Summary
Progesterone, estrogens, and testosterone are the well‐known steroidal sex hormones, which have been reported to have “nonreproductive “effects in the brain, specifically in the neuroprotection and neurotrophy. In the last one decade, there has been a surge in the research on the role of these hormones in neuroprotection and their positive impact on different brain injuries. The said interest has been sparked by a desire to understand the action and mechanisms of these steroidal sex hormones throughout the body. The aim of this article was to highlight the potential outcome of the steroidal hormones, viz. progesterone, estrogens, and testosterone in terms of their role in neuroprotection and other brain injuries. Their possible mechanism of action at both genomic and nongenomic level will be also discussed. As far as our knowledge goes, we are for the first time reporting neuroprotective effect and possible mechanism of action of these hormones in a single article.
Keywords: Estrogen, Neuroprotection, Neurotrophy, Progesterone, Steroids, Testosterone
Introduction
The best‐known steroidal sex hormones secreted primarily by the ovaries in females are progesterone, estrogens, and testosterone, from the testicles in males. The hypothalamus releases the peptide gonadotropin‐releasing hormone (GnRH) which ultimately controls the release of the peptides follicle‐stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary 1. These hormones control the development of follicular growth and corpus luteum in females, which ultimately results in the production of progesterone and estrogen 2. Several studies revealed that apart from reproductive roles, progesterone, estrogens, and testosterone have protective actions against different brain injuries 3, 4, 5.
There is growing evidence that suggests progesterone and its metabolite (allopregnanolone) could exert neuroprotective effects on the injured central nervous system (CNS). Over the last several years, preclinical studies around the world also suggested that progesterone, given in the acute stage of injury, could limit tissue damage and improves functional outcome after blunt traumatic brain injury (TBI), spinal cord injury, stroke, diabetic neuropathies, and other types of acute neural injury 6, 7, 8, 9, 10, 11. Progesterone has been reported for their role in brain neurogenesis regulation and regeneration, convulsions, myelination, TBI, cognition, mood alteration, inflammation, and mitochondrial function in different animal models 12, 13, 14, 15, 16, 17. Several clinical studies also highlighted their role in neuroprotection 18, 19. The neuroprotective mechanism of action of progesterone is still at the speculative stage because of vast complexity of the brain. Some scientific literature supports the involvement of progesterone receptors (PR) that include the classic nuclear progesterone receptors A (PRA), progesterone receptors B (PRB) and their spliced variants and seven transmembrane progesterone receptors β 20, 21. Scientific literature also suggested activation of different CNS signaling cascade pathways, viz. mitogen‐activated protein kinase (MAPK), extracellular‐regulated kinase (ERK), and up‐regulation of brain‐derived neurotrophic factor (BDNF) by progesterone 22, 23, 24, 25, 26, 27. All of these signaling cascades have been reported for their role in neuroprotection.
On the other hand, several studies reported the role of estrogen in stimulation, neuroprotection, and beneficial for cell survival 28, 29. The mechanism of action of estrogens also remains obscure, because of the complexity of the brain. Like progesterone, the data related to estrogens also suggest that estrogen receptor‐α (ERα) and estrogen receptor‐β (ERβ) may exert neuroprotection in the brain. At genomic level, estrogen has been reported to increase the expression of the anti‐apoptotic gene, B‐cell lymphoma 2 (bcl‐2), and inhibits the expression of the pro‐apoptotic gene. In vitro study also highlighted their role in the activation of extracellular signal‐regulated kinases (ERK) and phosphoinositol‐3‐kinase (PI3K)‐Akt pathways 30. The enhanced release of transforming growth factor (TGF)‐β1 is an important step of neuroprotection by estrogen 28. Some researchers also support the notion that testosterone could act against neurodegenerative disorders including Alzheimer's disease (AD), mild cognitive impairment (MCI), or depression 31. In the following section, we will try to cover these steroidal sex hormones at the individual level based on their neuroprotective action along with their mechanistic insight.
Progesterone and Their Neuroprotective Action
Progesterone has been reported for their neuroprotective action in various in vitro and in vivo models 27, 32, 33, 34. Several clinical studies also highlighted the neuroprotective potential of progesterone 18, 19, 35, 36, 37. Moreover, progesterone could target several regions of the brain particularly hypothalamus, hippocampus, and cortex. In most of the studies, progesterone has been suggested to have neuroprotective and/or regenerative effects in the hippocampal and cortical region 32, 33. It can be synthesized by both central and peripheral nervous system and can act not only on the brain but also on peripheral nerves. Baulieu et al. (1996) reported that progesterone promotes the formation of the myelin sheath in rat Schwann cells and increases the number of myelinated axons 38. In another study, the same group reported that blocking the action of progesterone impairs myelination in regenerating axons 39. Progesterone has been also reported to stimulate myelination in organotypic slice cultures of rats and mouse cerebellum 40. Several pieces of evidence in scientific literature also suggest that depletion in sex steroidal hormones, estrogen, and progesterone at menopause stage is a significant risk factor for the development of AD in women, which could be overcome by hormonal therapy 41, 42. Gonzalez et al. (2004) showed higher expression of BDNF at both mRNA and protein level in response to progesterone treatment in rats with spinal cord injury 43. Progesterone enhancement of endogenous neuronal BDNF could also provide a trophic environment within the lesioned spinal cord and might be part of progesterone‐activated pathways to provide neuroprotection 43. Moreover, progesterone treatment also significantly reduces the neuropathological and behavioral abnormalities associated with TBI in the rodent model 44. The antioxidant effect of progesterone also supports its potential in the treatment of brain injury 45. Progesterone has been reported for its neuroprotective action in symptomatic wobbler mice with spinal cord motor neuron degeneration, which could be mediated by the regulation of expression of some specific genes in neurons and glial cells 43. The therapeutic outcome associated with progesterone in the management of acute spinal cord injury was also reported to be good 46. Progesterone also acts on other brain region such as nucleus tractus solitarius (NTS), reverses the hypoxic injury in rats, and restores the normal respiratory rhythm 47. Progesterone and its derivatives (dihydroprogesterone [DHP] and tetrahydroprogesterone [THP]) have been reported to have the protective effect against diabetic neuropathy in experimental animal models 48. Scientific literature suggests the profound effect of progesterone on seizure processes 49, 50. Antiseizure effects of progesterone and its metabolite have been also reported in various animal models 51, 52. Progesterone has also shown to decrease epileptiform activity in mice 53, maximal electroshock (MES)‐induced seizures in rat 54, kainic acid‐induced seizures in rat 55, and amygdala‐kindled seizures in rats 56. Along with earlier mentioned studies, some clinical studies also reported a decrease in catamenial epilepsy in women in response to progesterone, which points antiseizure potential of progesterone 57.
