Abstract
Background
Cardiovascular disease is a major cause of mortality in patients with nonalcoholic fatty liver disease (NAFLD). However, the association of NAFLD with coronary microvascular dysfunction is, to our knowledge, unknown.
Purpose
To determine whether coronary microvascular dysfunction is more prevalent in patients with NAFLD and to determine whether coronary microvascular dysfunction predicts major adverse cardiac events (MACE) independently of NAFLD.
Materials and Methods
This retrospective study (2006–2014) included patients without evidence of obstructive epicardial coronary artery disease and healthy left ventricular ejection fraction (≥40%) at a clinical rest and stress myocardial perfusion PET/CT. NAFLD was defined by a mean hepatic attenuation of less than 40 HU at CT and coronary microvascular dysfunction as a coronary flow reserve (CFR) of less than 2.0. A composite of all-cause mortality, myocardial infarction, coronary revascularization, and hospitalization because of heart failure comprised MACE (130 of 886 patients; 14.7%). The relation between NAFLD and MACE was assessed by using multivariable Cox regression analysis.
Results
Among 886 patients (mean age, 62 years ± 12 [standard deviation]; 631 women [mean age, 62 years ± 12 years] and 255 men [mean age, 61 years ± 12]; and ejection fraction, 63% ± 9), 125 patients (14.1%) had NAFLD and 411 patients (46.4%) had coronary microvascular dysfunction. Coronary microvascular dysfunction was more prevalent (64.8% vs 43.4%; P < .001) and CFR was lower (1.9 ± 1.1 vs 2.2 ± 0.7; P < .001) in patients with NAFLD compared with those without NAFLD. NAFLD independently predicted coronary microvascular dysfunction (P = .01). The interaction of NAFLD and male sex predicted MACE (hazard ratio, 1.45; 95% confidence interval: 1.08, 1.69; P = .008) and coronary microvascular dysfunction remained associated with MACE (adjusted hazard ratio, 1.46; 95% confidence interval: 1.02, 2.07; P = .04).
Conclusion
Coronary microvascular dysfunction was more prevalent in patients with nonalcoholic fatty liver disease and predicted major adverse cardiac events independently of nonalcoholic fatty liver disease.
© RSNA, 2019
Online supplemental material is available for this article.
See also the editorial by Ambale-Venkatesh and Lima in this issue.
Summary
Patients who underwent myocardial perfusion PET/CT and showed evidence of nonalcoholic fatty liver disease at CT showed a higher risk of coronary microvascular dysfunction, which is an independent risk factor for major adverse cardiac events.
Key Points
■ Coronary microvascular dysfunction was more prevalent (64.8% vs 43.4%; P < .001) and coronary flow reserve lower (1.9 vs 2.2; P < .001) in patients with nonalcoholic fatty liver disease compared with those who did not have nonalcoholic fatty liver disease.
■ Men with nonalcoholic fatty liver disease had a higher risk of major adverse cardiac events (hazard ratio, 1.45; P = .008) as did patients with coronary microvascular disease (hazard ratio, 1.46; P = .04)
Introduction
More than 28 000 000 people in the United States and as many as 25% of the global population (1) are estimated to be affected by nonalcoholic fatty liver disease (NAFLD). Cardiovascular disease is a major cause of mortality in patients with NAFLD (2,3). Insulin resistance and inflammation are common threads in the pathogenesis of both NAFLD (4) and coronary artery disease (CAD) (5,6). Insulin-resistant states such as diabetes, hypertension, and obesity cause coronary atherosclerosis (ie, epicardial and microvascular CAD) and NAFLD. Characterized by similar pathophysiologic causes, insulin resistance, and inflammation, NAFLD is emerging as an independent risk factor for coronary atherosclerosis.
It is not surprising that calcified coronary atherosclerosis (7,8), obstructive CAD (9), and high-risk coronary plaque (10) are prevalent in patients with NAFLD, including asymptomatic patients (11). A small study (12) showed higher hepatic fat content as an independent predictor of myocardial insulin resistance and lower coronary microvascular vasodilator capacity in patients with diabetes. However, whether NAFLD is associated with coronary microvascular dysfunction independent of epicardial coronary stenosis, and in larger cohorts, has not been evaluated. To our knowledge, the relation of NAFLD, coronary microvascular dysfunction, and major adverse cardiac events (MACE) is not known.
Coronary microvascular function, assessed as coronary flow reserve (CFR; the ratio of stress to rest myocardial blood flow), provides an integrated assessment of epicardial and microvascular myocardial perfusion (13). Reduced CFR by quantitative myocardial perfusion imaging (MPI) with PET/CT is a powerful risk marker for MACE (14). Our hypothesis was that coronary microvascular dysfunction is more prevalent in patients with NAFLD and that coronary microvascular dysfunction determines MACE independent of NAFLD. Our purpose was to determine if coronary microvascular dysfunction is more prevalent in a cohort of patients undergoing PET MPI with incidental evidence of NAFLD at CT, but without evidence of epicardial CAD (normal PET/CT MPI), and to assess if coronary microvascular dysfunction, determined from PET MPI–derived CFR, predicts MACE independently of NAFLD.
