enhanced connectivity with the cerebellum/dentate nucleus in the TD patients compared with healthy controls (Figure 3A and Table 2). Moreover, this connectivity was correlated with the severity of parkinsonian tremor (Figure 4). The Vim nucleus chiefly receives excitatory glutamatergic input from the cerebellum/dentate nucleus 24. And the dentate nucleus, acting as a major output source of cerebellar, projects to the motor, premotor, prefrontal, and parietal areas of cortex 25. As described previously, dysfunction of cerebello–thalamo–cortical pathway has been recognized to play a critical role in parkinsonian tremor genesis 2, 3, 4, 5. The parkinsonian tremor is associated with an increased metabolism in the cerebellum/dentate nucleus 4, which could be reduced by deep brain stimulation (DBS) of Vim 4, 26. Our findings indicate that the enhanced Vim–cerebellum/dentate nucleus connectivity is associated with parkinsonian tremor; this connectivity is more strengthened as tremor becomes more severe.
Increased connectivity is found between the Vim nucleus and cortical motor regions, for example, the M1, SMA, and PMC in TD patients (Figure 3A and Table 2). The Vim nucleus sends excitatory glutamatergic efferents to cortical motor regions. These cortical motor regions have been identified involving in tremor‐related network 2, 3, 4, 27. The M1 has been suggested as the cortical area that drives the spinal motor neuron pool in parkinsonian tremor; meanwhile, the activity in PMC and SMA primarily projects to the M1 3. Vim DBS could reduce the metabolism in the M1 4, 26, which further suggests the treatment pathology of Vim DBS in PD tremor.
In addition, the Vim nucleus had increased connectivity with the globus pallidus and putamen in the TD patients compared with controls (Figure 3A and Table 2). As the Vim nucleus does not receive direct projections from the basal ganglia 28, the functional connectivity between the Vim nucleus and basal ganglia should via other regions, for example, cortical motor cortex. Our finding is consistent with a previous report that the basal ganglia (globus pallidus and putamen) had increased connectivity with the Vim–motor cortex–cerebellum circuit through the motor cortex in the TD patients 5.
The Vim Nucleus in the Akinetic‐/Rigid‐Related Network
The Vim nucleus had enhanced connectivity only with the globus pallidus and limbic lobe in the ARD patients (Figure 3B and Table 2). Neuroimaging studies have shown that typical neural changes associated with akinesia include decreased activity in the putamen 29 and SMA 30, as well as increased activity in some other motor regions, like the PMC, parietal cortex, and cerebellum 31. The SMA is acknowledged its key role in learning, planning and initiation a motor task 32. The dysfunction of the SMA has been recognized as a crucial reason for akinesia 33. The nature of the hyperactivation in these motor regions remains unclear. One likely explanation is that this phenomenon presents a functional compensation for the defective basal ganglia 31, 34. In the ARD patients, the Vim nucleus did not show any increased or decreased connectivity with these akinesia‐related brain regions. Moreover, connectivity with the Vim nucleus showed no significant correlation against the akinetic–rigidity scores. These findings indicate that the Vim nucleus is not critical in the akinetic‐/rigid‐related network.
Vim DBS can immediately and almost completely suppress parkinsonian tremor 35, but has no effect on akinesia and rigidity. Vim DBS is considered only for TD and in the absence of pronounced rigidity or akinesia. Our finding showed that the Vim is heavily involved in the tremor‐related network, but not in the akinetic‐/rigid‐related network provides a reasonable explanation why Vim DBS has a selective effect on different subtypes of PD.
The results before and after motion correction were largely consistent (Figures 3 and 5). In the current study, we have carefully examined head motion in our subjects; no one had more than 1.5 mm maximum translation in x, y, or z, or 1.5° of maximum angular motion in the three axes. In addition, head motion was corrected with DPARSF. Thus, head motion had no significant influence on our results.
A limitation of this study is that tremor in PD patients may have influence on our results. Future studies with simultaneous electromyography monitoring during fMRI scanning may help to regress out the influence of tremor. Another limitation is that we included both TD and ARD subtypes; most patients participated in this study did not receive Vim DBS. Thus, the effect of Vim DBS on the pattern of functional connectivity in the Vim nucleus was not examined. This will need to be investigated in future studies.
In conclusion, this study demonstrates that the Vim nucleus has a critical role in the tremor‐related network, but not in the akinetic‐/rigid‐related network. Our finding is helpful to explain why Vim DBS can significantly improve tremor, but has no effect on akinesia and rigidity in PD.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81071012 and 81271429), and Seed Grant of International Alliance of Translational Neuroscience (PXM2014_014226_000015).
The first two authors contributed equally to the work.