Collectively, all these mechanisms might be important in protecting the brain against various neurodegenerative diseases and brain‐related dysfunctions.
In view of encouraging results of the preclinical studies, progesterone had been taken into the clinical trials for its neuroprotective effects especially against TBI, ischemic stroke, multiple sclerosis and even for the treatment of AD. Interestingly, the neuroprotective efficacy of progesterone was found to be remarkable at the earliest phase of animal models for TBI, traumatic spinal cord injury, middle cerebral artery occlusion, and neurodegeneration 58, 59, 60, 61, 62. The above‐mentioned reports enable progesterone to enter four phase II trials to test its protective efficacy after TBI including ProTECT II and SyNAPSe. It also came out with consistent and encouraging outcome 18, 19, 63, 64. However, the results of the large multicenter randomized and placebo‐controlled phase III trials were disappointing 65, 66, 67. Phase III trial in women with intractable partial epilepsy also did not show much effect by progesterone, but the post hoc analysis identified a subset of women with higher levels of perimenstrual seizure exacerbation that were responsive to treatment 68. Progesterone as a neuroprotective agent offers great promises still it failed at phase III clinical trials. The reason behind the failure of progesterone at phase III trial was believed to be due to faulty extrapolation of preclinical animal studies data and use of subjective items measures that neither reflects nature of deficits or long‐term quantitative recovery. In one of the report, Ioannidis (2005) suggested that clinical research in general typically fails because of the laboratory studies on which trials are based often do not replicate one another and have too many false‐positive findings 69. These published papers then lead to unwarranted and overenthusiastic estimations of effect sizes and are uncritically reported in the peer‐review literature and accepted and used by the clinical community to go forward with clinical trials. Future problems can be avoided by pooling preclinical data, require more coordinated and sequential phase II trials using standardized outcomes to replicate potential findings 70. Recently, Menon and Maas (2015) suggested that precise definitions of injury, better trial design, better patient selection procedures, better outcome measures, and better options for when to take them and how often are needed to get positive outcome during phase III clinical trials 71. It is also suggested to test the potential of these hormones in nonhuman primates so that a high number of model animal could be involved.
Mechanism of Neuroprotection by Progesterone
Nuclear progesterone receptor (PR) has been localized in several regions of the brain including hippocampus, hypothalamus, cortex, and cerebellum 20, 72, 73, 74. Typically, the steroid hormones such as progesterone, estrogen, and testosterone had been maintained inactive under hypotonic conditions due to the association with heat‐shock proteins (hsps). The interaction between PR and chaperons molecule (hsp70, hsp90, hsp40) is prerequisite for hormonal binding which further express their action by dissociation from the chaperons molecule, then dimerize and finally interact with progesterone response element at the promoter region of the target genes 75, 76. Two major isoforms of PR, a full length PR‐B and N‐terminal truncated PR‐A isoform, have been reported to exist 77, 78. Although PR is expressed in hippocampus and frontal cortex, progesterone shows its effect in PR knockout mice as well, indicating the involvement of some other receptor 79. A novel progesterone binding protein (distinct from conventional PR), exclusively localized in neural tissue including the cerebral cortex, cerebellum, caudate nucleus, thalamus, pituitary gland, and spinal cord, has been also reported 80. Overall, progesterone has established neuroprotective action that takes place via several mechanisms. They could cause a reduction in both IL‐β and TNF‐α level in post‐TBI 43. Progesterone has been also reported to inhibit the inflammatory cytokines in the medial frontal cortex of TBI 81, 82. They reduce cerebral edema by stabilizing the blood–brain barrier, thus preventing the flow of water, ions, and inflammatory molecule across the membrane 43, 45. In fact, progesterone, estrogen, and testosterone had been known to activate MAPK, ERK, and Akt signaling pathways, which are reported to be associated with neuroprotection 83. Earlier studies suggested the co‐regulation between BDNF and steroid hormones, viz. progesterone, estrogens, and testosterone. BDNF is a neurotrophin, abundantly expressed in several areas of the CNS and known to enhance specific learning and memory processes 84. Neuroprotection by progesterone is associated with up‐regulation of BDNF, a rise in the activity of choline acetyltransferase, and reduction in the mitochondrial dysfunction 85, 86, 87, 88. Progesterone has been also reported to suppress inflammatory response and expression of nitric oxide synthase‐2 in cerebral ischemia model 89. On the other hand, it is noteworthy that progesterone could block the estrogen‐induced increase in spine density in hippocampus and reverses the estrogen‐induced enhancement of spatial memory in rodents indicating its antagonistic relationship with estrogen 90, 91, 92.
Estrogens and Their Neuroprotective Action
Apart from the reproductive role, estrogens have been reported to exert complex and diverse action against neurodegenerative disease and injuries. In addition, estrogens have shown to have protective effect on the stroke in animal models 93, 94. Furthermore, it also improves histological, physiological, and behavioral outcomes after transient middle cerebral artery occlusion, global forebrain ischemia, and subarachnoid hemorrhage and may be beneficial in reducing the risk of cognitive decline in women with normal function 95. Wise et al. (2005) reported that 17‐beta‐estradiol (estrogen) slows down the progression of injury, diminishes the extent of cell death by suppressing apoptotic pathways, and enhances the expression of cell survival genes 96. Administration of this estrogen to male rats significantly reduces brain edema and neurological deficits 97. Brain edema was reported to be less significant in female compared with male rats. The estrogen receptor (ER) antagonist ICI182, 780 was found to be exacerbated in an intracerebral hemorrhage (ICH)‐induced brain edema in female but not in male rats, suggesting protective ER activation during ICH in female rats 97. Estrogens and related drugs (selective estrogen receptor modulator, ERα, and Erβ) agonist produce neuroprotection of focal and global ischemia induced in the rat, mice, and gerbils 98, Moreover, estrogen treatment protects the dorsal hippocampal neurons CA1 regions, which are susceptible to ischemic injury 98. Goodman et al. (1996) reported that pretreatment of estrogens could protect cultured hippocampal neurons against oxidative stress injury, glucose deprivation, glutamate, FeSO4, and amyloid beta‐peptide toxicities and promote cell survival 99. Despite several reports on neuroprotection, some contradictory results of worsening of neuropathology by estrogen have also been reported. However, it is generally believed that estrogen increases neuronal excitability and mediates proconvulsant effects 100. There are also clinical and animal data that show that estrogen has anticonvulsant effects 101. However, conflicting data also exist on the association between estrogen and epileptic seizures.