Materials and Methods
This retrospective study was approved by the institutional ethics committee, Partner’s Human Research Committee (Protocol Number 2010P001481). Informed consent for this retrospective study was waived by the institutional ethics committee. This study was Health Insurance Portability and Accountability Act compliant. The authors received no industry support or funding for this study and had full control of all data and information submitted for publication.
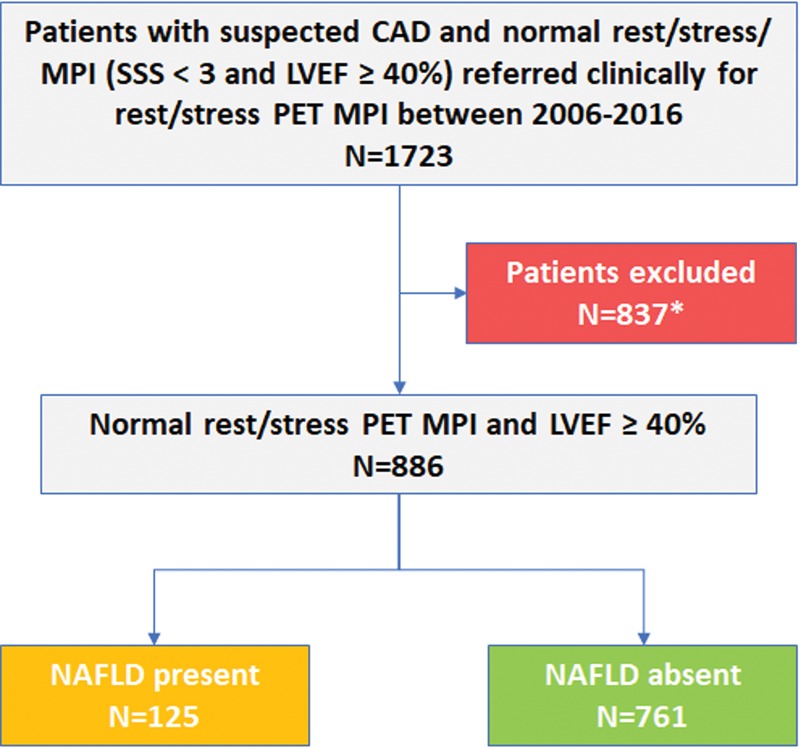
Our study cohort was selected from 1723 consecutive patients from the Brigham and Women’s Hospital (Boston, Mass) from 2006 to 2014 who were suspected of having CAD and who had undergone clinically performed rubidium 82 (82Rb) or N-13 ammonia PET/CT MPI that was normal (defined as no perfusion defects and summed stress perfusion score < 3) with a left ventricular ejection fraction of 40% or greater. From this cohort we excluded patients who may have had conditions that affected coronary microvascular dysfunction, mortality, or technical limitations (Fig 1), and included 886 patients in this analysis.
Figure 1:
Patient selection flowchart. LVEF = left ventricular ejection fraction, SSS = sum stress score, CAD = coronary artery disease, NAFLD = nonalcoholic fatty liver disease. * = Of the initial cohort of 1723 patients, 837 were excluded for one or more of the following criteria that may have affected survival, imaging technique, and/or CFR: active malignancy (n = 189), cirrhosis (n = 15), complex congenital heart disease (n = 17), human immunodeficiency virus (n = 8), infiltrative cardiomyopathy (n = 33), lung transplant (n = 12), missing/uninterpretable CT (n = 19), missing/uninterpretable PET (n = 24), renal dysfunction (estimated glomerular filtration rate, <45 mL/min/1.73 m2; n = 499), severe valve disease/surgery (n = 121).
Patients were imaged after an overnight fast with a whole-body PET/CT scanner (Discovery ST; GE Healthcare, Milwaukee, Wis) by using either 82Rb or N-13 ammonia as perfusion radiotracers as previously described (14). Initial scout (120 kV; 10 mA) and transmission (140 kV; 10 mA; free tidal breathing; nonelectrocardiography gated; noncontrast-agent enhanced; section thickness and collimation of 3 mm) CT transmission scans were obtained for attenuation correction from the carina to the bottom of the heart, with the z-axis field of view including the cranial half of the liver (∼6 cm). Further technical parameters of the MPI PET/CT acquisition technique are in Appendix E1 (online).
