Summary
Visceral pain is a global term used to describe pain originating from the internal organs of the body, which affects a significant proportion of the population and is a common feature of functional gastrointestinal disorders (FGIDs) such as irritable bowel syndrome (IBS). While IBS is multifactorial, with no single etiology to completely explain the disorder, many patients also experience comorbid behavioral disorders, such as anxiety or depression; thus, IBS is described as a disorder of the gut–brain axis. Stress is implicated in the development and exacerbation of visceral pain disorders. Chronic stress can modify central pain circuitry, as well as change motility and permeability throughout the gastrointestinal (GI) tract. More recently, the role of the gut microbiota in the bidirectional communication along the gut–brain axis, and subsequent changes in behavior, has emerged. Thus, stress and the gut microbiota can interact through complementary or opposing factors to influence visceral nociceptive behaviors. This review will highlight the evidence by which stress and the gut microbiota interact in the regulation of visceral nociception. We will focus on the influence of stress on the microbiota and the mechanisms by which microbiota can affect the stress response and behavioral outcomes with an emphasis on visceral pain.
Keywords: Brain‐Gut axis, Gut microbiota, Stress, Visceral Pain
Introduction
Irritable bowel syndrome (IBS) is a complex heterogeneous disorder associated with abdominal visceral pain, constipation, diarrhea, or a combination of both 1. It is the most common disorder seen by gastroenterologists and presents frequently with a number of intestinal and nonintestinal comorbidities 2. Notably, anxiety and depressive disorders account for 20–60% of these comorbidities 2, 3. The disorder is now viewed as one of altered gut–brain axis homeostasis 4, 5, 6. A distinguishing feature of IBS is that symptoms, including abdominal pain, are often triggered or exacerbated during periods of stress 7, 8. Stress is defined as the reaction by the body to a stimulus, either physical or psychological, that disrupts homeostasis. Stress has profound effects on the gastrointestinal (GI) tract including but not limited to alterations in intestinal motility 9, mucosal transport, gut barrier function 10, 11, 12, and visceral perception 13, 14. More recently, the role of the gut microbiota in the bidirectional communication along the gut–brain axis, and subsequent changes in behavior, has emerged 15, 16. Recent evidence proposes that stress can lead to long‐term changes in the gut microbiota 17; however, the importance of the gut microbiota and their role in visceral sensation and nociception remain to be further explored. The focus of this review will be to attempt to summarize a complex body of literature describing the relationship between stress and visceral pain and how the gut microbiota may interact through complementary or opposing factors to influence visceral nociceptive behaviors.
Stress
Stress was first described by Hans Selye almost 80 years ago as the general response of the body to any noxious stimulus 18. Selye elucidated the role of the hypothalamic–pituitary–adrenal (HPA) axis in mediating the biological effects of stress on the host. At the same time, Walter Cannon coined the phrase “fight or flight” response 19 with much of his work building upon Claude Bernard's description of homeostasis 20. The HPA and sympathomedullary axes are the two stress response pathways in mammals. The HPA axis is slower‐acting and adaptive, encompassing a network of anatomical constituents located both in the central nervous system (CNS) and in the periphery. The crucial components are the paraventricular nucleus (PVN) of the hypothalamus, the pituitary gland (anterior lobe), and the adrenal gland 21. The HPA axis responds to a stressor by releasing corticotropin‐releasing hormone (CRH) into the hypophyseal portal circulation, which travels to the anterior pituitary gland, where it binds to its respective receptor (CRH1). This event leads to the production of pro‐opiomelanocortin, which is subsequently cleaved within the pituitary corticotropes, to produce adrenocorticotropic hormone (ACTH), which in turn is released into systemic circulation. ACTH targets the adrenal cortex to stimulate the production and secretion of glucocorticoids such a cortisol (humans) or corticosterone (rats and mice) 21. Glucocorticoids are the main effector molecules of the HPA axis and, via binding to their intracellular receptors, function to regulate the physiological adaptations to stress 22, 23. Cortisol/corticosterone initiates negative feedback through binding to glucocorticoid (GR) and mineralocorticoid (MR) receptors 24, 25 in the hippocampus, PVN, and anterior pituitary 26. However, binding of cortisol/corticosterone to the amygdala promotes CRH expression and facilitation of the stress axis 27, 28. The HPA axis is under stringent regulation at both the neuronal and the endocrine levels; however, the body can also elicit maladaptive changes resulting in altered brain structure and function in response to chronic and uncontrollable stressors 29, 30, 31. The sympathomedullary axis is responsible for the acute fight or flight response, which is driven by the activation of preganglionic sympathetic neurons located within the intermediolateral cell column of the thoracolumbar spinal cord. The projections of these preganglionic sympathetic neurons are pre‐ and paravertebral ganglia. These project to terminal organs and to chromaffin cells of the adrenal medulla resulting in increased circulating levels of epinephrine (adrenal medulla) and norepinephrine (sympathetic nerves) 32. The release and action of these catecholamines results in alterations in physiology such as increased heart rate, vasoconstriction, and mobilization of energy stores, to allow the host to adequately adapt to the stressor.
