Summary
Aims
While a number of studies have shown that free magnesium (Mg) decline is a feature of traumatic brain injury (TBI), poor central penetration of Mg has potentially limited clinical translation. This study examines whether polyethylene glycol (PEG) facilitates central penetration of Mg after TBI, increasing neuroprotection while simultaneously reducing the dose requirements for Mg.
Methods
Rats were exposed to diffuse TBI and administered intravenous MgCl2 either alone (254 μmol/kg or 25.4 μmol/kg) or in combination with PEG (1 g/kg PEG) at 30‐min postinjury. Vehicle‐treated (saline or PEG) and sham animals served as controls. All animals were subsequently assessed for blood‐brain barrier permeability and edema at 5 h, and functional outcome for 1 week postinjury.
Results
Optimal dose (254 μmol/kg) MgCl2 or Mg PEG significantly improved all outcome parameters compared to vehicle or PEG controls. Intravenous administration of 10% MgCl2 alone (25.4 μmol/kg) had no beneficial effect on any of the outcome parameters, whereas 10% Mg in PEG had the same beneficial effects as optimal dose Mg administration.
Conclusion
Polyethylene glycol facilitates central penetration of Mg following TBI, reducing the concentration of Mg required to confer neuroprotection while simultaneously reducing the risks associated with high peripheral Mg concentration.
Keywords: Blood–brain barrier, Edema, Magnesium, Neurotrauma, Treatment
Introduction
Brain free magnesium (Mg) decline has been identified as a ubiquitous secondary injury factor leading to neurological deficits following traumatic brain injury (TBI) 1, 2, 3. While extensive evidence for a neuroprotective role for Mg in experimental TBI has been reported 4, 5, 6, this has not been consistently translated to the clinical setting. In a small clinical trial with a well‐defined patient population 7, acute Mg administration following TBI resulted in a significant improvement in outcome as assessed by the Glasgow Outcome Scale at 3 months. However, in a larger trial with a more heterogeneous patient pool 8, there were no beneficial effects associated with prolonged Mg administration, with higher doses of Mg administration being associated with deleterious side effects. Reports have suggested that the lack of central penetration of Mg may account for the trial inconsistencies 9, 10, 11.
Polyethylene glycol (PEG) is a hydrophilic polymer that attaches to various protein medications increasing their membrane permeability, decreasing rate of clearance, and facilitating longer acting medicinal effects, longer dosing intervals, and reducing toxicity 12. While a limited number of studies have utilized PEG as a membrane sealing agent in the treatment of TBI 13, 14, none have used the compound to facilitate blood–brain barrier (BBB) penetration. In contrast, previous studies have used combined Mg and PEG in traumatic spinal cord injury 15, 16 with the studies of Ditor et al. 16 demonstrating that MgSO4 in a PEG formulation resulted in improved neuroprotection around the injury site and greater locomotor recovery in spinal cord trauma compared to administration of MgSO4 alone 16. Later, Kwon et al. 17 established the optimal dose for Mg in a PEG formulation to help guide the treatment parameters for a clinical trial of a Mg salt in PEG formulation in acute human spinal cord injury. The comparison between MgSO4 and MgCl2 in PEG showed no statistical difference between the two salts, but the authors noted that the MgCl2‐treated group appeared to have slightly better locomotor recovery early on compared to the MgSO4 treated group.
In the current study, we have examined the efficacy of MgCl2 with or without PEG on edema, BBB permeability, and functional outcome after TBI. Additionally, we examined the effect of a 10% Mg dose in PEG versus 10% MgCl2 alone on the same outcome parameters to determine whether PEG facilitates CNS entry of Mg.
Methods
All experimental protocols were approved by the Animal Ethics Committee of the University of Adelaide. Injury was induced using the Marmarou impact acceleration model of diffuse traumatic brain injury as described in detail elsewhere 18, 19. Briefly, adult male Sprague rats (300–400 g; n = 96) were initially anaesthetized with 5% isoflurane in room air before being intubated and maintained on 1.5–2% isoflurane in room air using a rodent ventilator. A midline incision was then made to expose the skull, and a 10‐mm stainless steel disk (3 mm depth) fixed centrally between lambda and bregma using a polyacrylamide adhesive. The rat was then placed on a 10‐cm foam bed, and injury was induced by dropping a 450‐g brass weight from a height of 2 m onto the stainless steel disk as previously described. This level of injury has been associated with the production of moderate‐to‐severe functional deficits 4, 18. The animal and 10‐cm foam bed was immediately removed after impact, thus preventing any potential second impact with the weight rebound. An additional 20 animals served as sham surgical (uninjured) controls. The steel disk was removed from the skull and midline incision closed using surgical clips before being weaned from the ventilator over the next 10 min. Body temperature in all animals was maintained at 37°C using a thermostatically controlled heating pad.