Measures of Coronary Microvascular Function
The presence of coronary microvascular dysfunction was defined as a CFR less than 2.0, which was calculated as the ratio of myocardial blood flow at stress over rest for the entire left ventricle. Regional and global rest and peak stress absolute mean myocardial blood flow (milliliter per minute per gram of tissue) was computed from the dynamic rest and stress images by fitting the 82Rb/N-13 ammonia time-activity curves to a two-compartment tracer kinetic model (15) by using commercial software (Corridor4DM; Invia Medical Imaging Solutions, Ann Arbor, Michigan) as previously validated (16). The results of myocardial blood flow or CFR were not included in the clinical report, and thus did not influence clinical care. A CFR of less than 2.0 was considered abnormal and indicative of microvascular dysfunction, and 2.0 or greater was considered normal on the basis of previous studies (16).
Assessment of NAFLD
The presence of NAFLD was determined by assessing for the presence of hepatic steatosis by measuring mean CT attenuation values (in Hounsfield units) at the CT for attenuation correction scan from the PET/CT acquisition. We used a previously described (17,18) quantitative method of measuring mean liver Hounsfield units, and mean hepatic attenuation less than 40 HU denoted the presence of NAFLD. We performed a validation of hepatic Hounsfield unit measurement at CT for attenuation correction compared with diagnostic noncontrast CT in 32 patients, which showed good correlation (Appendix E1 [online]). Liver Hounsfield units were obtained by manually tracing four equal circular regions of interest on the liver at two separate axial levels, referred to as upper and lower, by using commercially available software (Osirix MD; Pixmeo, Berenex, Switzerland). The individual patient hepatic attenuation value was calculated as the mean of the eight hepatic regions of interest at the two levels. Care was taken to sample homogeneous areas representative of the liver parenchyma by avoiding blood vessels, bile ducts, hila, and the capsular surface margins. We showed the presence of NAFLD on the basis of an assessment of the cranial portion of the liver included at the field of view of the CT for attenuation correction scan, and we believe it is justified because NAFLD is generally a diffuse process. Patients with evidence of liver parenchymal disease at the CT for attenuation correction scan (nodular, shrunken liver contour suggestive of cirrhosis, extensive liver cysts, liver masses, and previous hepatic resection) were excluded. Tracings were performed by three readers (T.V., a cardiovascular imaging fellow, D.J.M., a radiology fellow, and A.K., a radiology fellow; authors had 4, 6, and 7 years of imaging experience, respectively) who were blinded to the clinical and PET MPI results.
Patients were evaluated for incident cardiovascular events after the PET scan (Appendix E1 [online]). The primary end point included a composite of all-cause mortality, myocardial infarction, percutaneous or surgical coronary revascularization, and admissions for heart failure exacerbation. If a patient underwent more than one event, only the first event was recorded.
Statistical Analysis
Continuous data (mean ± standard deviation, standard deviation, or nonparametric statistics when appropriate) and categorical data (numbers or percentages) were compared by using a t test or χ2 tests, as appropriate. The independent associations between NAFLD, myocardial blood flow, and clinical events were studied by using linear regression analysis with multivariable adjustments. P values less than .05 indicated statistical significance.
Annualized MACE rates were calculated by dividing the event proportion for each event (ie, number of events/cohort size) by the mean follow-up time (in years), converted to percentages. After verifying the baseline proportionality assumption, multivariable Cox regression analyses were used to analyze the relation between NAFLD and MACE. All model variables were chosen on the basis of a multivariable stepwise forward selection to determine the independent predictors of MACE. We derived hazard ratios and 95% confidence intervals from the Cox models. Statistical analyses were performed (T.V., S.D., N.S.B.) with statistical software (Stata/IC 13.1 for Mac; StataCorp, College Station, Tex).
Results
The study cohort included 886 patients (mean age, 62 years ± 12) with normal PET MPI examinations and included 71.2% women (631 of 886; mean age, 62 years ± 12) and 28.8% men (255 of 886; mean age, 61 years ± 12). Coronary risk factors were prevalent in this cohort with history of hypertension, dyslipidemia, and diabetes in 72.2% (640 of 886), 59.4% (526 of 886), and 29.8% (264 of 886) of patients, respectively. The overall left ventricular ejection fraction (mean, 63% ± 9) and CFR (mean, 2.15 ± 0.7) were normal. The mean liver attenuation was 55 HU ± 15, and NAFLD (liver attenuation, <40 HU) was found in 14.1% of the study cohort (125 of 886). The baseline characteristics of the patients stratified by the presence (n = 125; 14.1%) or absence (n = 761; 85.9%) of NAFLD are summarized in Table 1. Patients with NAFLD had higher body mass index; higher prevalence of hypertension and diabetes; and were more frequently prescribed and administered statins, oral antidiabetic medications, and insulin. Indexed left ventricular volumes and left ventricular ejection fraction were similar in patients with and without NAFLD. The probability of CAD as estimated by the Morise score (including age, sex, symptoms, history of previous myocardial infarction, smoking, dyslipidemia, diabetes, and ST segment changes at rest electrocardiography) (19) was higher in the NAFLD group compared with the non-NAFLD group (mean Morise score, 10.43 ± 3.25 vs 9.25 ± 2.83, respectively; P < .001).