Visceral Pain
Visceral pain is a generic term that is applied to pain arising from the internal organs contained within the thorax and abdomen 33. Acute visceral pain usually has an identifiable cause, such as infection or tissue damage, which can be typically treated with an appropriate therapeutic agent. In contrast, chronic visceral pain, such as other types of chronic pain, is long‐lasting and can be difficult to treat with current pharmaceuticals. The lack of identifiable pathology in some types of chronic visceral pain has led to the use of the term functional gastrointestinal disorders (FGIDs) to describe 45 adult and pediatric disorders, including IBS, functional dyspepsia, infant colic and abdominal migraine, that affect discrete regions of the GI tract 34.
Within the GI tract, extrinsic nociceptors can respond to multimodal stimuli, depending on receptor expression, including stretch, pH, bacterial products, substances released from immune cells, and neurotransmitters released from the enteric nervous system or enterochromaffin cells 35. The nociceptors have nerve endings throughout the layers of the GI tract (mucosal, submucosal, muscular), and their cell bodies are located in the dorsal root ganglion (DRG). The first synapse is in the superficial layers of the dorsal horn of the spinal cord. The nociceptive signal is then transmitted to the contralateral side of the spinal cord via decussating fibers, and pain signals reach the brain via the spinothalamic tract, which has a somatotropic arrangement in the anterolateral aspect of the spinal cord. Although vagal afferents were not previously thought to be involved in the mediation of visceral pain 36, recent evidence suggests a role for vagal transmission of anti‐/pro‐nociceptive signals, which bypasses the spinal cord 37, 38, 39, 40. Within the brain, the signal is then relayed to cortical areas for localization and to limbic areas for the emotional component of the pain response. Output from the cortical and limbic regions in response to the pain activates descending inhibitory circuitry within the brainstem that causes release of inhibitory neurotransmitters within the dorsal horn of the spinal cord. While the mechanisms responsible for chronic pain are still under investigation, and likely are dependent on the nature of the initiating stimulus, sensitization can occur at a number of different sites. These include but not limited to local mediators within the GI tract, remodeling of ascending afferents within the dorsal horn, hyperactivity of central pain circuitry, and/or loss of descending inhibition (Figure 1).
Figure 1.
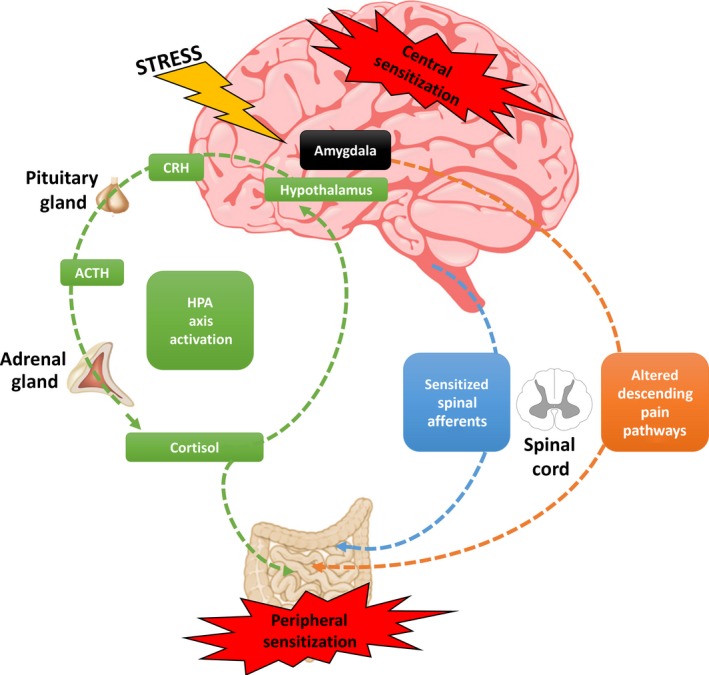
Central and Peripheral Pain Sensitization. Heightened pain perception can occur due to a combination of both central sensitization and peripheral sensitization. The hypothalamic–pituitary–adrenal (HPA) axis is activated by stress. In brief, the hypothalamus secretes corticotropin‐releasing hormone (CRH) into the hypophyseal portal system for the activation of the anterior pituitary and subsequent release of adrenocorticotropic hormone (ACTH) into the systemic circulation. In response to ACTH, the adrenal cortex releases cortisol (corticosterone in rodents), which can directly activate resident immune cells and extrinsic primary afferents within the gastrointestinal tract to promote peripheral sensitization. While cortisol binding to the hypothalamus promotes feedback inhibition of the stress response, cortisol binding to the amygdala facilitates further stress‐induced secretion of CRH, promoting central sensitization of stress pathways. The amygdala also promotes CRH signaling in the brain stem to further promote central sensitization by altering descending inhibition within the spinal cord. Direct injury to the GI tract can lead to sensitization of spinal afferents (lower threshold for activation and/or longer lasting responses) that can persist following recovery from the injury.