At 30 min after injury induction, animals were administered with an intravenous dose of MgCl2 (254 μmol/kg), Mg PEG (254 μmol/kg MgCl2 in 1 g/kg PEG), PEG (1 g/kg), an equal volume of saline (vehicle), 10% MgCl2 (25.4 μmol/kg), or 10% Mg PEG (25.4 μmol/kg MgCl2 in 1 g/kg PEG). The optimal dose of MgCl2 (254 μmol/kg) was taken from previous work by our laboratory 4.
For BBB permeability 19, 2 mL/kg of 4% Evan's blue dye was injected via the tail vein at 4.5 h after injury, allowed to circulate for 30 min before the animals were saline‐perfused, decapitated, and their brains removed. The right and left hemispheres were individually weighed before being homogenized with phosphate‐buffered solution and trichloroacetic acid. Samples were then cooled overnight and centrifuged for 30 min at 1000 g the following day. The supernatants were removed, and the amount of Evan's blue was measured at 610 nm using a spectrophotometer. Evan's blue is expressed as μg/g of brain tissue against a previously obtained standard curve for Evan's blue absorbance.
Brain water content (edema) was calculated on the basis of wet weight versus dry weight using a Mettler Toledo halogen moisture analyzer as previously described by Plesnila and colleagues 20. Briefly, at 5 h after injury, brains were rapidly removed and sectioned into left and right hemispheres, which were placed on separate slides. Another slide was placed on top of the tissue on each slide, and the tissue was then crushed into a paste between the two slides. The slides were the placed into the moisture analyzer in which they were weighed before being rapidly heated to 180°C for 10 min and subsequently reweighed. The final % water reading is displayed automatically at the conclusion of the assessment.
Neurological outcome was assessed up to 7 days following TBI using the rotarod test for motor deficits 4, 21 and the object recognition test for cognitive deficits as previously described 22, 23. The rotarod test requires an animal to walk on a motorized rotating assembly of 18 rods, each 1 mm in diameter. The rotational speed of the assembly is increased from 0 to 30 revolutions per minute (rpm) in intervals of 3 rpm every 10 second. The duration in seconds at the point at which the animal completed the 2‐min task, fell from the rods, or gripped the rods and spun for two consecutive turns rather than actively walking on the rods was recorded as the final score.
The object recognition cognitive test consists of an open box (80 cm long, 80 cm wide and 80 cm high) with objects placed within the box. The objects to be discriminated are made of a biologically neutral material such as glass, plastic, or metal and weighted so that the animals will not move them around. The test consists of a sample phase (3 min) and a choice phase (3 min) with 15‐min retention interval between the two phases. In the sample phase, two identical objects are placed in the box. The animal is then placed in the box, and the total time spent exploring each object is recorded. After 3 min of exploration, the rat is removed from the open box and returned to its cage. After a delay of 15 min, the rat is reintroduced to the open box and a choice phase is started for a further 3 min. In the choice phase, one of the objects is replaced with a new novel object. The time spent in exploring each object is again recorded. The time is recorded only when the rat touches the object with its nose or the rat's nose is directed toward an object at a distance of ≤2 cm. Turning around or sitting on the object is not considered as exploratory behavior. Uninjured animals spend more time exploring the new object rather that the familiar one.
Histology was performed on coronal slices taken at −4.5 mm from bregma, focusing on the left and right hippocampi, which are thought to be associated with behavioral outcome. Animals were perfusion‐fixed at the end of the 7‐day neurological assessment period using 10% buffered formalin (pH 7.4). Their brains were then removed and stored in 10% buffered formalin for 3 days before being cut into 2‐mm coronal slices, using a Kopf rodent blocker, and embedded in paraffin. Paraffin‐embedded blocks were cut into 5‐μm sections and stained using hematoxylin and eosin (H&E) to assess dark cell change as previously described elsewhere 24. Slides were digitally scanned using a NanoZoomer (Hamamatsu, Japan) and viewed with the associated proprietary viewing software.