Table 1:
Patient Demographics
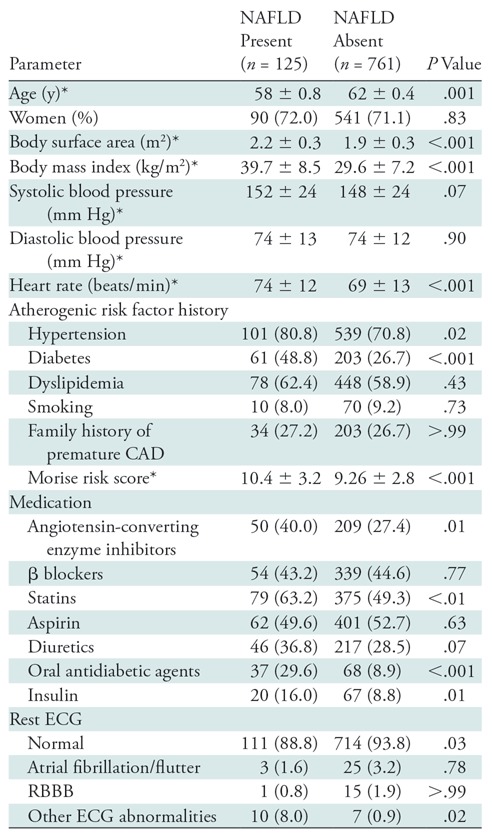
Note.—Unless otherwise indicated, data are numbers of patients and data in parentheses are percent. CAD = coronary artery disease, ECG = electrocardiography, NAFLD = nonalcoholic fatty liver disease, RBBB = right bundle branch block.
*Data are mean ± standard deviation.
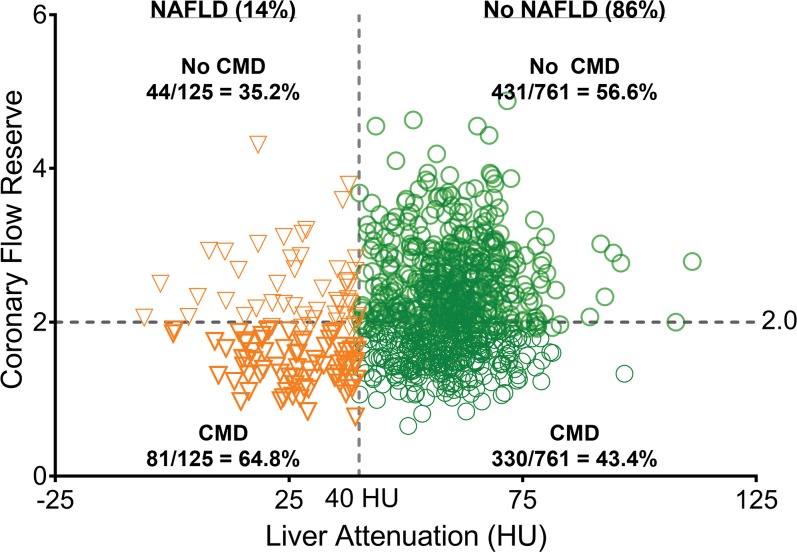
Mean CFR was 2.15 ± 0.69, and coronary microvascular dysfunction (CFR, <2.0) was found in 46.4% of the overall study cohort (411 of 886). As shown in Table 2, patients with NAFLD versus without NAFLD had lower rest myocardial blood flow and lower mean hyperemic myocardial blood flow (1.92 mL/min/g ± 0.71 vs 2.50 mL/min/g ± 0.92, respectively; P < .001) and CFR (1.9 ± 1.1 vs 2.2 ± 0.7, respectively; P < .001). Coronary microvascular dysfunction was observed more frequently in patients with compared with patients without NAFLD (64.8% [81 of 125] vs 43.4% [330 of 761], respectively; P < .001) with a risk ratio of coronary microvascular dysfunction in patients with NAFLD of 1.5 (95% confidence interval: 1.2, 1.7; P < .001; Fig 2). An example of images in a patient with NAFLD and coronary microvascular dysfunction is in Figure 3.
Table 2:
Comparison in Patients with and without Nonalcoholic Fatty Liver Disease
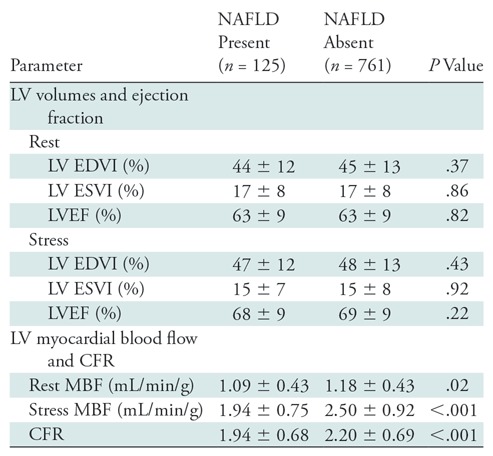
Note.—Unless otherwise indicated, data are mean ± standard deviation. CFR = coronary flow reserve, EDVI = indexed end-diastolic volume, ESVI = indexed end-systolic volume, LV = left ventricle, LVEF = left ventricular ejection fraction, MBF = myocardial blood flow, NAFLD = nonalcoholic fatty liver disease.