In the laboratory setting, GI hypersensitivity, or an exaggerated sensitivity, is typically measured in response to distension of the particular area of the GI tract. In the case of clinical studies, the subject has a balloon catheter inserted into the colorectal region and graded isobaric or isovolumetric distensions are performed with the individual reporting their perception of the stimulus. Thresholds can be determined for perception of the balloon, discomfort, and pain, with many patients with IBS reporting lowered thresholds for all stimuli compared to healthy volunteers, and hence, visceral hypersensitivity is apparent in a subset of patients with IBS 41, 42. In preclinical studies, the same experimental paradigm used in patients to assess visceral sensitivity has been adapted to rats and mice 43, 44. Graded colorectal distension (CRD) produces a visceromotor behavioral response (VMR) in rodents that is typically positively correlated with distension pressure/volume. The VMR can be quantified visually without additional instrumentation in freely moving animals, or as quantification of the electromyogenic (EMG) signal following implantation of electrodes in the overlying abdominal muscle, which requires the animal to be partially restrained to minimize movement artifact with the EMG signal. A nonsurgical assessment of VMR via manometric recordings using sensors within the colon to measure changes in intracolonic pressure has been established in mouse models 45, 46, 47. This method has the advantage that animals do not have to be restrained while assessing colonic sensitivity; however, the disadvantage is that the measurements can contain artifacts due to colonic contractions during normal colonic motility and unrelated to measures of colonic sensitivity. For further reading on rodent CRD, the reader is referred to a review on the assessment of visceral pain in rodent models by O'Mahony and colleagues 45.
Multiple techniques have been developed to produce acute and chronic colonic hypersensitivity as measured by an increase in VMR to distension. Exposing neonatal pups to adverse experiences (separation from the dam, direct colonic irritation/inflammation) can produce a heightened VMR in adulthood 48, 49. In adult animals, acute stressors (physical or psychological) typically produce transient increases in the VMR, while repeated homotypic or heterotypic stressors can produce a chronic hypersensitivity 48, 49. Similarly, active inflammation induced by chemical irritants or a pathogen causes acute hypersensitivity, with a subset of rodents developing a persistent hypersensitivity following recovery from the inflammation 48, 49. While each of these models can demonstrate an increase in VMR to distension, which has good construct validity for the clinical paradigm used to evaluate visceral pain, one deficit with these preclinical models is the lack of the ability to monitor spontaneous visceral nociceptive behaviors. A study by Chen et al. aimed to develop an alternative approach to assess the affective responses of visceral pain. In an experimental model of rodent colitis, a conditioned place preference paradigm was established whereby animals were conditioned with intraluminal administration of 2% lidocaine hydrochloride or vehicle followed by confinement to an assigned choice compartment for 30 min. A clear preference was observed for the chamber paired with intracolonic lidocaine treatment in colitic rats, whereas no preference was seen in control animals 50. Continuing to develop novel translationally relevant animal models will undoubtedly aid in our understanding of the heterogeneous pathophysiology of visceral pain.
Stress and Visceral Pain
Maladaptive stress responses have been associated with an array of pathologies including FGIDs, affective disorders, autoimmune disease, and hypertension 21, 22, 51, 52, 53. Moreover, evidence suggests that stress can also have profound effects on pain processes 48, 54, 55, 56, 57 including visceral pain 49. Here we will discuss evidence from the literature to show a centrally mediated mechanism of stress‐induced visceral pain in IBS in both clinical and preclinical studies.
Evidence from Clinical Studies
The emergence and rapid advancement of imaging technologies has aided our understanding of the neurocircuitry underlying visceral hypersensitivity in patients with IBS 58. Imaging studies have allowed us to visualize aberrant circuitry within the brain in regions involved in the stress response in patients with IBS. Silverman et al. were one of the first to show altered brain activation patterns using positron emission tomography (PET) in patients with IBS not only in response to actual and simulated rectal distension but also in the anticipation of rectal pain, specifically decreased activation within the anterior cingulate cortex (ACC) of patients with IBS compared to controls 59. A similar study using functional magnetic resonance imaging (fMRI) 60 also showed altered activation patterns within specific brain regions. Again, altered ACC activity was observed in patients with IBS; however, in this study, increased activation within the ACC was observed. It is important to note that these two earlier studies showed opposite effects of rectal distension on ACC activity in patients with IBS, which may in part be explained by methodological procedures. Other studies have highlighted the important role of deactivation of brain areas and circuits in the normal processing of pain signals, with controls having greater frontal deactivation than patients with IBS 61. This was in line with others showing that patients with IBS exhibited significant deactivation within the right insula, the right amygdala, and the right striatum 62. Moreover, patients with IBS exhibit not only altered brain patterns to noxious rectal distension but also subliminal, liminal, and supraliminal distensions 63, 64. Taken together, it is becoming clear that patients with IBS process visceral stimuli differently to that of control subjects at a central level, with IBS patients showing altered activation and deactivation patterns to both nonnoxious and noxious stimuli, which is characteristic of visceral hypersensitivity. Following on from these earlier studies, there has been an abundance of literature to support a role of altered central circuitry in IBS and we are now beginning to build a consensus on the numerous brain regions altered in IBS patients. The ACC is one specific brain region that has consistently been shown to distinguish patients with IBS from controls in imaging studies; however, other regions include the amygdala, insula, prefrontal cortex, thalamus, somatosensory cortex, posterior cingulate, hippocampus, periaqueductal gray, and cerebellum 65, 66, 67, 68, 69. These studies also showed gender‐related differences in brain activation patterns65, 70. The progression and development of imaging tools such as structural MRI and diffusion tensor imaging has allowed us to understand the aberrant connectivity between these brain regions in patients with IBS 71, 72, 73, 74, 75, 76, 77, 78. We are now beginning to appreciate that different networks within the brain are altered in the IBS population, which may in part explain the heterogeneous nature of IBS symptomology 79. These data implicate a “top‐down” mechanism of altered discrete neurocircuits, mainly involving brain regions and nuclei implicated in nociception and affect, in the mediation and presentation of visceral hypersensitivity in IBS.