All data are expressed as mean ± SD and were analyzed using Graphpad Prism® (La Jolla, CA, USA). Statistical differences for BBB and edema (n = 5–6 per group) were determined using one‐way analysis of variance followed by Bonferroni post hoc tests. Functional outcome (n = 5–8 per group) was analyzed by repeated‐measure analysis of variance followed by Tukey's multiple comparison tests. A P value of 0.05 was considered significant.
Results
Blood–Brain Barrier Permeability
The permeability of the BBB was assessed using Evan's blue dye, which binds to albumin. Under normal circumstances, the BBB is intact thereby preventing large molecules from crossing. However, upon disruption of this barrier, large protein molecules like albumin may enter the brain parenchyma. In sham animals, the amount of Evan's blue in the brain parenchyma was negligible (3.4 ± 0.3 μg/g), consistent with the presence of an intact BBB (Figure 1). In animals subject to TBI and administered saline (vehicle) at 30‐min postinjury, brain Evan's blue concentration was 52.2 ± 1.0 μg/g (P < 0.0001 vs. sham), reflecting increased BBB permeability. Treatment with Mg PEG or MgCl2 reduced barrier permeability to near sham levels following TBI to 3.9 ± 0.4 μg/g and 4.8 ± 0.5 μg/g, respectively, indicating a relatively intact BBB. On the other hand, treatment with PEG alone did not improve BBB permeability, with brain Evan's blue concentration at almost vehicle levels (50.5 ± 1.1 μg/g; P < 0.0001). When the dose of the Mg salt was reduced to 10% of optimal, brain Evan's blue concentration was 52.0 ± 0.6 μg/g, which was no different to the vehicle‐treated animals. In contrast, when the reduced magnesium concentration was administered in combination with PEG (10% Mg PEG), brain Evans blue was again reduced to sham levels (3.8 ± 0.1 μg/g; P < 0.0001).
Figure 1.
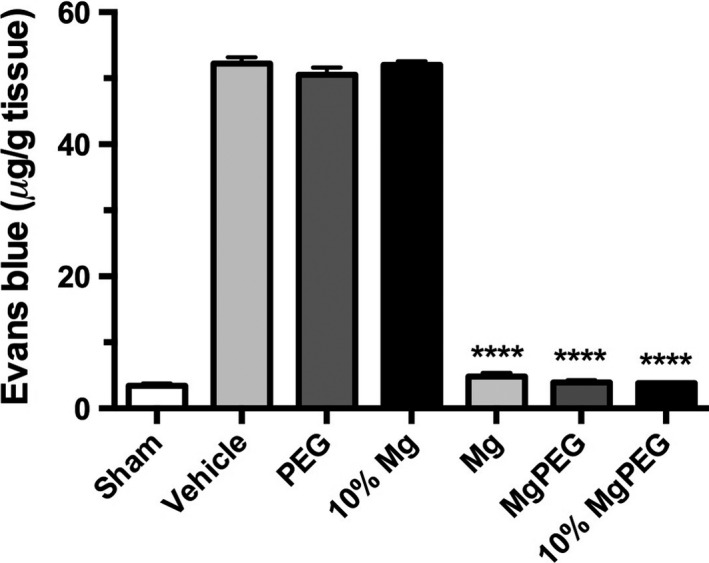
BBB permeability in rats at 5 h following moderate diffuse TBI. Animals were treated at 30 min after TBI with 254 μmol/kg MgCl2 (optimal dose), Mg PEG (254 μmol/kg MgCl2 in 1 g/kg PEG), PEG alone, an equal volume of vehicle (saline), 10% MgCl2 (25 μmol/kg), or 10% Mg PEG. ****P < 0.0001 versus shams.
Edema
Sham or uninjured animals demonstrated a brain water content at 5‐h posttrauma of 78.4 ± 0.2% (Figure 2), which is consistent with values reported by others in rat experiments 25, 26. After TBI, brain water content in saline‐treated (vehicle) animals was 79.13 ± 0.19% (P < 0.001), supporting the development of mild edema, which is typical in this type of injury (Figure 2). Treatment with PEG alone did not significantly reduce brain water content compared with saline‐treated (vehicle) animals, with a value of 79.08 ± 0.38% (P < 0.001) recorded at 5 h. The brain water content following administration of optimal doses of Mg PEG and MgCl2 was 78.82 ± 0.14% and 78.83 ± 0.22%, respectively, which is significantly less than vehicle‐ and PEG‐treated controls (P < 0.001) and similar to sham levels. Reducing the dose of MgCl2 to 10% of the optimum dose negated the beneficial effects of Mg administration, with brain water values of 79.16 ± 0.10% recorded, which is similar to vehicle controls. In contrast, administration of 10% MgCl2 in PEG significantly reduced brain water to 78.61 ± 0.14%, which is significantly less than PEG alone (P < 0.05).