Figure 2:
Scatter plot of coronary microvascular dysfunction in patients with nonalcoholic fatty liver disease (NAFLD) and without NAFLD. CMD = coronary microvascular dysfunction.
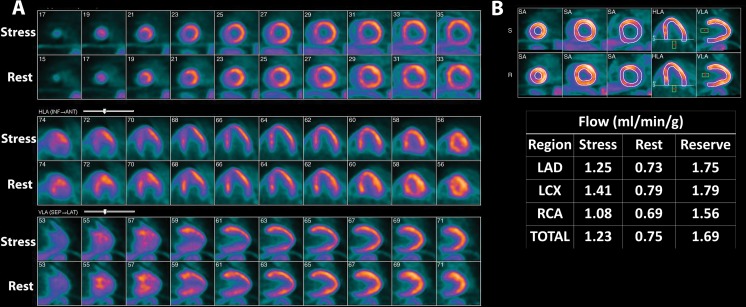
Figure 3:
Example case of a 62-year-old man with evidence of coronary microvascular dysfunction and nonalcoholic fatty liver disease at myocardial perfusion PET/CT. A, Stress and rest myocardial N-13 ammonia myocardial perfusion PET with blood flow quantification produced a normal examination without perfusion defects and globally reduced coronary flow reserve of 1.7, with, B, severely reduced peak stress myocardial blood flow of 1.2 mL/min/g. The overall mean liver density for this patient was 26.2 HU, consistent with nonalcoholic fatty liver disease (images not shown). HLA = horizontal long axis, LAD = left anterior descending coronary artery, LCX = left circumflex coronary artery, RCA = right coronary artery, SA = short axis, VLA = vertical long axis.
Predictors of NAFLD
The univariable and multivariable predictors of NAFLD are in Table E1 (online). Age, body mass index, rest heart rate, history of diabetes, history of hypertension, medication use (ie, angiotensin converting enzyme inhibitors, lipid lowering therapy, oral hypoglycemic agents and insulin), left ventricular mass (P < .001), and Morise score (P < .001) were univariable predictors of NAFLD. At multivariable analyses, body mass index (P ≤ .001), resting heart rate (P = .003), oral hypoglycemic agents (P < .001), left ventricular mass (P = .04), and indexed left ventricular end-diastolic volume were independently associated with NAFLD. We did not find an interaction between left ventricular mass and left ventricular end-diastolic volume (P = .12).
NAFLD and Microvascular Dysfunction
To determine the independent association of NAFLD with CFR, we developed step-wise linear regression models (Table 3) by including demographic variables medication use, ECG changes, and left ventricular function in the first step, and by including NAFLD in the second step. In the final model (R = 0.08; P < .001), age (P < .001), heart rate (P < .001), diastolic blood pressure (P < .001), and stress left ventricular ejection fraction (P = .006) predicted CFR. NAFLD remained an independent predictor of CFR (P = .001), independent of individual risk factors or Morise score in separate models. The mean CFR value declined by 0.25 with a diagnosis of NAFLD.