Taken together, imaging studies have provided strong evidence for altered neurocircuitry in the IBS population, which may underpin centrally mediated visceral pain as well as altered descending modulation of visceral pain in this patient population. The implications of such studies suggest that a cognitive or behavioral intervention may be of therapeutic benefit to patients with IBS 80 as has recently been shown using mindfulness‐based techniques 81, 82, 83, 84 and cognitive behavioral therapy 85, 86, 87. Reverse translation models employing optogenetic technologies will also allow us to further delineate the circuitry underlying visceral hypersensitivity and allow for a more precise understanding of exact brain regions, nuclei, cell types, and mediators that are involved and are amenable to pharmacological manipulation for future drug discovery.
Evidence from Preclinical Studies
The majority of evidence implicating stress as a crucial player in the pathophysiology of visceral pain comes from experimental stress models, which have been developed to target critical periods throughout the life span to assess the vulnerability, potential triggers, and perpetuation influences of stress and the future development of visceral pain 47. Here we will discuss the most commonly used animal models of early‐life stress and adulthood stress‐induced visceral pain.
Early‐life psychological stressors in the form of maternal neglect or abuse (maternal separation, limited nesting, odor attachment learning), or physical stressors in the form of injury (colonic irritation) can enhance the susceptibility of individuals to develop altered visceral pain responses in adulthood, a key symptom of IBS 47. The maternal separation, limited nesting, and odor attachment models are based on the premise that by altering the dam–pup relationship during sensitive HPA axis phases in the first 2 weeks of life, this will have long‐lasting effects on the stress response and subsequent visceral pain sensitivity. Indeed, numerous independent research groups have consistently found heightened visceral sensitivity in these models 13, 48, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100. Moreover, maternal separation has also been associated with altered intestinal permeability and motility 88, 101, 102, 103, 104, 105. Early‐life stress in the form of physical stressors has also been shown to be a valid preclinical model of visceral pain. The neonatal GI tract is highly sensitive to both mechanical and chemical stressors resulting in a proinflammatory phenotype characterized by mucosal inflammation and tissue irritation. Animal models of daily irritation of the neonatal colon by mechanical irritation (CRD) or chemical irritation (mustard oil) have been shown to increase visceral pain behaviors from adolescence to adulthood 106, 107, 108, 109. The mechanisms by which these physical stressors exert their long‐term effects on visceral sensation are varied with contributions from both local effects within the gut and spinal and supraspinal processes seen to induce plasticity and maintenance of the hypersensitive profile 107, 110, 111, 112.
Life‐threatening stressors, psychological stressors (acute and chronic stress), or physical stressors (intestinal infection or inflammation) during adulthood have all been implicated in the development of IBS. Water avoidance stress (WAS) and restraint stress are among the most widely used acute stress paradigms to model features of IBS preclinically. These stressors are based on an aversive surrounding environment. In the case of WAS, animals are placed on a raised platform surrounded by water for 1 h/day, whereas restraint‐stressed animals are placed in a device, which restricts movement for 1–2 h/day. Both of these paradigms can be performed in an acute or repetitive manner. In recent studies, WAS has been described as a form of psychological stressor to assess modulation of visceral pain 46, 51, 92, 113, 114, 115; however, earlier work showed that WAS also leads to stress‐related alterations in gut motor function 116, 117. Restraint stress for 2 h has also been shown to induce an immediate visceral hypersensitivity in male 118 and female Wistar rats 97. Convergent reports suggest that daily stress predicts the intensity and severity of visceral pain 7, 8, 119, 120, 121. In rat models, data show that a 1 h daily WAS for 7/10 consecutive days induced visceral hypersensitivity in male rats 113, 114, 122. In mice, the data are inconsistent, showing visceral hyperalgesia 46, visceral analgesia 46 or to have no effect on visceral sensitivity 123 following WAS. Recently, a clinically relevant model of IBS has been described whereby animals undergo stress in the form of forced swim stress for 3 days as well as estradiol treatment and have existing chronic somatic pain (craniofacial muscle injury). This model displayed chronic visceral hypersensitivity that persisted for months and also exhibited other key features of IBS, specifically central sensitization 124. Moreover, estrogen‐dependent visceral hypersensitivity has also been developed as an animal model of visceral pain with clinical relevance due to the female preponderance seen in patients with IBS 125. Other models such as the chronic psychosocial stress paradigm model the unpredictable nature of life's stressors with unpredictable and randomized sessions of social defeat and cage overcrowding for 19 days. This model has been shown to induce a heightened response to CRD 11, 14, 126 and anxiety‐ and depression‐related behaviors 127. As a model of corticosterone‐induced pain targeting only the amygdala, implantation of corticosterone micropellets on the dorsal surface of the central nucleus of the amygdala has also been shown to increase colonic sensitivity to CRD 128. Although patients with IBS report that psychological stress is a key factor in the onset and exacerbation of symptoms, a significant proportion of IBS cases occur after an illness, particularly an infection of the GI tract. A transient Trichinella spiralis infection was shown to induce sustained visceral hypersensitivity in a mouse model 129, 130. Moreover, similar findings were reported in a rat model of Nippostrongylus brasiliensis infection 131. Although the vast majority of human postinflammatory hypersensitivity symptoms are observed after bacterial infection (Campylobacter, Shigella, Salmonella, or Escherichia coli infections), there has been limited animal models of postinfectious visceral hypersensitivity 132, 133. Inflammation is one of the leading causes/mechanisms thought to underpin IBS and its associated symptomatology 134, 135, 136, 137. In animal models, acetic acid 138, mustard oil 139, 140, zymosan 141, 142, trinitrobenzenesulfonic acid (TNBS) 143, 144, and dextran sulfate sodium 145 evoke visceral hypersensitivity associated with colonic inflammation.