Figure 2.
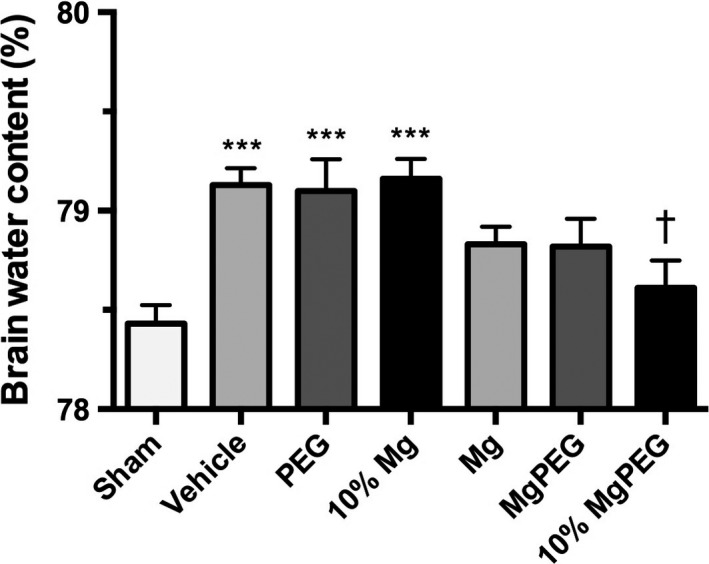
Edema at 5 h after moderate diffuse TBI in rats. Animals were treated at 30 min after TBI with 254 μmol/kg MgCl2, Mg PEG (254 μmol/kg MgCl2 in 1 g/kg PEG), PEG alone, an equal volume of vehicle (saline), 10% MgCl2 (25 μmol/kg), or 10% Mg PEG. ***P < 0.001 versus shams; † P < 0.05 versus vehicle.
Motor Outcome
Sham animals demonstrated consistently high rotarod scores averaging 120 second over the 7‐day assessment period (Figure 3). After diffuse TBI, the saline‐treated (vehicle), PEG‐treated and 10% MgCl2 (25 μmol/kg)‐treated animals all demonstrated significant motor deficits (P < 0.0001) versus sham animals, recording rotarod scores of between 20 and 40 second for the entire 7‐day assessment period. In contrast, animals treated with either optimal dose MgCl2 (254 μmol/kg), optimal dose MgCl2 in PEG (Mg PEG), or low dose MgCl2 (25 μmol/kg) in PEG showed significantly higher rotarod scores than vehicle‐treated animals (P < 0.0001). Indeed, animals treated with both low and optimal dose magnesium in PEG recorded rotarod scores that were comparable to the sham values between days 4–7 following TBI.
Figure 3.
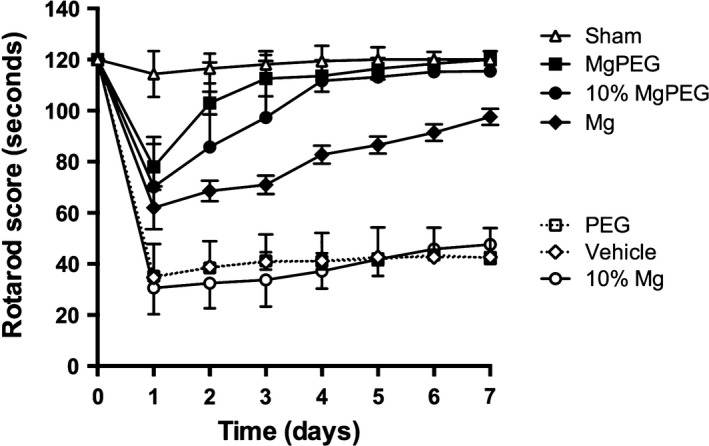
Motor function over 7 days following TBI as assessed using the rotarod. Animals treated with 254 μmol/kg MgCl2, Mg PEG (254 μmol/kg MgCl2 in 1 g/kg PEG), or 10% MgCl2 (25 μmol/kg) in PEG performed significantly better (P < 0.0001) than vehicle‐, PEG‐ or 10% MgCl2 (25 μmol/kg)‐treated controls. For clarity, significance symbols have been omitted.
