Dear Editor
Fingolimod (Gilenya®, Novartis) is an effective and safe treatment for multiple sclerosis (MS). Through the sphingosine 1‐phosphate receptor, this drug affects peripheral lymphocytes and dendritic cells, as well as neurons, oligodendrocytes, and astrocytes in the central nervous system 1. A few cases of tumefactive and necrotizing lesions seen on MRI among patients using fingolimod have been described 2, 3, 4, 5, 6, 7, 8, 9. We now add to the discussion by presenting two new cases of very extensive lesions on MRI in brain and spinal cord associated with use of fingolimod. Patients' disabilities are described in terms of their EDSS (Expanded Disability Scale Score) 10. Both patients were investigated for infections or vasculitis that might have explained the new lesions. The Ethics Committee of Universidade Metropolitana de Santos approved studies and reports on fingolimod in Brazilian patients with MS under CAAE 39658414.9.0000.5509. These patients gave written consent to have their case presented for medical discussion, and confidentiality on data was guaranteed.
Case 1 – The patient was a 28‐year‐old female who presented suboptimal response to glatiramer acetate and interferon beta‐1a, and for whom use of natalizumab was inconvenient (she was frequently traveling for work). After 36 months of using natalizumab with good disease control (JCV‐negative), the medication was changed to fingolimod to facilitate administration. At that time, she presented EDSS 2.0. After 20 months of fingolimod without relapses or increased disability, the patient underwent new MRI. At this time, she complained of a tingling feeling in her limbs. A very large lesion with concentric layers was detected in the frontal lobe (Figure 1). Several lesions in the spinal cord were also observed (Figure 1). The symptoms did not evolve, and no cognitive dysfunction was observed at any time (EDSS = 2.0). Fingolimod was withdrawn, and the patient is, at present, using monthly pulses of corticosteroids. After 2 months of fingolimod withdrawal, the lesions were markedly reduced in MRI. The patient has returned to natalizumab.
Figure 1.
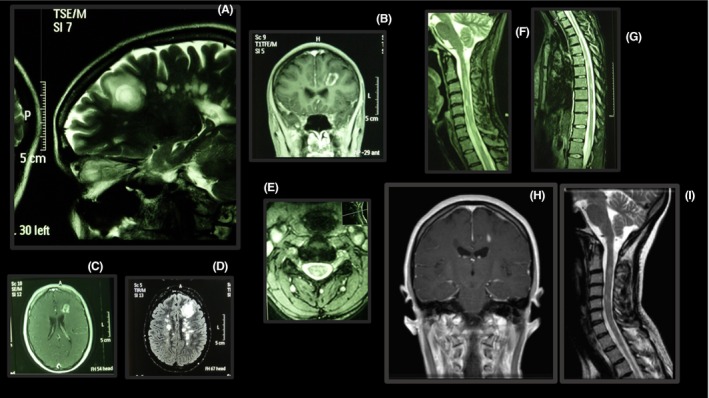
Lesions in the brain and spinal cord of a patient using fingolimod. The frontal lesion seems to have concentric layers in the white matter substance (A) and is enhanced by gadolinium, forming a ring (B, C). This frontal lesion does not invade the cortical grey matter layer (A). Spinal cord lesions affect both cervical and thoracic segments (E, F, G). Two months after fingolimod withdrawal, the lesions were markedly reduced (H, I).
Case 2 – The patient was a 35‐year‐old female with suboptimal response to glatiramer acetate and interferon beta‐1a. She was treated with natalizumab for 24 months, but because she was JCV‐positive, the medication was changed to fingolimod (EDSS = 2.0). After 15 months of treatment with this drug, she developed motor and ataxic symptoms without cognitive dysfunction (EDSS = 3.5). She was JCV‐negative in her spinal fluid, and the lesion improved with fingolimod withdrawal. Thus, progressive multifocal leukoencephalopathy (PML) was ruled out. An extensive tumefactive lesion was observed on MRI (Figure 2). After 2 months of fingolimod withdrawal, the tumefactive lesion reduced in MRI. The patient has returned to natalizumab treatment and remains stable with EDSS 3.0.