References
- 1. Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto‐rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol 2009;22:387–393. [DOI] [PubMed] [Google Scholar]
- 2. Volkmann J, Joliot M, Mogilner A, et al. Central motor loop oscillations in parkinsonian resting tremor revealed by magnetoencephalography. Neurology 1996;46:1359–1370. [DOI] [PubMed] [Google Scholar]
- 3. Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain 2003;126:199–212. [DOI] [PubMed] [Google Scholar]
- 4. Mure H, Hirano S, Tang CC, et al. Parkinson's disease tremor‐related metabolic network: characterization, progression, and treatment effects. NeuroImage 2011;54:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 2011;69:269–281. [DOI] [PubMed] [Google Scholar]
- 6. DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007;64:20–24. [DOI] [PubMed] [Google Scholar]
- 7. Hirai T, Jones EG. A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev 1989;14:1–34. [DOI] [PubMed] [Google Scholar]
- 8. Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp 2009;30:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu T, Long X, Wang L, et al. Functional connectivity of cortical motor areas in the resting state in Parkinson's disease. Hum Brain Mapp 2011;32:1443–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico‐striatal connectivity in Parkinson's disease. Cereb Cortex 2010;20:1175–1186. [DOI] [PubMed] [Google Scholar]
- 11. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang AE, Fahn S. Assessment of Parkinson's disease In: Munsat TL, editor. Quantification of Neurological Deficit. Boston, MA: Butterworths, 1989;285–309. [Google Scholar]
- 13. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 14. Schiess MC, Zheng H, Soukup VM, Bonnen JG, Nauta HJ. Parkinson's disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat Disord 2000;6:69–76. [DOI] [PubMed] [Google Scholar]
- 15. Lewis MM, Du G, Sen S, et al. Differential involvement of striato‐ and cerebello–thalamo–cortical pathways in tremor‐ and akinetic/rigid‐predominant Parkinson's disease. Neuroscience 2011;177:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Wei L, Hu X, et al. Akinetic‐rigid and tremor‐dominant Parkinson's disease patients show different patterns of intrinsic brain activity. Parkinsonism Relat Disord 2015;21:23–30. [DOI] [PubMed] [Google Scholar]
- 17. Yan CG, Zang YF. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual‐Leone A. Resting‐state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A 2014;111:E4367–E4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL. The creation of a brain atlas for image guided neurosurgery using serial histological data. NeuroImage 2006;30:359–376. [DOI] [PubMed] [Google Scholar]
- 20. Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the Human Brain. Stuttgart: Georg Thieme, 1977. [Google Scholar]
- 21. Sadikot AF, Chakravarty MM, Bertrand G, Rymar VV, Al‐Subaie F, Collins DL. Creation of computerized 3D MRI‐integrated atlases of the human basal ganglia and thalamus. Front Syst Neurosci 2011;5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 2013;76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson ME, Turner RS. Activity of neurons in cerebellar‐receiving and pallidal‐receiving areas of the thalamus of the behaving monkey. J Neurophysiol 1991;66:879–893. [DOI] [PubMed] [Google Scholar]
- 25. Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol 2003;89:634–639. [DOI] [PubMed] [Google Scholar]
- 26. Fukuda M, Barnes A, Simon ES, et al. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. NeuroImage 2004;21:608–615. [DOI] [PubMed] [Google Scholar]
- 27. Pollok B, Makhloufi H, Butz M, et al. Levodopa affects functional brain networks in Parkinsonian resting tremor. Mov Disord 2009;24:91–98. [DOI] [PubMed] [Google Scholar]
- 28. Percheron G, Francois C, Talbi B, Yelnik J, Fenelon G. The primate motor thalamus. Brain Res Brain Res Rev 1996;22:93–181. [PubMed] [Google Scholar]
- 29. Herz DM, Eickhoff SB, Lokkegaard A, Siebner HR. Functional neuroimaging of motor control in Parkinson's disease: a meta‐analysis. Hum Brain Mapp 2014;35:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haslinger B, Erhard P, Kampfe N, et al. Event‐related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain 2001;124:558–570. [DOI] [PubMed] [Google Scholar]
- 31. Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain 2005;128:2250–2259. [DOI] [PubMed] [Google Scholar]
- 32. Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre‐supplementary motor areas. Nat Rev Neurosci 2008;9:856–869. [DOI] [PubMed] [Google Scholar]
- 33. Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol 2004;14:715–719. [DOI] [PubMed] [Google Scholar]
- 34. Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain 1999;122(Pt 3):483–495. [DOI] [PubMed] [Google Scholar]
- 35. Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg 1996;84:203–214. [DOI] [PubMed] [Google Scholar]