Cognitive Outcome
In the sample phase of the object recognition test, all animals were exposed to two identical objects, which they equally explored (Figure 4). In the choice phase, sham‐injured animals spent significantly more time (P < 0.0001) exploring the novel object, reflecting the ability to recall the prior object and showing more interest in the novel object. In contrast, vehicle‐treated animals explored both objects equally, being unable to differentiate between the objects after TBI. Similarly, animals subject to TBI and treated with either PEG or 10% MgCl2 could not differentiate between the familiar and novel object in the choice phase, indicating significant cognitive deficits. On the other hand, animals treated with the optimal dose MgCl2, optimal dose MgCl2 in PEG, or 10% MgCl2 in PEG spent significantly more time (P < 0.0001) exploring the novel object, demonstrating cognitive ability equal to sham animals.
Figure 4.
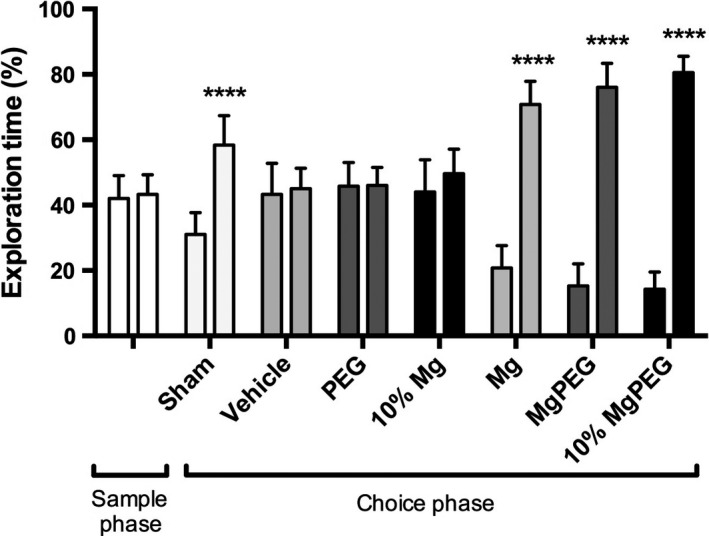
Object recognition test outcomes after diffuse TBI in rats. Animals were treated with 254 μmol/kg MgCl2, Mg PEG (254 μmol/kg MgCl2 in 1 g/kg PEG), PEG alone, an equal volume of vehicle (saline), 10% MgCl2 (25 μmol/kg), or 10% Mg PEG at 30‐min postinjury. ****P < 0.0001 compared to original object.
Histology
In contrast to sham animals (Figure 5A), sections from vehicle‐treated animals demonstrated extensive dark cell change and vacuolization in the CA3 region of both hippocampi, together with a loss of layer architecture. These changes are typical of what has been previously described at 7 days following rodent TBI and reflect the presence of persistent cell injury and neuronal cell loss 24, 27. Treatment with optimal dose MgCl2 (Figure 5C) markedly attenuated the occurrence of CA3 dark cell change and vacuolization relative to the vehicle‐treated animals, although the 10% magnesium dose had no such neuroprotective benefit. In contrast, 10% MgCl2 in PEG (Figure 5F) was highly neuroprotective compared to PEG treatment alone.
Figure 5.
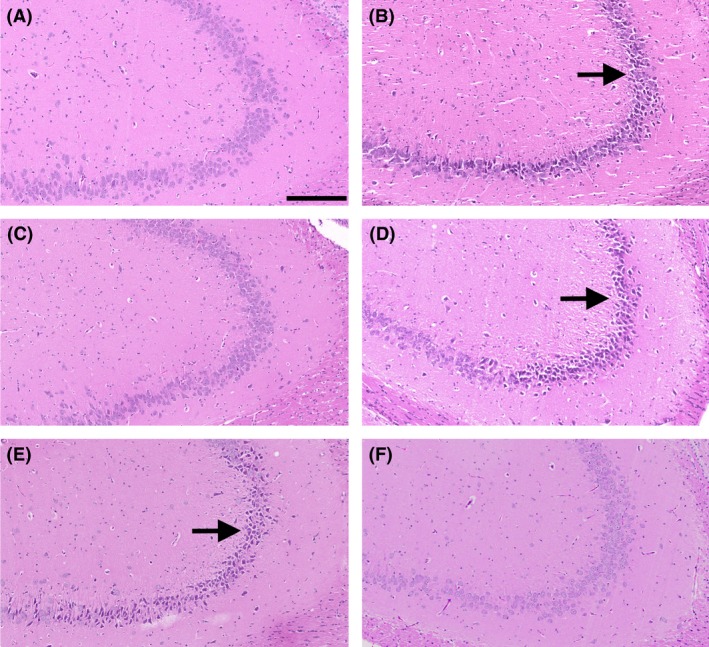
H&E‐stained hippocampal (CA3) sections at 7 days following diffuse TBI in rats. A: sham; B saline vehicle; C: 254 μmol/kg MgCl2; D: 10% MgCl2 (25 μmol/kg); E: PEG alone; F: 10% Mg PEG. Arrows indicate the CA3 field with extensive dark cell change visible. Scale bar = 200 μm.