Promising preclinical studies on estrogen enable it to test in clinical trials for its neuroprotective potential. A pilot clinical trial with estriol administration in women with multiple sclerosis showed promising results with significant reduction in pathological lesions 102. Subsequent phase II study had been enrolled, and one study published with encouraging results as the estriol combined with glatiramer acetate in women with relapsing‐remitting multiple sclerosis and treatment was well tolerated over 24 months 103. Even though several phase II and phase III clinical trials have been registered for estrogen to investigate the protective effects on neurodegeneration, the results are yet to be released.
Mechanism of Neuroprotection by Estrogen
Along with the well‐recognized reproductive effect, estrogens could also influence numerous nonreproductive functions such as bone and mineral metabolism, cardiac and vascular function, memory and cognition, mood alterations, and progression of age‐related disease 104. Several studies have explored the role of different estrogen receptors (ER) in the neuroprotection 94, 95, 105, 106, 107, 108, 109, 110, 111. Two isoforms of estrogen receptor ERα and ERβ are expressed in adult brain. ER antagonist ICI 182780 increases infract size in middle cerebral artery occlusion of cerebral ischemia in female rats 112, 113. Estrogen could also block estrogen‐induced neuroprotection in global ischemia and cortical explants studies 114. The neuroprotective potential of estrogen was found to be lost in OVX estrogen receptor‐α knockout (ERKO) mice but at the same time, it protects the brain of OVX estrogen receptor‐β knockout (BERKO) mice, suggesting the involvement of ERα in neuroprotection 106, 109. Interestingly, few studies reported the involvement of only ERβ isoform and/or both ERα and ERβ in the protection of CA1 neurons from global ischemia‐induced death 115. Under the genomic level, estrogen is known to overexpress anti‐apoptotic gene bcl‐2 in ischemic part following global ischemia 105, 116, 117. Furthermore, it also inhibits the expression of BAD gene, which is the antagonist of bcl‐2 gene 105, 116, 117, 118. Estrogen also induces certain pathways such as rapid activation of extracellular signal‐regulated kinases (ERK) and phosphoinositol‐3‐kinase (PI3K)‐Akt pathways in cortical and hippocampal cells in vitro, which also play a role in the neuroprotective action 22, 119, 120. ER antagonist ICI182, 780 has been reported to block estradiol benzoate treatment‐induced phosphorylation of Akt in the CA1 region of the hippocampus following cerebral ischemia, which is associated with inhibition of pro‐apoptotic MLK3‐MKK4/7‐JNK1/2 (mixed lineage kinase‐3/MAP kinase kinase‐4‐7/c‐jun‐N‐terminal kinase) pathway 22. The interaction of ERα with cytoskeleton protein, p130Cas (a complex containing Src and PI3K), could lead to the activation of ERK and Akt pathways 121. In addition, ERα has also been reported to interact with the calmodulin binding protein (striatin), in vascular cells, which facilitates cell membrane targeting and is critical for estrogen‐mediated Akt and eNOS [endothelial nitric oxide synthase] activation 122. The neuroprotective roles of TGF‐β to cortical, hippocampal neurons, and cerebral ischemia have been also reported in scientific literature 123, 124. Moreover, specific PI3K inhibitors or Akt inhibitor could directly prevent Akt activation and completely block the induction of TGF‐β1 release by estrogen 125.
Androgens/Testosterone and Their Neuroprotective Action
Testosterone, the gonadal hormone, has been reported for its various effects on numerous body tissues, including CNS 31. One of the less known actions of testosterone is neuroprotection that takes place via activation of androgen pathways. Because of its lipophilic nature, testosterone could cross the blood–brain barrier and influence neuronal cells 126. Moreover, testosterone has also been reported to have antioxidant and anti‐apoptotic potential, which provides neuroprotective effect 127, 128, 129. Testosterone acts via androgen receptors, which are present in neurons throughout the CNS 31, 129, 130. Many of the therapeutic effects of testosterone, viz. libido, cognition, and mood alterations, are mediated through CNS 131. The decrease in testosterone level in men may lead to neurological disorders like AD, in which β amyloid (Aβ) protein is directly related to testosterone level 132, 133.
Androgens are also positive regulators of neuronal plasticity in the spinal nucleus of the bulbocavernosus 134, excitability in the CA1 region of the hippocampus 135, and spine density in the hippocampus 136. Moreover, androgens also prevent retraction 137 or increases the length 138 and size 139 of neuritis from motor neurons. Other neurotrophic effects of testosterone include cell differentiation 140, neurogenesis 141, 142, development of neurons in the hippocampus 143, motor 144, 145, and autonomic systems 146.
Regulation of neuronal viability is one of the important actions of androgens. During development, androgen metabolites determine neuron number in specific sexual dimorphic nuclei via apoptosis regulation 147, 148. Androgens could also regulate central and peripheral motor neurons survival following injury 149, 150. As far as our knowledge goes from the available scientific literature, we did not come across any clinical trial study on testosterone that might be currently undergoing. We have summarized the neuroprotective effects and their proposed mechanism of action of progesterone, estrogen, and testosterone in Table 1.
Table 1.