Central Mechanisms of Stress‐Induced Visceral Pain
Numerous mediators involved in the HPA axis response to stress have been implicated in the pathophysiology of IBS. Most notably, the role of CRH has been extensively investigated in preclinical studies 101, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157. Although we acknowledge that CRH has many peripheral functions 158, here we will briefly focus on the centrally mediated effects of CRH. Numerous reviews discuss this topic in more detail 156, 159. CRH is a 41‐amino acid peptide with two endogenous G‐protein‐coupled receptors, CRH1 and CRH2 160. The receptors have a common signal transduction pathway coupled to Gs‐adenylate cyclase; however, they have very different effects depending on localization and cell type 161, 162. One of the earliest demonstrations that CRH was involved in stress‐related visceral pain was shown by Gué et al. 118 where intracerebroventricular administration of CRH recapitulated stress‐induced visceral hypersensitivity in a similar manner to that induced by restraint stress. Moreover, they also showed that antagonism of CRH receptors using α‐helical CRF9‐41 reversed both the CRH‐ and the stress‐induced visceral pain 118. There have been limited studies since then to specifically elucidate the central role of CRH and its receptors on visceral hypersensitivity with many studies showing changes in expression but lack pharmacological interventions 163, 164, 165, 166, 167, 168. We have shown that stress‐induced visceral pain is associated with increased CRH expression in the central nucleus of the amygdala (CeA) and that oligonucleotide knockdown of CRH within the CeA reverses corticosterone‐ and stress‐induced visceral hypersensitivity 169. Moreover, we have shown in a model of high anxiety that CP 376395, a specific CRH1 antagonist administered to the CeA, reversed visceral hypersensitivity 170. Others have also implicated the CeA as a critical nucleus involved in the mediation of CRH effects on visceral sensitivity. Su et al. demonstrated that direct CRH administration to the CeA increased visceral pain, while CP 376395 attenuated this effect 171. Furthermore, intrahippocampal administration of CRF9‐41 or JTC‐017, a specific CRH1 antagonist, attenuated visceral pain 172. There has been an abundance of studies showing peripheral administration of CRH1 antagonists, which cross the blood–brain barrier, have positive effects in animal models of visceral pain 92, 146, 148, 155; however, to date, no CRH1 antagonist has shown evidence of efficacy in patients with IBS, and further research is required to understand the relationship of CRH and its receptors on visceral pain.
The Gut Microbiota
The gut microflora has recently emerged as one of the most fascinating entities in modern biomedical research. The gut microbiome has been implicated in a whole host of physiological functions from energy metabolism to psychiatric well‐being 173, 174, 175. The abundance of microbiota residing in the human intestine is estimated as 1014 microorganisms, which amounts to three times the number of human cells in the body. The bulk of the intestinal microbiota is of bacterial origin that varies in stability, diversity, and number throughout development, from birth to old age 176, 177, 178. Technological advances in the methods used to identify and quantify the gut microbiome have allowed us to better understand its complexity, not only population complexity but also functional complexity. Sequencing‐based approaches have rapidly developed and are reviewed in many recent publications 179, 180, 181, 182. The human GI tract is dominated by two phyla: Firmicutes and Bacteroidetes, together with members of Actinobacteria, Verrucomicrobia, Proteobacteria, Fusobacteria, and Cyanobacteria phyla 183. The relative abundance of microbial populations stabilizes after the first 3 years of life 184 and appears to be relatively stable throughout adult life, but can be altered during disease states. Specifically, disorders directly affecting the GI tract such as inflammatory bowel disease 185 and celiac disease 186 have been shown to exhibit microbial dysbiosis. Moreover, dysbiosis has also been implicated in other nonintestinal disorders such as autoimmune diseases 187, allergy, asthma, metabolic syndrome, cardiovascular disease, and obesity 188. Although there is a general consensus that an overall decrease in the diversity of the microbiota populations present within the GI tract is associated with disease states, as well as reduced Lactobacillus and Bifidobacterium species, no specific dysbiotic signature has emerged across studies.