Discussion
In this study, a diffuse closed head injury model was used to investigate the effect of combined MgCl2 and PEG on edema, BBB permeability, and functional and histological outcome after TBI. Furthermore, we examined whether PEG facilitated CNS entry of Mg. To the best of our knowledge, this is the first study to demonstrate the effects of Mg PEG on edema, BBB permeability, and functional and histological outcome after TBI as well as to demonstrate that coadministration of PEG effectively reduces the dose of MgCl2 required for neuroprotective efficacy.
Previous studies with magnesium salts have demonstrated Mg's beneficial effects on the BBB 26, 28. In the current study, we used the 5‐h time point to determine the effects of the various therapies on BBB permeability given that this time point has been shown to have maximal BBB permeability in previous studies of TBI, with maximum development of vasogenic edema 19. Our results demonstrate that the saline (vehicle)‐treated animals had an increase in uptake of the Evan's blue dye indicating increased BBB permeability compared to the sham animals. Similarly, animals treated with PEG also demonstrated high BBB permeability after TBI. The animals treated with optimal dose Mg, with or without PEG, significantly attenuated BBB permeability such that they were no longer different from the shams. Furthermore, animals treated with 10% of the optimal Mg dose were not different from vehicles, whereas those treated with 10% of the optimal Mg dose in PEG were significantly improved relative to vehicle animals, and similar to optimal dose Mg‐treated animals.
Similar results were observed with respect to edema. While a number of studies have demonstrated that Mg attenuates edema following TBI 26, 29, 30, the current study shows that Mg in a PEG formulation also reduces edema and that 10% of the optimal Mg dose in PEG dose has the same beneficial effects as the optimal dose of Mg.
Polyethylene glycol is a nontoxic molecule that is considered a membrane sealant that freely enters the rat brain parenchyma 13, 14, as well as being capable of attaching to various protein medications increasing their membrane permeability, decreasing rate of clearance, and facilitating longer acting medicinal effects, longer dosing intervals, and reducing toxicity 12. While earlier reports suggest that in itself, PEG may be beneficial in TBI 13, 14, the compound had no significant effects on any of the outcome parameters used in the current study, similar to results previously reported in spinal cord injury 16, 17. Nonetheless, when combined with a low dosage of Mg, PEG enhanced central penetration of the Mg. Indeed, the 10% Mg PEG dose had the same beneficial effects as the optimal Mg dose, while in contrast, the 10% Mg dose alone was completely ineffective and the outcomes identical to that of the saline‐treated animals. Previous studies with Mg salts alone have demonstrated beneficial effects of Mg on the BBB and edema after TBI 26, 28. This is the first study to show the beneficial effects of MgCl2 in a PEG solution, at doses of Mg that are ineffective on their own.
Our current results also showed that Mg, Mg PEG, and 10% Mg PEG attenuated functional deficits following severe diffuse TBI. The rotarod test was the preferred test for motor function because it has previously been shown to be the most sensitive after TBI 4, 21. The object recognition test was preferred for cognitive assessment and recognition memory in the current study because it is highly reproducible 22, 23. The motor and cognitive results are consistent with a number of previous studies that have shown significant improvement in functional outcome after TBI with Mg administration 3, 4, 6, 31.