Table 3:
Univariable and Multivariable Associates of CFR
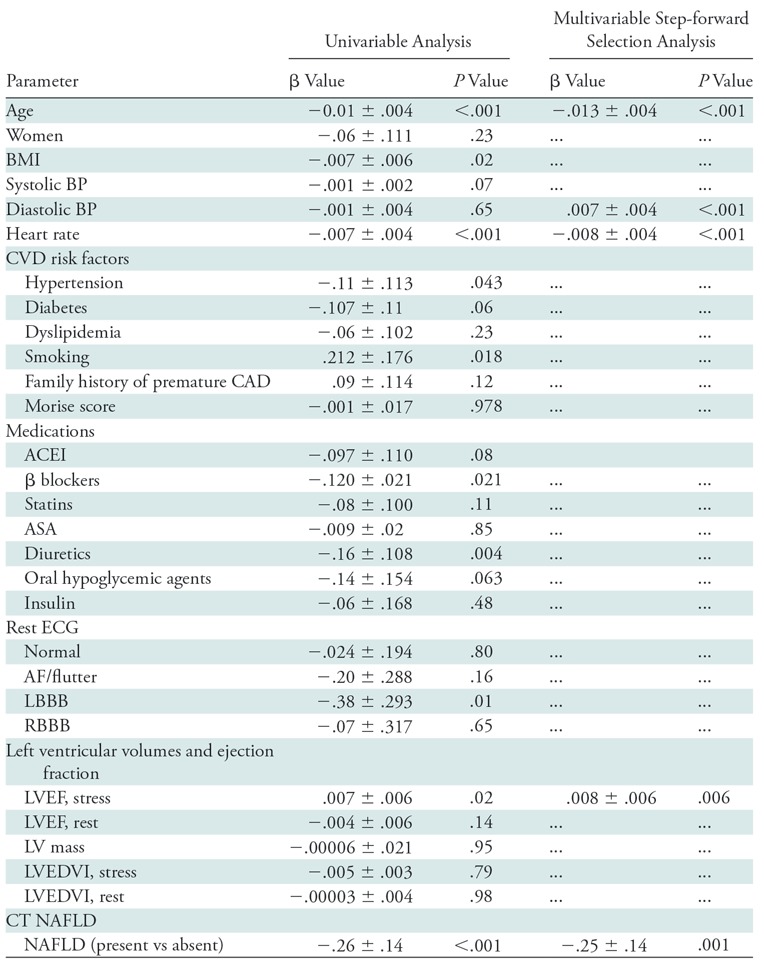
Note.—Unless otherwise indicated, data are ± standard error. Only significant variables from univariable analyses are shown in the multivariable models. ACEI = angiotensin converting enzyme inhibitors, AF = atrial fibrillation, ASA = acetylsalicylic acid, BMI = body mass index, BP = blood pressure, CAD = coronary artery disease, CFR = coronary flow reserve, CVD = cardiovascular disease, ECG = electrocardiography, LBBB = left bundle branch block, LV = left ventricle, LVEDVI = indexed left ventricular end-diastolic volume, LVEF = left ventricular ejection fraction, NAFLD = nonalcoholic fatty liver disease, RBBB = right bundle branch block.
Predictors of MACE
After a median follow-up of 5.6 years (interquartile range, 3.0–7.1 years), 14.7% of patients (130 of 886) underwent an MACE, which included deaths in 9.5% of patients (84 of 886), nonfatal myocardial infarctions in 4.5% (40 of 886), heart failure hospitalizations in 5.1% (45 of 886), and coronary revascularizations in 2.4% (21 of 886) (Table E2 [online]). The annualized event rates did not suffer significantly in patients with and without NAFLD. NAFLD was not a univariable predictor of MACE (P = .08). Risk factors of age (P < .001), sex (P = .02), hypertension (P < .001), smoking status (P = .01), family history of premature CAD (P = .005), use of β blockers (P < .001), use of diuretics (P = .04), left bundle branch block at electrocardiography (P = .04), and atrial fibrillation and/or flutter at electrocardiography (P = .003) demonstrated a significant univariable association with MACE. The Morise score was a significant univariable predictor of MACE. As expected, rest left ventricular ejection fraction (P < .001), stress myocardial blood flow (P = .001), and CFR (P = .004) demonstrated significant univariable association with MACE. For every unit increase of CFR there was a 46% reduction in the probability of MACE.
Multivariable Cox survival models, by using step-forward methods, were constructed to evaluate the incremental prognostic value of CFR over NAFLD. In these models an interaction term for sex and liver attenuation were included (Table 4). Abnormal CFR was strongly associated with MACE with an adjusted hazard ratio of 1.35 (95% confidence interval: 1.02, 2.07; P = .04), in addition to age (P < .001), rest left ventricular ejection fraction (P < .001), history of hypertension (P < .01), and history of tobacco use (P < .001). In these models, there was no relationship between Morise score and MACE (P > .99). Likewise, there was no association between NAFLD and MACE (P = .08). We observed a significant interaction between NAFLD and men in the multivariate model on risk of MACE (P < .01); men with NAFLD had an almost 1.5-fold higher risk of developing MACE (hazard ratio, 1.45; 95% confidence interval: 1.08, 1.69; Table 4).
Table 4:
Multivariable Cox Proportional Hazards Models for Prediction of Major Adverse Cardiac Events
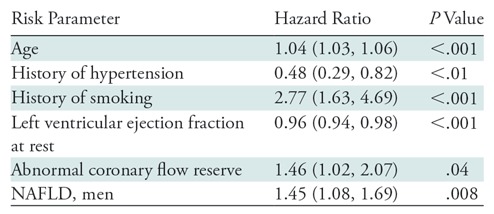
Note.—Data in parentheses are 95% confidence intervals. Model used was χ2 74.72, P < .001. For categorical variables, hazard ratio is listed for the presence of risk parameter by using absence of the risk parameter as the reference category. Variables included in this stepwise model but not significant include the following: male sex, body mass index, history of diabetes, history of dyslipidemia, family history of premature coronary artery disease, angiotensin converting enzyme use, diuretic use, oral hypoglycemic use, insulin use, statin use, systolic blood pressure (rest), diastolic blood pressure (rest), heart rate (rest), normal electrocardiography, atrial fibrillation/flutter, left bundle branch block, ST segment depression at electrocardiography, nonalcoholic fatty liver disease, men without nonalcoholic fatty liver disease, women with nonalcoholic fatty liver disease, and women without nonalcoholic fatty liver disease. NAFLD = nonalcoholic fatty liver disease.