Stress and the Gut Microbiota
The origin and development of a “healthy” gut microbiota starts in early life, which is also designated as an important neurodevelopmental time window. This provides an opportunity for the colonizing microbiota to influence immature systems such as the pain pathways in the CNS and make a permanent impact. This is abetted by the bidirectional communication provided by the microbiota–gut–brain axis 189, 190. During early life, this axis is also developing and is in itself open to modification by the gut microbiota, which has been shown in several studies 191, 192.
Over 40 years ago, Tannock and Savage demonstrated that both environmental stress and dietary stress were capable of markedly altering the gut microbiota in mice, affecting factors that regulate the localization and population levels of microorganisms along the GI tract 193, possibly allowing for the establishment of pathogenic bacterial species 193, 194. Studies have shown that both prenatal and postnatal stress after birth can impact on microbial colonization 195. Furthermore, appropriate bacterial colonization postnatally impacts on pain pathways, as germ‐free mice display impaired ability to respond to inflammatory pain 196, and we have shown that treatment with antibiotics in early life is associated with visceral hypersensitivity 191. Also, adult mice exposed to a social disruption stressor showed an altered gut microbiota as well as increased circulating levels of cytokines 197. In particular, this stress led to a decrease in L. reuteri, an immunomodulatory species of bacteria. Social stress increases the risk of inflammation‐related diseases, promoting proinflammatory gene expression and monocyte differentiation 190, 198. Therefore, inflammatory alterations leading to an altered gut microbiota can enhance the ability of enteric pathogens to colonize the intestine 199. It has also been shown that both acute stress and repeated stress affect levels of intestinal secretory IgA, impacting intestinal homeostasis, inflammatory response, and possibly dysbiosis 200.
Stress can affect the gut microbiota not only through the immune system but also leads to changes in catecholamine levels, which also have a significant impact on the gut microbiota 201. Moreover, stress affects recovery from bacterial infections due to the fact that gut bacteria respond to neurotransmitters and other stress‐related mediators 201. Animal models of IBS have shown altered gut microbial populations 90, 202, 203, and recovery of the IBS‐like symptoms has occurred upon probiotic administration 203, 204, 205. Recently, it has been shown that the gut microbiota is necessary for the expression of the anxiety‐like and depressive‐like behaviors induced by maternal separation as germ‐free mice separated from their mothers in early life did not show the typical phenotype induced by this early‐life stress 206. Maternal separation stress also appears to alter the gut environment, which can potentially lead to changes in the bacterial population 207.
Hence, it is possible that stress changes the internal environment of the GI tract through immune, neurochemical, and physical mechanisms to make it a less habitable space for certain bacteria, yet leads to the enhancement of more pathological species. This can potentially increase pain and pain signaling mechanisms from the GI tract contributing to visceral hypersensitivity.
The Gut Microbiota and Visceral Pain
Evidence from Clinical Studies
In recent years, the role of the gut microbiota in the pathophysiology of IBS has been investigated with numerous independent research groups showing divergent gut microbiota populations in IBS patient cohorts, when compared to healthy controls 208, 209, 210, 211, 212, 213, 214. This topic has been the focus of many recent reviews 4, 10, 215, 216, 217. Patients with IBS were shown to have reduced Bacteroides and Parabacteroides sp. when compared to healthy volunteers 218. Other groups have also shown similar findings with decreased abundance of Bacteroides/Prevotella group and Veillonella genus and increased Lactobacillus, Bacillus, Bifidobacteria, Clostridium, and Eubacterium rectale 219. However, conflicting results have emerged with Bacteroidetes phylum significantly increased in patients with IBS 220, 221. Moreover, others have demonstrated that subtypes of IBS cluster by microbiota composition revealing certain subgroups of patients with IBS display normal‐like microbiota composition compared with healthy controls, while others were characterized by an increase in Firmicutes‐associated taxa and a depletion of Bacteroidetes‐related taxa 208.
Recent evidence suggests that probiotic interventions appear to be beneficial for patients with FGIDs 222, 223, 224, further implicating a role of the gut microbiota in the pathophysiology of FGIDs. Symprove, a probiotic containing Lactobacillus rhamnosus NCIMB 30174, L. plantarum NCIMB 30173, L. acidophilus NCIMB 30175, and Enterococcus faecium NCIMB 30176, was shown to significantly improve overall symptom severity in patients with IBS 225. Moreover, Bifidobacterium bifidum MIMBb75 was shown to alleviate global IBS symptomology, as measured on the Likert scale, and also significantly improve symptoms such as pain/discomfort, distension/bloating, urgency, and digestive disorder. In addition, probiotic intervention also improved the quality of life of patients with IBS 226. Furthermore, another multispecies probiotic cocktail containing Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus also showed positive effects over placebo in terms of relief from IBS symptoms 224. More recently, studies are suggesting that probiotic treatment itself does not alter the gut microbiota populations, indicating a more complex relationship between the microbiota and IBS pathophysiology 227, 228, 229. Moreover, there is now increasing evidence that alterations in the gut microbiota can modulate both peripheral and central nervous systems functions, thus altering neurochemistry and subsequently behavior 230, 231, 232. This is of particular relevance to visceral pain as both peripheral sensitization and central sensitization are thought to play significant roles in the onset and development of visceral hypersensitivity 233.