Neuroprotective action and proposed mechanism of action of progesterone, estrogens, and testosterone
| Hormone | Neuroprotective effects | Mechanism of action | References |
|---|---|---|---|
| Progesterone |
Promotes formation of myelin sheath Increases the number of myelinated axons Modifies glial tube organization Stabilizing the blood–brain barrier |
Interact with heat‐shock protein [hsp70, hsp90, and hsp40] ↓IL‐β and TNF‐α in post‐TBI ↑MAPK, ERK, and Akt signaling pathways ↓ Inflammatory response ↓Expression of nitric oxide synthase‐2 |
Baulieu et al. 1996 Ghoumari et al., 2003 Gaichino et al., 2003 Baulieu and Schumacher, 2000 Evans et al. 2004 Wali et al. 2007 Gruenbaum et al. 2011 |
| Estrogens |
Enhances the expression of genes that optimizes cell survival Protection from stroke in experimental animal models Reduces the risk of cognitive decline brain edema and neurological deficits in women Protect neurons against oxidative stress injury, glutamate toxicity, glucose deprivation, FeSO4 toxicity, and amyloid beta‐peptide toxicity Anticonvulsant effects |
↑Expression of anti‐apoptotic gene bcl‐2
↓Expression of BAD gene ↑Extracellular signal‐regulated kinases (ERK) ↑ Phosphoinositol‐3‐kinase (PI3K)‐Akt activation ↑Interaction with p130Cas ↑Interaction with striatin ↑Transforming growth factor‐β (TGF‐β) |
Sampei et al., 2000; Hurn and Macrae, 2000 Simpkins et al., 1997 Catherine et al., 2005 Wise et al., 2005 Nakamura et al., 1996 Goodman et al., 1996 Veliskiova et al., 2007 Gloria et al., 2006 |
| Testosterone |
Neuronal plasticity in the spinal nucleus Excitability in the CA1 region of hippocampus Prevents retraction or increase the length of neuritis from motor neurons Antioxidant and anti‐apoptotic effects |
↑Expression of hsp 70 ↓Apoptotic and rapid cell signaling pathways ↑Signal transduction pathways that have relevance to cell viability in both neuronal and non‐neuronal cells ↑MAPK/ERK signaling |
Nguyen et al., 2010 Tehranipour and Moghimi, 2010 Spritzer and Galea, 2007 Zhang et al., 2010 Ottem et al., 2007 Marron et al., 2005 Nguyen et al., 2005 Yao et al., 2015 |
Mechanism of Neuroprotection by Testosterone
The activation of gene pathways that increase or decrease the expression with cell survival is the general mechanism for androgen receptor (AR)‐dependent neuroprotection. Androgen neuroprotection could be blocked by anti‐androgen flutamide, which antagonizes AR‐dependent neuroprotection. The genomic androgen pathway includes members of heat‐shock protein family that could provide cellular protection during stress 151. Zhang et al. (2004) reported neuroprotection by androgen via increased expression of hsp70 152. Moreover, Pike (2001) suggested AR‐dependent neuroprotection through inhibition of apoptotic and rapid cell signaling pathways 153. Additionally, androgen could also activate signal transduction pathways that have relevance to cell viability in both neuronal and non‐neuronal cells. In one study, Lin et al. (1999) reported increased cell survival of human prostate LNCaP cell following treatment with dihydro‐testosterone via P13K/Akt signaling 154. Similar result was also reported by Yao et al. (2015) in the C6 glial cells 155.
Some researchers explored the activation of MAPK/ERK signaling cascade by androgens in neurons that are believed to contribute neuroprotection. Their role was confirmed by inhibiting MAPK/ERK signaling by MEK inhibitors, which blocked both androgen‐induced ERK phosphorylation and neuroprotection. Nguyen et al. (2005) also reported androgen‐activated neuroprotection via MAPK/ERK signaling in PC12 cells 156. They also reported that activation of MAPK/ERK cascade led to the Rsk‐1 activation and ultimately phosphorylation of BAD gene at Ser112 region. It is also believed by the neuroscientist that MAPK/ERK‐Rsk signaling in androgen neuroprotection might regulate neuronal viability. A schematic diagram showing the various mechanistic action of progesterone, estrogen, and testosterone has been also provided in Figure 1.
Figure 1.
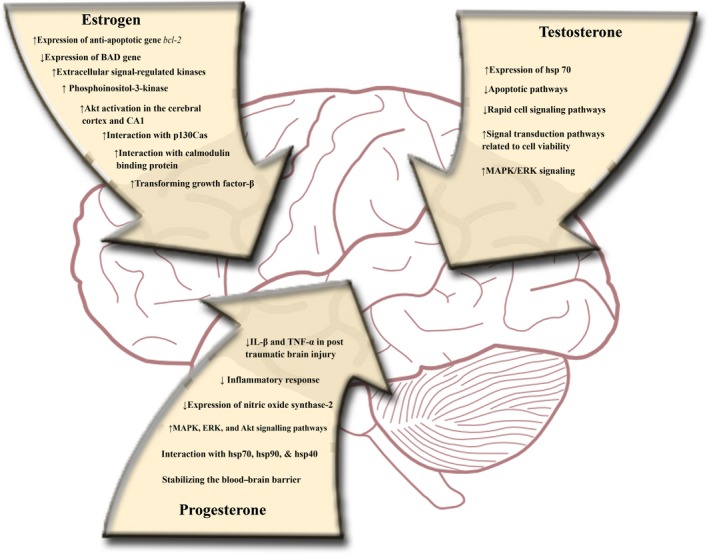
Neuroprotective mechanism of action of progesterone, estrogen, and testosterone.
Conclusion
Based on our review article, it is quite clear that progesterone, estrogen, and testosterone possess neuroprotective potential. Their possible mechanisms of action have been also reported in scientific literature. However, some of the contradictory reports are also available in the literature. We believe more research is required to pinpoint certain mechanism of action of these individual sex steroids.
Conflict of Interest
None to declare.
Acknowledgments
The authors gratefully acknowledge the research facility provided by Jamia Hamdard University, New Delhi, India, and King Fahd Medical Research Center (KFMRC), King Abdulaziz University, Jeddah, Saudi Arabia. Thanks are also due to Mohammad S Gazdar (Librarian, KFMRC) for providing assistance with the literature.