Figure 2.
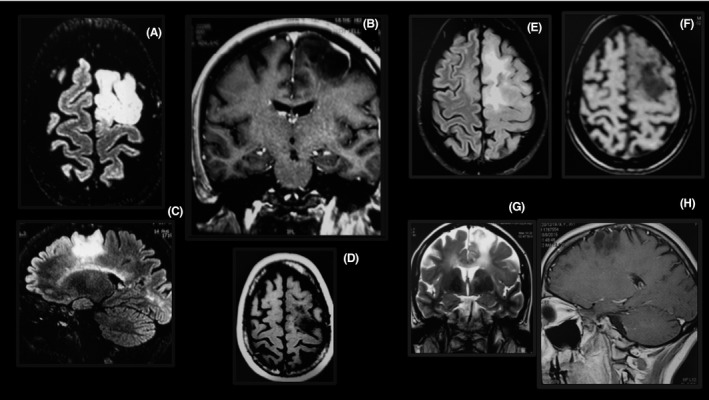
Tumefactive lesion in the brain of a patient using fingolimod. The lesion is large (A–D), enhanced by gadolinium forming a ring (B), and restricted to the white matter (A–C). Two months after fingolimod withdrawal, the lesions were markedly reduced (E–H).
The cases presented here are similar to those recently described by other researchers in this field 2, 3, 4, 5, 6. It is interesting to note that the lesions are restricted to the white matter and do not invade the gray matter cortex. The effects of fingolimod are different in the peripheral blood and in the brain, and observational follow‐up from “real‐life” clinical settings is as important as clinical trials. Close follow‐up of the present cases is of essence to uncover the underlying pathogenesis of these lesions. An increasing number of new drugs for treating MS is becoming available and, therefore, enhanced clinical alertness to cases like these is extremely relevant.
Conflict of interest
The authors have no conflict of interests to declare.
References
- 1. Groves A, Kihara Y, Chun J. Fingolimod: Direct CNS effects of sphingosine 1‐phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci 2013;328:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Visser F, Wattjes MP, Pouwels PJ, et al. Tumefactive multiple sclerosis lesions under fingolimod treatment. Neurology 2012;79:2000–2003. [DOI] [PubMed] [Google Scholar]
- 3. Leypoldt F, Munchau A, Moeller F, et al. Hemorrhaging focal encephalitis under fingolimod (FTY720) treatment: A case report. Neurology 2009;72:1022–1024. [DOI] [PubMed] [Google Scholar]
- 4. Jander S, Turowski B, Kieseier BC, et al. Emerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimod. Mult Scler 2012;18:1650–1652. [DOI] [PubMed] [Google Scholar]
- 5. Kinney MO, McDonnell G. Tumefactive multiple sclerosis lesions under fingolimod treatment. Neurology 2013;81:403. [DOI] [PubMed] [Google Scholar]
- 6. Pilz G, Harrer A, Wipfler P, et al. Tumefactive MS lesions under fingolimod: A case report and literature review. Neurology 2013;81:1654–1658. [DOI] [PubMed] [Google Scholar]
- 7. Hellmann MA, Lev N, Lotan I, et al. Tumefactive demyelination and a malignant course in an MS patient during and following fingolimod therapy. J Neurol Sci 2014;344:193–197. [DOI] [PubMed] [Google Scholar]
- 8. Harirchian MH, Taalimi A, Siroos B. Emerging tumefactive MS after switching therapy from interferon‐beta to fingolimod: A case report. Mult Scler Relat Disord 2015;4:400–402. [DOI] [PubMed] [Google Scholar]
- 9. Boangher S, Goffette S, Van Pesch V, Mespouille P. Early relapse with tumefactive MS lesion upon initiation of fingolimod therapy. Acta Neurol Belg. 2015. [Epub ahead of print] doi: 10.1007/s13760-015-0495-z. [DOI] [PubMed] [Google Scholar]
- 10. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology (Cleveland) 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]