Evidence from Preclinical Studies
Manipulation of the gut microbiota through the use of probiotic and prebiotic treatments has shown that visceral hypersensitivity can be reversed in preclinical models 222. A mixture of 8 probiotic bacterial strains (VSL#3) was shown to have protective effects against development of visceral hypersensitivity driven by neonatal maternal separation 234. Moreover, the same cocktail of probiotics was shown to prevent visceral hypersensitivity induced by inflammation via intracolonic instillation of 4% acetic acid when given prophylactically 235. Bifidobacterium species, particularly Bifidobacterium infantis 35624, has been shown to be particularly effective at ameliorating visceral hyperalgesia in both stress‐induced visceral hypersensitivity and colitis 236, 237, 238. Lactobacillus species have also displayed efficacy in visceral pain models 89, 239, 240, 241. Indeed, Lactobacillus rhamnosus CNCM I‐3690 was shown to exhibit protective effects on intestinal barrier function in a mouse model of increased colonic permeability by restoring barrier integrity and increasing the levels of tight junction proteins, occludin, and E‐cadherin 242. The modulation of the intestinal barrier by the gut microbiota has been the focus of recent reviews 243, 244. Furthermore, antibiotic‐induced visceral hypersensitivity again underpins a role of the gut microbiota in the pathophysiology of visceral pain, which appears to be dependent on the time of exposure 191, 239. When animals are exposed to antibiotic treatment in early life, they subsequently develop visceral hypersensitivity in adulthood 191. Further evidence has also shown that antibiotic treatment in adulthood attenuated visceral pain‐related responses elicited by intraperitoneal acetic acid or intracolonic capsaicin 245, or indeed increased visceral sensitivity to CRD 239. Interestingly, rifaximin, a semisynthetic, nonabsorbable antibiotic, has also shown positive effects in the treatment of IBS 246, 247, 248, 249, 250, 251, 252, 253, 254 and received FDA approval in early June 2015 for the treatment of diarrhea predominant IBS. Finally, the concept of fecal microbiota transplantation as a potential treatment for FGIDs has recently been the topic of numerous reviews 255, 256, 257. Preclinical evidence has shown that visceral hypersensitivity could be transferred to rats by transplantation with IBS fecal microbiota 258. These findings add to the growing literature that microbiota dysfunction may be a key player in the pathophysiology of IBS and may lead to future novel therapeutic interventions.
Peripheral Mechanisms of Stress‐Induced Visceral Pain
The clinical literature suggests that altered microbial populations are evident in patients with IBS; however, the implications of such changes remain to be elucidated. The metabolic profile of the gut microbiota is also altered in patients with IBS 213, 259, 260, 261, 262, 263, 264, 265, 266, which may in part explain changes in symptomology. Numerous independent groups have shown changes in the metabolites produced by the gut microbiota, including bile acids 266, organic acids such as acetic acid and propionic acid 213, volatile organic metabolites 264, fecal proteases 263, formate, glucose, lactate, pyruvate 260, amino acids (alanine, pyroglutamic acid 262, tyrosine, lysine, leucine 260), phenols (hydroxyphenyl acetate, hydroxyphenyl propionate 262), polyunsaturated fatty acids (PUFAs) 267, and short‐chain fatty acids (SCFAs) 268. These metabolites themselves can act as signaling molecules to exert effects locally within the gut but also have the potential to elicit effects distant to the site of production. The gut microbiota are also involved in the production of a range of neuroactive compounds including neurotransmitters: gamma‐aminobutyric acid (GABA), serotonin, norepinephrine, and dopamine.
To date, there have been only a limited number of studies investigating the direct interactions of the gut microbiota and its metabolites on pain and nociceptive processes. Formyl peptides have been show to directly stimulate primary afferent nerves 269, while other bacterial products such as lipopolysaccharide (LPS) can directly activate colonic DRG neurons 270. The mechanism of therapeutic effects of probiotic interventions has also been investigated with Lactobacillus reuteri, demonstrating inhibitory effects on lumbar DRG neurons 240. In a recent study by Cenac et al. 267, the authors investigated alterations in PUFA content in colonic biopsies from patients with IBS. PUFAs are known to be endogenous agonists of transient receptor potential (TRP) channels, which are key in nociceptive signaling. TRPV4 in particular is involved in numerous processes associated with visceral hypersensitivity, including protease‐, serotonin‐, and histamine‐induced visceral pain 271, 272. In this study, they show increased levels of 5,6‐EET, a TRPV4 agonist, in IBS colonic biopsies, which correlated with increased pain scores 267. They also show that this specific PUFA activates mouse sensory neurons in vitro and that 35% of human DRG neurons express TRPV4, implicating it as a critical channel in the mediation of visceral pain.
Proteases produced by the gut microbiota and activated mast cells have been implicated in the pathophysiology of visceral hypersensitivity 273, 274, 275, 276, 277, 278. Proteases exert their effects through proteinase‐activated receptors (PARs), which are found on both enteric neurons and extrinsic nerves that innervate the gut and have been implicated in the mediation of pain 279. Despite this, the role of mast cells and their mediators, histamine, tryptase, and chymase, to serve as therapeutic targets using mast cell stabilizers remains controversial due to other sites of action outside of the gut 280.