Discussion
Our study showed that incidental evidence of NAFLD at CT was found in 14.1% of patients (125 of 886), and 64.8% of patients (81 of 125) with NAFLD demonstrated coronary microvascular dysfunction. Patients with NAFLD had high atherogenic risk factor burden; but NAFLD, an integrated effect of organ damage from atherogenic risk factors, was a more powerful and independent predictor of microvascular dysfunction than individual risk factor burden. Notably, among patients with NAFLD, the risk of coronary microvascular dysfunction was 1.5-fold higher compared with those who did not have NAFLD. Lower CFR and left ventricular ejection fraction, but not NAFLD, were independent predictors of MACE. Whereas in the overall cohort the risk of MACE was similar in patients with and without NAFLD, there was a significant interaction with sex; male patients with NAFLD had significantly higher risk of MACE. Our findings suggest that coronary microvascular dysfunction may explain some of the higher risk of adverse cardiovascular events in patients with NAFLD.
To our knowledge, our study is the largest to evaluate the association of NAFLD and coronary microvascular dysfunction. Yilmaz et al (20) measured CFR by transthoracic Doppler electrocardiography on the left anterior descending coronary artery in 59 patients with NAFLD with 77 matched control participants, and they found an association between NAFLD and microvascular dysfunction. Lautamäki et al (12) examined the effect of hepatic steatosis on microvascular dysfunction in 55 patients with type 2 diabetes mellitus and known CAD by using 15 oxygen water PET to measure CFR, and fluorine 18 fluorodeoxyglucose PET to measure myocardial glucose uptake. They quantified the extent of hepatic steatosis by using MR proton spectroscopy and found that patients with higher liver fat content had higher levels of myocardial insulin resistance, lower myocardial glucose extraction rates, and lower CFR than did patients who had lower quantities of hepatic fat. Our results confirmed that the link between NAFLD and coronary microvascular dysfunction holds true when applied to a large general cohort of patients without known CAD, and when controlled for other cardiovascular risk factors.
Although NAFLD has been recognized as a potential risk factor for cardiovascular disease, there remains conflicting evidence on the exact nature of the link. NAFLD has been demonstrated as an independent prognostic factor for CAD and vascular disease in some series (21,22), but this association has not been consistently identified in all series (23), including two studies in diabetic patients (24,25). Some of the differences among these studies may be explained by the variety of investigations used to diagnose NAFLD, including liver enzymes, US, CT (by using several different criteria), MRI, and liver biopsy; to our knowledge there is no single defined noninvasive reference standard test for diagnosis of NAFLD. In the multiethnic study of atherosclerosis study cohort, Shah et al (18) did not find an association between hepatic fat and overall cardiovascular disease events or mortality, but they did find that patients with hepatic steatosis were more likely to have features of adverse left ventricular remodeling. Shah et al used the same definition of hepatic steatosis as we did in our study (mean liver < 40 HU), and our results mirror their results; we also did not find an independent association between NAFLD and MACE or overall mortality, but we did find that NAFLD is predictive of coronary microvascular dysfunction, which is associated with poorer cardiovascular disease prognosis and mortality (16).
The findings of our study suggest that the higher risk of cardiovascular disease in NAFLD found in various series may partially be explained by the greater prevalence of clinical risk factors such as smoking, higher body mass index, and diabetes. This relationship between NAFLD and cardiovascular disease risk factors is established; a prospective study of 4401 men by Hamaguchi et al (26) found that the metabolic syndrome is a strong predictor for the development of NAFLD. A prospective study of 2103 patients with type 2 diabetes mellitus by Targher et al (27) found that patients with NAFLD were at increased risk of future cardiovascular disease events independent of classic cardiovascular disease risk factors and the metabolic syndrome. Our results suggest that coronary microvascular dysfunction may explain some of this increased cardiovascular disease risk in patients with NAFLD. In the overall cohort and in the different subgroups, patients who had an abnormal CFR had higher annualized event rates compared with those who had a normal CFR. In addition, when adjusted for important predictor factors such as rest left ventricular ejection fraction, age, and risk clinical factors, abnormal CFR provided strong cardiac prognostication to cardiovascular events. Our findings corresponded with results from other groups in which abnormal CFR portended a higher risk regarding clinical and metabolic factors (28).