Finally, the histological examination of the CA3 region of the hippocampi demonstrated that optimal dose MgCl2 and 10% Mg PEG markedly attenuated H&E‐associated dark cell change, in contrast to 10% MgCl2 alone which has no positive effect relative to saline (vehicle)‐ or PEG‐treated animals. Dark cell change is a phenomenon found within the brain following many conditions including TBI, hypoglycemia, and ischemia and is thought to result from Ca2+ influx, with subsequent energy depletion, phospholipase and other enzyme activation, and potentially leading to cell death 32, 33. While dark cell change has been shown in both the cortex and hippocampus after TBI, the changes are particularly apparent in the CA3 region of the hippocampus where they persist for at least 7 days 32. Moreover, these changes in the hippocampus have been shown to be associated with cognitive deficits in rodent TBI models 34. Our present results indicate that the 10% magnesium dose in PEG markedly reduced the dark cell change in the hippocampus, consistent with the improved outcome noted in the cognitive tests.
In conclusion, this study demonstrates that administration of Mg PEG 30 min after TBI reduces edema and BBB permeability and improves functional (motor and cognitive) and histological outcome. Moreover 10% Mg PEG showed significant beneficial effects when compared with the 10% MgCl2 dose, which in itself has no beneficial effect. These findings suggest that when used in combination with Mg, PEG facilitates central penetration of the Mg salt after TBI. Accordingly, lower doses of Mg may be used in combination with PEG thereby reducing the possibility of any potential side effects associated with high blood Mg concentration.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
Supported, in part, by the Neurosurgical Research Foundation, Australia.
References
- 1. Vink R, McIntosh TK, Demediuk P, Weiner MW, Faden AI. Decline in intracellular free Mg2+ is associated with irreversible tissue injury after brain trauma. J Biol Chem 1988;263:757–761. [PubMed] [Google Scholar]
- 2. Vink R, Cernak I. Regulation of intracellular free magnesium in central nervous system injury. Front Biosci 2000;5:D656–D665. [DOI] [PubMed] [Google Scholar]
- 3. Vink R, Nechifor M, editors. Magnesium in the central nervous system. Adelaide: Adelaide University Press, 2012. [PubMed] [Google Scholar]
- 4. Heath DL, Vink R. Optimization of magnesium therapy after severe diffuse axonal brain injury in rats. J Pharmacol Exp Ther 1999;288:1311–1316. [PubMed] [Google Scholar]
- 5. Vink R, Nimmo AJ. Novel therapies in development for the treatment of traumatic brain injury. Expert Opin Investig Drugs 2002;11:1375–1386. [DOI] [PubMed] [Google Scholar]
- 6. Sen AP, Gulati A. Use of magnesium in traumatic brain injury. Neurotherapeutics 2010;7:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhandapani SS, Gupta A, Vivekanandhan S, Sharma BS, Mahapatra AK. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. Ind J Neurotrauma 2008;5:27–33. [Google Scholar]
- 8. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: A randomised controlled trial. Lancet Neurol 2007;6:29–38. [DOI] [PubMed] [Google Scholar]
- 9. Schouten JW. Neuroprotection in traumatic brain injury: A complex struggle against the biology of nature. Curr Opin Crit Care 2007;13:134–142. [DOI] [PubMed] [Google Scholar]
- 10. Stippler M, Fischer MR, Puccio AM, et al. Serum and cerebrospinal fluid magnesium in severe traumatic brain injury outcome. J Neurotrauma 2007;24:1347–1354. [DOI] [PubMed] [Google Scholar]
- 11. Brewer RP, Parra A, Borel CO, Hopkins MB, Reynolds JD. Intravenous magnesium sulfate does not increase ventricular CSF ionized magnesium concentration of patients with intracranial hypertension. Clin Neuropharmacol 2001;24:341–345. [DOI] [PubMed] [Google Scholar]
- 12. Milton Harris J, Martin NE, Modi M. Pegylation: A novel process for modifying pharmacokinetics. Clin Pharmacokinet 2001;40:539–551. [DOI] [PubMed] [Google Scholar]
- 13. Koob AO, Colby JM, Borgens RB. Behavioral recovery from traumatic brain injury after membrane reconstruction using polyethylene glycol. J Biol Eng 2008;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smucker PMD, Hekmatyar SK, Bansal N, Rodgers RB, Shapiro AMD, Borgens RB. Intravenous polyethylene glycol successfully treats severe acceleration induced brain injury in rats as assessed by magnetic resonance imaging. Neurosurgery 2009;64:984–990. [DOI] [PubMed] [Google Scholar]