References
- 1. Reindollar RH, Moretuzzo RW, Ellegood JO, Mahesh VB, McDonough PG. The effects of single‐dose luteinizing hormone‐releasing hormone on ovulatory menstrual function: Support for a single luteinizing hormone‐ and follicle‐stimulating hormone‐releasing factor. Fertil Steril 1985;43:185–195. [DOI] [PubMed] [Google Scholar]
- 2. Baird DT, McNeilly AS. Gonadotrophic control of follicular development and function during the oestrous cycle of the ewe. J Reprod Fertil Suppl 1981;30:119–133. [PubMed] [Google Scholar]
- 3. Berent‐Spillson A, Briceno E, Pinsky A, et al. Distinct cognitive effects of estrogen and progesterone in menopausal women. Psychoneuroendocrinology 2015;59:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia‐Ovejero D, González S, Paniagua‐Torija B, et al. Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. J Neurotrauma 2014;31:857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh M, Su C, Ng S. Non‐genomic mechanisms of progesterone action in the brain. Front Neurosci 2013;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med 2006;47:381–389. [DOI] [PubMed] [Google Scholar]
- 7. Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci 2007;25:151–159. [PubMed] [Google Scholar]
- 8. Roglio I, Giatti S, Pesaresi M, et al. Neuroactive steroids and peripheral neuropathy. Brain Res Rev 2008;57:460–469. [DOI] [PubMed] [Google Scholar]
- 9. Veiga S, Leonelli E, Beelke M, Garcia‐Segura LM, Melcangi RC. Neuroactive steroids prevent peripheral myelin alterations induced by diabetes. Neurosci Lett 2006;402:150–153. [DOI] [PubMed] [Google Scholar]
- 10. Labombarda F, González S, Lima A, et al. Progesterone attenuates astro‐ and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp Neurol 2011;231:135–146. [DOI] [PubMed] [Google Scholar]
- 11. Jarahi M, Sheibani V, Safakhah HA, Torkmandi H, Rashidy‐Pour A. Effects of progesterone on neuropathic pain responses in an experimental animal model for peripheral neuropathy in the rat: a behavioral and electrophysiological study. Neuroscience 2014;256:403–411. [DOI] [PubMed] [Google Scholar]
- 12. Brinton RD, Thompson RF, Foy MR, et al. Progesterone receptors: Form and function in brain. Front Neuroendocrinol 2008;29:313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garay L, Gonzalez Deniselle MC, Lima A, Roig P, De Nicola AF. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J Steroid Biochem Mol Biol 2007;107:228–237. [DOI] [PubMed] [Google Scholar]
- 14. Aggarwal R, Medhi B, Pathak A, Dhawan V, Chakrabarti A. Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. J Pharm Pharmacol 2008;60:731–737. [DOI] [PubMed] [Google Scholar]
- 15. Roglio I, Bianchi R, Gotti S, et al. Neuroprotective effects of dihydroprogesterone and progesterone in an experimental model of nerve crush injury. Neuroscience 2008;155:673–685. [DOI] [PubMed] [Google Scholar]
- 16. Ibanez C, Shields SA, El‐Etr M, Leonelli E, Magnaghi V, Li WW, et al. Steroids and the reversal of age‐associated changes in myelination and remyelination. Prog Neurobiol 2003;71:49–56. [DOI] [PubMed] [Google Scholar]
- 17. VanLandingham JW, Cutler SM, Virmani S, et al. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology 2006;51:1078–1085. [DOI] [PubMed] [Google Scholar]
- 18. Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit Care 2008;12:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wright DW, Kellermann AL, Hertzberg VS, et al. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med 2007;49:391–402, e1‐2. [DOI] [PubMed] [Google Scholar]
- 20. Guerra‐Araiza C, Reyna‐Neyra A, Salazar AM, Cerbon MA, Morimoto S, Camacho‐Arroyo I. Progesterone receptor isoforms expression in the prepuberal and adult male rat brain. Brain Res Bull 2001;54:13–17. [DOI] [PubMed] [Google Scholar]
- 21. Guerra‐Araiza C, Villamar‐Cruz O, Gonzalez‐Arenas A, Chavira R, Camacho‐Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol 2003;15:984–990. [DOI] [PubMed] [Google Scholar]
- 22. Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular‐signal regulated kinase in explants of the cerebral cortex. Endocrine 2001;14:407–415. [DOI] [PubMed] [Google Scholar]
- 23. Singh M, Su C. Progesterone, brain‐derived neurotrophic factor and neuroprotection. Neuroscience 2013;239:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toung TJ, Chen TY, Littleton‐Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab 2004;24:1160–1166. [DOI] [PubMed] [Google Scholar]
- 25. Sommer B. Alzheimer's disease and the amyloid cascade hypothesis: Ten years on. Curr Opin Pharmacol 2002;2:87–92. [DOI] [PubMed] [Google Scholar]
- 26. Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain‐derived neurotrophic factor expression. Endocrinology 2009;150:3162–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen‐activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 2007;72:381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alexaki V‐I, Charalampopoulos I, Kampa M, et al. Estrogen exerts neuroprotective effects via membrane estrogen receptors and rapid Akt/NOS activation. FASEB J 2004;18:1594–1596. [DOI] [PubMed] [Google Scholar]
- 30. Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF‐1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol 2008;68:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bialek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol 2004;56:509–518. [PubMed] [Google Scholar]
- 32. Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J Neurosurg 2000;92:848–852. [DOI] [PubMed] [Google Scholar]
- 33. Morali G, Letechipia‐Vallejo G, Lopez‐Loeza E, Montes P, Hernandez‐Morales L, Cervantes M. Post‐ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett 2005;382:286–290. [DOI] [PubMed] [Google Scholar]
- 34. Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res 2005;1049:112–119. [DOI] [PubMed] [Google Scholar]
- 35. Junpeng M, Huang S, Qin S. Progesterone for acute traumatic brain injury. Cochrane Database Syst Rev 2012:CD008409. [DOI] [PubMed] [Google Scholar]
- 36. Vandromme M, Melton SM, Kerby JD. Progesterone in traumatic brain injury: Time to move on to phase III trials. Crit Care 2008;12:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. B W, I S, Dg S. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci 2010;29:61–71. [DOI] [PubMed] [Google Scholar]
- 38. Baulieu EE, Schumacher M, Koenig H, Jung‐Testas I, Akwa Y. Progesterone as a neurosteroid: Actions within the nervous system. Cell Mol Neurobiol 1996;16:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baulieu EE, Schumacher M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Hum Reprod 2000;15:1–13. [DOI] [PubMed] [Google Scholar]
- 40. Ghoumari AM, Ibanez C, El‐Etr M, et al. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem 2003;86:848–859. [DOI] [PubMed] [Google Scholar]
- 41. Paganini‐Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol 1994;140:256–261. [DOI] [PubMed] [Google Scholar]
- 42. Tang M‐X, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 1996;348:429–432. [DOI] [PubMed] [Google Scholar]