Our study had limitations. First, it was a retrospective observational study performed at a single center, and therefore our results are most applicable to similar patient cohorts. Second, the presence of NAFLD was assessed at CT for the attenuation correction scan component of PET/CT. NAFLD is a manifestation of insulin resistance, but parameters to estimate metabolic syndrome or calcium score were not available in all of our patients. Whereas selection of patients with a normal PET scan and preserved left ventricular ejection fraction excludes epicardial CAD in most cases and makes it feasible to evaluate microvascular dysfunction, the presence of balanced ischemia in our cohort, although rare, was possible. Our approach provided an opportunity to explore the association between NAFLD, coronary microvascular dysfunction, and adverse clinical outcomes in a large unselected group of patients. However, these associations are all cross sectional and may simply be as a result of shared risk factors and pathways, which was not considered in our study.
Among patients without imaging evidence of obstructive coronary artery disease, nonalcoholic fatty liver disease is an independent risk factor for coronary microvascular dysfunction. Coronary microvascular dysfunction, not nonalcoholic fatty liver disease, was independently associated with higher risk of future major adverse cardiac events.
APPENDIX
T.V., N.S.B., and P.B. supported by the National Institutes of Health training grant (T32-HL094301). M.T.O. supported by National Institutes of Health (T32HL076136, KL2TR002542) and American Heart Association (18CDA34110366).
T.V. and D.J.M. contributed equally to this work.
Disclosures of Conflicts of Interest: T.V. disclosed no relevant relationships. D.J.M. disclosed no relevant relationships. M.T.O. disclosed no relevant relationships. N.S.B. disclosed no relevant relationships. A.K. disclosed no relevant relationships. S.J. disclosed no relevant relationships. A.J.D.M. disclosed no relevant relationships. A.N. disclosed no relevant relationships. P.VB. disclosed no relevant relationships. J.H. disclosed no relevant relationships. C.F.B. disclosed no relevant relationships. M.L.S. disclosed no relevant relationships. V.R.T. disclosed no relevant relationships. H.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed advisory board service on and stock options in OptimizeRx. Other relationships: disclosed no relevant relationships. R.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed payment to author’s institution for grants/grants pending from Astellas and Amgen. Other relationships: disclosed no relevant relationships. M.F.D.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for scientific/medical advisory board membership from Sanofi and General Electric; disclosed money to author’s institution for grants/grants pending from Spectrum Dynamics and Gilead. Other relationships: disclosed no relevant relationships. S.D. disclosed no relevant relationships.
Abbreviations:
- CAD
- coronary artery disease
- CFR
- coronary flow reserve
- MACE
- major adverse cardiac events
- MPI
- myocardial perfusion imaging
- NAFLD
- nonalcoholic fatty liver disease
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 3.Athyros VG, Tziomalos K, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: An update. World J Gastroenterol 2015;21(22):6820–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66(6):1138–1153. [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996;334(15):952–957. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352(16):1685–1695. [DOI] [PubMed] [Google Scholar]
- 7.Jaruvongvanich V, Wirunsawanya K, Sanguankeo A, Upala S. Nonalcoholic fatty liver disease is associated with coronary artery calcification: A systematic review and meta-analysis. Dig Liver Dis 2016;48(12):1410–1417. [DOI] [PubMed] [Google Scholar]
- 8.Sinn DH, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut 2017;66(2):323–329. [DOI] [PubMed] [Google Scholar]
- 9.Tomizawa N, Inoh S, Nojo T, Nakamura S. Relationship of hepatic steatosis severity and coronary artery disease characteristics assessed by coronary CT angiography. Int J Cardiovasc Imaging 2016;32(Suppl 1):73–82. [DOI] [PubMed] [Google Scholar]
- 10.Puchner SB, Lu MT, Mayrhofer T, et al. High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology 2015;274(3):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SB, Park GM, Lee JY, et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: An observational cohort study. J Hepatol 2018;68(5):1018–1024. [DOI] [PubMed] [Google Scholar]
- 12.Lautamäki R, Borra R, Iozzo P, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 2006;291(2):E282–E290. [DOI] [PubMed] [Google Scholar]
- 13.Dorbala S, Di Carli MF. Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med 2014;44(5):344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124(20):2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Fakhri G, Kardan A, Sitek A, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 2009;50(7):1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017;136(24):2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyce CJ, Pickhardt PJ, Kim DH, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol 2010;194(3):623–628. [DOI] [PubMed] [Google Scholar]
- 18.Shah RV, Anderson A, Ding J, et al. Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging 2017;10(9):1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med 1993;118(2):81–90. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz Y, Kurt R, Yonal O, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis 2010;211(1):182–186. [DOI] [PubMed] [Google Scholar]
- 21.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol 2016;65(3):589–600. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep 2016;6(1):33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MK, Ahn CW, Nam JS, Kang S, Park JS, Kim KR. Association between nonalcoholic fatty liver disease and coronary artery calcification in postmenopausal women. Menopause 2015;22(12):1323–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKimmie RL, Daniel KR, Carr JJ, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol 2008;103(12):3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143(10):722–728. [DOI] [PubMed] [Google Scholar]
- 27.Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005;54(12):3541–3546. [DOI] [PubMed] [Google Scholar]
- 28.Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58(7):740–748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.