- 15. Lee JH, Roy J, Sohn HM, et al. Magnesium in a polyethylene glycol formulation provides neuroprotection after unilateral cervical spinal cord injury. Spine Phila 1976;35:2041–2048. [DOI] [PubMed] [Google Scholar]
- 16. Ditor DS, John SM, Roy J, Marx JC, Kittmer C, Weaver LC. Effects of polyethylene glycol and magnesium sulfate administration on clinically relevant neurological outcomes after spinal cord injury in the rat. J Neurosci Res 2007;85:1458–1467. [DOI] [PubMed] [Google Scholar]
- 17. Kwon BK, Roy J, Lee JH, et al. Magnesium chloride in a polyethylene glycol formulation as a neuroprotective therapy for acute spinal cord injury: Preclinical refinement and optimization. J Neurotrauma 2009;26:1379–1393. [DOI] [PubMed] [Google Scholar]
- 18. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg 1994;80:291–300. [DOI] [PubMed] [Google Scholar]
- 19. O'Connor CA, Cernak I, Vink R. The temporal profile of edema formation differs between male and female rats following diffuse traumatic brain injury. Acta Neurochir Suppl 2006;96:121–124. [DOI] [PubMed] [Google Scholar]
- 20. Kim SW, Chen G, Plesnila N. New method to optimize brain edema measurement In: Katayama Y, editor. The XVth International Symposium of Brain Edema and Cellular Injury. Tokyo: Nihon University, 2011;82. [Google Scholar]
- 21. Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: An evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma 1994;11:187–196. [DOI] [PubMed] [Google Scholar]
- 22. Bevins RA, Besheer J. Object recognition in rats and mice: A one‐trial non‐matching‐to‐sample learning task to study ‘recognition memory’. Nat Protoc 2006;1:1306–1311. [DOI] [PubMed] [Google Scholar]
- 23. Donkin JJ, Cernak I, Blumbergs PC, Vink R. A substance P antagonist reduces axonal injury and improves neurologic outcome when administered up to 12 hours after traumatic brain injury. J Neurotrauma 2011;28:218–224. [DOI] [PubMed] [Google Scholar]
- 24. O'Connor CA, Cernak I, Johnson F, Vink R. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp Neurol 2007;205:145–153. [DOI] [PubMed] [Google Scholar]
- 25. Bareyre F, Wahl F, McIntosh TK, Stutzmann JM. Time course of cerebral edema after traumatic brain injury in rats: Effects of riluzole and mannitol. J Neurotrauma 1997;14:839–849. [DOI] [PubMed] [Google Scholar]
- 26. Esen F, Erdem T, Aktan D, et al. Effects of magnesium administration on brain edema and blood‐brain barrier breakdown after experimental traumatic brain injury in rats. J Neurosurg Anesthesiol 2003;15:119–125. [DOI] [PubMed] [Google Scholar]
- 27. Harford‐Wright E, Thornton E, Vink R. Angiotensin‐converting enzyme (ACE) inhibitors exacerbate histological damage and motor deficits after experimental traumatic brain injury. Neurosci Lett 2010;481:26–29. [DOI] [PubMed] [Google Scholar]
- 28. Imer M, Omay B, Uzunkol A, et al. Effect of magnesium, MK‐801 and combination of magnesium and MK‐801 on blood‐brain barrier permeability and brain edema after experimental traumatic diffuse brain injury. Neurol Res 2009;31:977–981. [DOI] [PubMed] [Google Scholar]
- 29. Okiyama K, Smith DH, Gennarelli TA, Simon RP, Leach M, McIntosh TK. The sodium channel blocker and glutamate release inhibitor BW1003C87 and magnesium attenuate regional cerebral edema following experimental brain injury in the rat. J Neurochem 1995;64:802–809. [DOI] [PubMed] [Google Scholar]
- 30. Feldman Z, Gurevitch B, Artru AA, et al. Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. J Neurosurg 1996;85:131–137. [DOI] [PubMed] [Google Scholar]
- 31. Hoane MR. Assessment of cognitive function following magnesium therapy in the traumatically injured brain. Magnes Res 2007;20:229–236. [PubMed] [Google Scholar]
- 32. Thornton E, Vink R, Blumbergs PC, Van Den Heuvel C. Soluble amyloid precursor protein alpha reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res 2006;1094:38–46. [DOI] [PubMed] [Google Scholar]
- 33. Auer RN. Hypoglycemic brain damage. Forensic Sci Int 2004;146:105–110. [DOI] [PubMed] [Google Scholar]
- 34. Hicks RR, Smith DH, Lowenstein DH, Saint Marie R, McIntosh TK. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J Neurotrauma 1993;10:405–414. [DOI] [PubMed] [Google Scholar]