- 43. Schumacher M, Guennoun R, Robert F, et al. Local synthesis and dual actions of progesterone in the nervous system: Neuroprotection and myelination. Growth Horm IGF Res 2004;14:S18–S33. [DOI] [PubMed] [Google Scholar]
- 44. Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol 2002;178:59–67. [DOI] [PubMed] [Google Scholar]
- 45. Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol 1997;31:1–11. [DOI] [PubMed] [Google Scholar]
- 46. Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine 1999;24:2134–2138. [DOI] [PubMed] [Google Scholar]
- 47. Pascual O, Morin‐Surun M‐P, Barna B, Denavit‐Saubié M, Pequignot J‐M, Champagnat J. Progesterone reverses the neuronal responses to hypoxia in rat nucleus tractus solitarius in vitro. J Physiol (Lond) 2002;544:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leonelli E, Bianchi R, Cavaletti G, et al. Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: A multimodal analysis. Neuroscience 2007;144:1293–1304. [DOI] [PubMed] [Google Scholar]
- 49. Frye CA. Effects and mechanisms of progestogens and androgens in ictal activity. Epilepsia 2010;51:135–140. [DOI] [PubMed] [Google Scholar]
- 50. Murialdo G, Magri F, Tamagno G, et al. Seizure frequency and sex steroids in women with partial epilepsy on antiepileptic therapy. Epilepsia 2009;50:1920–1926. [DOI] [PubMed] [Google Scholar]
- 51. Lonsdale D, Burnham WM. The anticonvulsant effects of allopregnanolone against amygdala‐kindled seizures in female rats. Neurosci Lett 2007;411:147–151. [DOI] [PubMed] [Google Scholar]
- 52. Singh S, Hota D, Prakash A, Khanduja KL, Arora SK, Chakrabarti A. Allopregnanolone, the active metabolite of progesterone protects against neuronal damage in picrotoxin‐induced seizure model in mice. Pharmacol Biochem Behav 2010;94:416–422. [DOI] [PubMed] [Google Scholar]
- 53. Frye CA, Rhodes ME, Walf A, Harney J. Progesterone reduces pentylenetetrazol‐induced ictal activity of wild‐type mice but not those deficient in type I 5alpha‐reductase. Epilepsia 2002;43:14–17. [DOI] [PubMed] [Google Scholar]
- 54. White HS, Woodhead JH, Franklin MR, Swinyard EA, Wolf HA. General principles. Experimental selection, quantification, and evaluation of antiepileptic drugs. Antiepileptic Drugs, 4th edn New York, NY: Raven Press; 1995;99–110. [Google Scholar]
- 55. Nicoletti F, Speciale C, Sortino MA, et al. Comparative effects of estradiol benzoate, the antiestrogen clomiphene citrate, and the progestin medroxyprogesterone acetate on kainic acid‐induced seizures in male and female rats. Epilepsia 1985;26:252–257. [DOI] [PubMed] [Google Scholar]
- 56. Lonsdale D, Burnham WM. The anticonvulsant effects of progesterone and 5alpha‐dihydroprogesterone on amygdala‐kindled seizures in rats. Epilepsia 2003;44:1494–1499. [DOI] [PubMed] [Google Scholar]
- 57. Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res 2009;85:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gibson CL, Gray LJ, Bath PMW, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain 2008;131:318–328. [DOI] [PubMed] [Google Scholar]
- 59. Schumacher M, Sitruk‐Ware R, De Nicola AF. Progesterone and progestins: Neuroprotection and myelin repair. Curr Opin Pharmacol 2008;8:740–746. [DOI] [PubMed] [Google Scholar]
- 60. De Nicola AF, Labombarda F, Deniselle MCG, et al. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front Neuroendocrinol 2009;30:173–187. [DOI] [PubMed] [Google Scholar]
- 61. Stein DG. Progesterone in the treatment of acute traumatic brain injury: A clinical perspective and update. Neuroscience 2011;191:101–106. [DOI] [PubMed] [Google Scholar]
- 62. Schumacher M, Mattern C, Ghoumari A, et al. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog Neurobiol 2014;113:6–39. [DOI] [PubMed] [Google Scholar]
- 63. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: A randomized clinical trial with placebo group. Adv Biomed Res 2012;1:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shakeri M, Boustani MR, Pak N, et al. Effect of progesterone administration on prognosis of patients with diffuse axonal injury due to severe head trauma. Clin Neurol Neurosurg 2013;115:2019–2022. [DOI] [PubMed] [Google Scholar]
- 65. Wright DW, Yeatts SD, Silbergleit R, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med 2014;371:2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Skolnick BE, Maas AI, Narayan RK, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med 2014;371:2467–2476. [DOI] [PubMed] [Google Scholar]
- 67. Stein DG. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj 2015;29:1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Herzog AG, Fowler KM, Smithson SD, et al. Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial. Neurology 2012;78:1959–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ioannidis JPA. Why most published research findings are false. PLoS Med 2005;2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schwamm LH. Progesterone for traumatic brain injury — resisting the sirens’ song. N Engl J Med 2014;371:2522–2523. [DOI] [PubMed] [Google Scholar]
- 71. Menon DK, Maas AIR. Traumatic brain injury in 2014: Progress, failures and new approaches for TBI research. Nat Rev Neurol 2015;11:71–72. [DOI] [PubMed] [Google Scholar]
- 72. Guerra‐Araiza C, Cerbon MA, Morimoto S, Camacho‐Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci 2000;66:1743–1752. [DOI] [PubMed] [Google Scholar]
- 73. Guerra‐Araiza C, Coyoy‐Salgado A, Camacho‐Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull 2002;59:105–109. [DOI] [PubMed] [Google Scholar]
- 74. Camacho‐Arroyo I, Guerra‐Araiza C, Cerbon MA. Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. NeuroReport 1998;9:3993–3996. [DOI] [PubMed] [Google Scholar]
- 75. Krusekopf S, Chauchereau A, Milgrom E, Henderson D, Cato AC. Co‐operation of progestational steroids with epidermal growth factor in activation of gene expression in mammary tumor cells. J Steroid Biochem Mol Biol 1991;40:239–245. [DOI] [PubMed] [Google Scholar]
- 76. Moore MR, Zhou JL, Blankenship KA, Strobl JS, Edwards DP, Gentry RN. A sequence in the 5′ flanking region confers progestin responsiveness on the human c‐myc gene. J Steroid Biochem Mol Biol 1997;62:243–252. [DOI] [PubMed] [Google Scholar]
- 77. Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 2003;21:1973–1979. [DOI] [PubMed] [Google Scholar]
- 78. Elledge RM, Green S, Pugh R, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand‐binding assay compared with ER, PgR and pS2, by immuno‐histochemistry in predicting response to tamoxifen in metastatic breast cancer: A Southwest Oncology Group Study. Int J Cancer 2000;89:111–117. [PubMed] [Google Scholar]
- 79. Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane‐associated progesterone‐binding protein, 25‐Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci U S A 2000;97:12816–12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A 2003;100:2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS. Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen–glucose deprivation. Eur J Neurosci 2006;24:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre‐frontal cortex. Neuroscience 2004;123:349–359. [DOI] [PubMed] [Google Scholar]
- 83. Kaur P, Jodhka PK, Underwood WA, et al. Progesterone increases brain‐derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen‐activated protein kinase‐ and phosphoinositide‐3 kinase‐dependent manner in cerebral cortical explants. J Neurosci Res 2007;85:2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yamada K, Mizuno M, Nabeshima T. Role for brain‐derived neurotrophic factor in learning and memory. Life Sci 2002;70:735–744. [DOI] [PubMed] [Google Scholar]
- 85. Chourbaji S, Hellweg R, Brandis D, et al. Mice with reduced brain‐derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res 2004;121:28–36. [DOI] [PubMed] [Google Scholar]
- 86. Saha RN, Liu X, Pahan K. Up‐regulation of BDNF in astrocytes by TNF‐alpha: A case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol 2006;1:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yadav A, Agarwal S, Tiwari SK, Chaturvedi RK. Mitochondria: Prospective targets for neuroprotection in Parkinson's disease. Curr Pharm Des 2014;20:5558–5573. [DOI] [PubMed] [Google Scholar]
- 88. Zhang R, Zhang J, Fang L, et al. Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer's disease‐like lesions. Int J Mol Sci 2014;15:14396–14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase‐2 expression following cerebral ischemia. Exp Neurol 2005;193:522–530. [DOI] [PubMed] [Google Scholar]
- 90. Murphy DD, Segal M. Progesterone prevents estradiol‐induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology 2000;72:133–143. [DOI] [PubMed] [Google Scholar]
- 91. Bimonte‐Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle‐aged ovariectomized female rats. Eur J Neurosci 2006;24:229–242. [DOI] [PubMed] [Google Scholar]
- 92. Carroll JC, Rosario ER, Pike CJ. Progesterone blocks estrogen neuroprotection from kainate in middle‐aged female rats. Neurosci Lett 2008;445:229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab 2000;20:631–652. [DOI] [PubMed] [Google Scholar]
- 94. Sampei K, Goto S, Alkayed NJ, et al. Stroke in estrogen receptor‐alpha‐deficient mice. Stroke 2000;31:738–743. [DOI] [PubMed] [Google Scholar]
- 95. Simpkins JW, Rajakumar G, Zhang YQ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg 1997;87:724–730. [DOI] [PubMed] [Google Scholar]
- 96. Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the women's health initiative. Endocr Rev 2005;26:308–312. [DOI] [PubMed] [Google Scholar]
- 97. Nakamura T, Xi G, Keep RF, et al. Effects of endogenous and exogenous estrogen on intracerebral hemorrhage‐induced brain damage in rats. Acta Neurochir Suppl 2006;96:218–221. [DOI] [PubMed] [Google Scholar]
- 98. Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine 2006;29:217–231. [DOI] [PubMed] [Google Scholar]
- 99. Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta‐peptide toxicity in hippocampal neurons. J Neurochem 1996;66:1836–1844. [DOI] [PubMed] [Google Scholar]
- 100. Velísková J. The role of estrogens in seizures and epilepsy: The bad guys or the good guys? Neuroscience 2006;138:837–844. [DOI] [PubMed] [Google Scholar]
- 101. Velísková J. Estrogens and epilepsy: Why are we so excited? Neuroscientist 2007;13:77–88. [DOI] [PubMed] [Google Scholar]
- 102. Sicotte NL, Liva SM, Klutch R, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol 2002;52:421–428. [DOI] [PubMed] [Google Scholar]
- 103. Voskuhl RR, Wang H, Wu TCJ, et al. Estriol combined with glatiramer acetate for women with relapsing‐remitting multiple sclerosis: A randomised, placebo‐controlled, phase 2 trial. Lancet Neurol 2016;15:35–46. [DOI] [PubMed] [Google Scholar]
- 104. Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M. Minireview: Neuroprotective effects of estrogen‐new insights into mechanisms of action. Endocrinology 2001;142:969–973. [DOI] [PubMed] [Google Scholar]
- 105. Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl‐2 in cerebral ischemia: A potential role for estrogen receptors. J Neurosci 1999;19:6385–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dubal DB, Zhu H, Yu J, et al. Estrogen receptor alpha, not beta, is a critical link in estradiol‐mediated protection against brain injury. Proc Natl Acad Sci U S A 2001;98:1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fillit HM. The role of hormone replacement therapy in the prevention of Alzheimer disease. Arch Intern Med 2002;162:1934–1942. [DOI] [PubMed] [Google Scholar]
- 108. Gulinello M, Lebesgue D, Jover‐Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav 2006;49:246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Merchenthaler I, Dellovade TL, Shughrue PJ. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann N Y Acad Sci 2003;1007:89–100. [DOI] [PubMed] [Google Scholar]
- 110. Wilson ME, Dubal DB, Wise PM. Estradiol protects against injury‐induced cell death in cortical explant cultures: A role for estrogen receptors. Brain Res 2000;873:235–242. [DOI] [PubMed] [Google Scholar]
- 111. Yang SH, Shi J, Day AL, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke 2000;31:745–749. [DOI] [PubMed] [Google Scholar]
- 112. Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor‐alpha (ERalpha) and estrogen receptor‐beta (ERbeta) messenger ribonucleic acid in the wild‐type and ERalpha‐knockout mouse. Endocrinology 1997;138:4613–4621. [DOI] [PubMed] [Google Scholar]
- 113. Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor‐alpha and ‐beta mRNA in the rat central nervous system. J Comp Neurol 1997;388:507–525. [DOI] [PubMed] [Google Scholar]
- 114. Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia‐induced cell death: Multiple cellular pathways of neuroprotection. Steroids 2009;74:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia‐induced cell death. Endocrinology 2005;146:3070–3079. [DOI] [PubMed] [Google Scholar]
- 116. Wu TW, Wang JM, Chen S, Brinton RD. 17Beta‐estradiol induced Ca2 + influx via L‐type calcium channels activates the Src/ERK/cyclic‐AMP response element binding protein signal pathway and BCL‐2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen‐induced neuroprotection. Neuroscience 2005;135:59–72. [DOI] [PubMed] [Google Scholar]
- 117. Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl‐2 expression in primary hippocampal neurons. Brain Res 2004;1010:22–34. [DOI] [PubMed] [Google Scholar]
- 118. Alkayed NJ, Goto S, Sugo N, et al. Estrogen and Bcl‐2: Gene induction and effect of transgene in experimental stroke. J Neurosci 2001;21:7543–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Singer CA, Figueroa‐Masot XA, Batchelor RH, Dorsa DM. The mitogen‐activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci 1999;19:2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Singh M, Setalo G Jr, Guan X, Warren M, Toran‐Allerand CD. Estrogen‐induced activation of mitogen‐activated protein kinase in cerebral cortical explants: Convergence of estrogen and neurotrophin signaling pathways. J Neurosci 1999;19:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cabodi S, Moro L, Baj G, et al. p130Cas interacts with estrogen receptor alpha and modulates non‐genomic estrogen signaling in breast cancer cells. J Cell Sci 2004;117:1603–1611. [DOI] [PubMed] [Google Scholar]
- 122. Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci U S A 2004;101:17126–17131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dhandapani KM, Hadman M, De Sevilla L, Wade MF, Mahesh VB, Brann DW. Astrocyte protection of neurons: Role of transforming growth factor‐beta signaling via a c‐Jun‐AP‐1 protective pathway. J Biol Chem 2003;278:43329–43339. [DOI] [PubMed] [Google Scholar]
- 124. Sortino MA, Chisari M, Merlo S, et al. Glia mediates the neuroprotective action of estradiol on beta‐amyloid‐induced neuronal death. Endocrinology 2004;145:5080–5086. [DOI] [PubMed] [Google Scholar]
- 125. Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte‐derived transforming growth factor‐{beta} mediates the neuroprotective effects of 17{beta}‐estradiol: Involvement of nonclassical genomic signaling pathways. Endocrinology 2005;146:2749–2759. [DOI] [PubMed] [Google Scholar]
- 126. Iqbal MJ, Dalton M, Sawers RS. Binding of testosterone and oestradiol to sex hormone binding globulin, human serum albumin and other plasma proteins: Evidence for non‐specific binding of oestradiol to sex hormone binding globulin. Clin Sci (Lond) 1983;64:307–314. [DOI] [PubMed] [Google Scholar]
- 127. Chisu V, Manca P, Lepore G, Gadau S, Zedda M, Farina V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3‐nitro‐L‐tyrosine incorporation into alpha‐tubulin in a mouse neuroblastoma cell line. Arch Ital Biol 2006;144:63–73. [PubMed] [Google Scholar]
- 128. Nguyen TV, Jayaraman A, Quaglino A, Pike CJ. Androgens selectively protect against apoptosis in hippocampal neurones. J Neuroendocrinol 2010;22:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tehranipour M, Moghimi A. Neuroprotective effects of testosterone on regenerating spinal cord motoneurons in rats. J Mot Behav 2010;42:151–155. [DOI] [PubMed] [Google Scholar]
- 130. Belle MD, Lea RW. Androgen receptor immunolocalization in brains of courting and brooding male and female ring doves (Streptopelia risoria). Gen Comp Endocrinol 2001;124:173–187. [DOI] [PubMed] [Google Scholar]
- 131. Banks WA, Morley JE, Niehoff ML, Mattern C. Delivery of testosterone to the brain by intranasal administration: Comparison to intravenous testosterone. J Drug Target 2009;17:91–97. [DOI] [PubMed] [Google Scholar]
- 132. Gouras GK, Xu H, Gross RS, et al. Testosterone reduces neuronal secretion of Alzheimer's beta‐amyloid peptides. Proc Natl Acad Sci U S A 2000;97:1202–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ramsden M, Nyborg AC, Murphy MP, et al. Androgens modulate beta‐amyloid levels in male rat brain. J Neurochem 2003;87:1052–1055. [DOI] [PubMed] [Google Scholar]
- 134. Matsumoto A, Prins GS. Androgenic regulation of expression of androgen receptor protein in the perineal motoneurons of aged male rats. J Comp Neurol. 2002;443:383–387. [PubMed] [Google Scholar]
- 135. Pouliot WA, Handa RJ, Beck SG. Androgen modulates N‐methyl‐D‐aspartate‐mediated depolarization in CA1 hippocampal pyramidal cells. Synapse 1996;23:10–19. [DOI] [PubMed] [Google Scholar]
- 136. Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci 2004;24:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen‐dependent regulation of brain‐derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology 2007;148:3655–3665. [DOI] [PubMed] [Google Scholar]
- 138. Marron TU, Guerini V, Rusmini P, et al. Androgen‐induced neurite outgrowth is mediated by neuritin in motor neurones. J Neurochem 2005;92:10–20. [DOI] [PubMed] [Google Scholar]
- 139. Brooks BP, Merry DE, Paulson HL, Lieberman AP, Kolson DL, Fischbeck KH. A cell culture model for androgen effects in motor neurons. J Neurochem 1998;70:1054–1060. [DOI] [PubMed] [Google Scholar]
- 140. Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: A novel source of sex dimorphism? Eur J Neurosci 2008;27:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. [DOI] [PubMed] [Google Scholar]
- 142. Zhang JM, Tonelli L, Regenold WT, McCarthy MM. Effects of neonatal flutamide treatment on hippocampal neurogenesis and synaptogenesis correlate with depression‐like behaviors in preadolescent male rats. Neuroscience 2010;169:544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nunez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing Gamma‐Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience 2009;158:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Huppenbauer CB, Tanzer L, DonCarlos LL, Jones KJ. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: Equal efficacy of testosterone, dihydrotestosterone, and 17‐beta estradiol. J Neurosci 2005;25:4004–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Matsumoto A. Hormonally induced neuronal plasticity in the adult motoneurons. Brain Res Bull 1997;44:539–547. [DOI] [PubMed] [Google Scholar]
- 146. Keast JR, Saunders RJ. Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs of the male rat. Neuroscience 1998;85:543–556. [DOI] [PubMed] [Google Scholar]
- 147. Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol 2002;52:312–321. [DOI] [PubMed] [Google Scholar]
- 148. Schwarz JM, McCarthy MM. Cellular mechanisms of estradiol‐mediated masculinization of the brain. J Steroid Biochem Mol Biol 2008;109:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Fargo KN, Foecking EM, Jones KJ, Sengelaub DR. Neuroprotective actions of androgens on motoneurons. Front Neuroendocrinol 2009;30:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yu WH. Administration of testosterone attenuates neuronal loss following axotomy in the brain‐stem motor nuclei of female rats. J Neurosci 1989;9:3908–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kelly S, Yenari MA. Neuroprotection: Heat shock proteins. Curr Med Res Opin 2002;18:s55–s60. [PubMed] [Google Scholar]
- 152. Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1‐42 toxicity through heat shock protein 70. J Neurosci 2004;24:5315–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pike CJ. Testosterone attenuates beta‐amyloid toxicity in cultured hippocampal neurons. Brain Res 2001;919:160–165. [DOI] [PubMed] [Google Scholar]
- 154. Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3′‐kinase pathway is a dominant growth factor‐activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res 1999;59:2891–2897. [PubMed] [Google Scholar]
- 155. Yao K, Wu J, Zhang J, Bo J, Hong Z, Zu H. Protective effect of DHT on apoptosis induced by U18666A via PI3K/Akt signaling pathway in C6 glial cell lines. Cell Mol Neurobiol 2015; DOI: 10.1007/s10571-015-0263-x (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen‐activated protein kinase signaling: Role in neuroprotection. J Neurochem 2005;94:1639–1651. [DOI] [PubMed] [Google Scholar]