Many patients with IBS report that their diet is a key contributor to their symptoms 281. Specific dietary interventions such as the low fermentable substrate diet (LFSD) and the FODMAP diet, which are known to alter the gut microbiota profile 282, have shown beneficial effects with reduced abdominal pain in patients with IBS both in childhood and adulthood 283, 284, 285, 286. Short‐chain fermentable carbohydrates increase luminal H2 and CH4 production, which increases gas production and bloating in patients with IBS leading to luminal distension and increased small intestinal water volume, which may worsen abdominal pain 287.
Finally, a novel area of speculation is the role of SCFAs in the communication of the gut microbiota with the brain. SCFAs produced in the gut, such as butyrate, are known to have histone deacetlyase (HDAC) inhibitor activity. Thus, epigenetic processes such as histone acetylation may also be altered by the gut microbiota (reviewed in 288). Moreover, there is evidence to suggest that epigenetic changes at the level of the spinal cord are involved in early‐life stress‐induced visceral pain 100, adulthood stress‐induced visceral pain 115, and estrogen‐induced visceral pain 289. Indeed, supraspinal epigenetic mechanisms have also been implicated in stress‐induced visceral hypersensitivity 113, 290.
The Microbiota–Gut–Brain Axis
From the evidence discussed above, it is apparent that IBS is a multifaceted disorder with both central and peripheral factors at play; thus, it is most commonly described as a biopsychosocial disorder of the gut‐brain axis. The gut–brain axis encompasses a number of fundamental elements, including the CNS, the autonomic nervous system (ANS) (sympathetic and parasympathetic), the enteric nervous system (ENS), the neuroendocrine (HPA axis), and neuroimmune systems, and more recently has expanded to include the gut microbiota, which fulfill key roles in bidirectional communication thus leading us to now refer to it as the microbiota–gut–brain axis 15, 17, 291, 292, 293, 294, 295, 296. This axis is pivotal in maintaining homeostasis and is involved in the control of a plethora of physiological functions including motor, sensory, autonomic, and secretory functions of the gastrointestinal tract to regulate an array of processes from energy metabolism to mood regulation 296, 297. The network of communication throughout the axis is facilitated by an extensive neuronal web of afferent fibers projecting from peripheral tissues to higher‐order processing centers in cortical CNS structures and efferent projections from the CNS to the smooth muscle in the intestinal wall 4.
As mentioned previously, the spinal cord is fundamental in mediating sensory signals between peripheral organs and the integrative cortices within the brain. Sensitization of peripheral afferents plays a key role in the peripheral sensitization leading to visceral hypersensitivity in patients with IBS. This occurs as a result of local inflammatory processes as well as alterations in sensory motor gut function 298. Indeed, clinical evidence has shown low‐grade mucosal inflammation 134 and enhanced intestinal permeability 299 in patients with IBS, which may be responsible for the local sensitization and facilitation of visceral pain responses. The process of peripheral sensitization includes a plethora of different receptor types that include the TRPV family, PARs, cholecystokinin receptors, serotonin receptors, cannabinoid receptors, as well as an array of ion channels including ATP‐gated ion channels, voltage‐gated sodium and calcium channels, and acid‐sensing ion channels 298. The ligands for these receptors such as luminal contents, epithelial metabolites, immune mediators, lipids, and gut hormones are found within the GI tract. Binding of these endogenous ligands to their respective receptors leads to the release of neurotransmitters such as acetylcholine, somatostatin, substance P (SP), neurokinin A, and calcitonin gene‐related peptide, and a cascade of events that are all associated with pain signaling and neurogenic inflammation 300.
Moreover, the CNS and GI tract are in constant bidirectional communication through the vagus nerve and its branches. The essential role of the vagus nerve in the microbiota–gut–brain axis signaling has been shown previously 301, 302, in preclinical models whereby vagotomy prevented the anxiolytic effects of probiotic treatment, an effect thought to be mediated by altered GABAergic receptor expression 232, 303, 304.
Gut hormones released from the enteroendocrine cells such as cholecystokinin (CCK), glucagon‐like peptide (GLP), peptide YY (PYY), and serotonin are mediators that sense the local environment in the gut and respond appropriately. They are involved in many functions including digestion and protective processes. Indeed, CCK has been implicated in visceral pain in both preclinical and clinical studies 38, 305, 306, 307. Serotonin is predominantly known for its role in the brain, where it functions as a neurotransmitter; however, approximately 95% of serotonin in the body is contained within the gut, specifically, in the enterochromaffin cells of the mucosa and in the nerve terminals of the ENS neurons 295. Serotonin's peripheral functions involve regulation of GI motility, secretion, and sensory perception 308, 309. Its central functions include the regulation of mood, cognitive functioning, and central processing of sensory signals involved in pain processes 310, 311, 312. Thus, it is apparent that serotonin signaling is a key linker in communication along this axis 313, dysfunction of which may underlie the pathological symptoms present in both GI and mood disorders, and may also explain the high comorbidity of these disorders 3. Moreover, therapeutic compounds that modulate serotonergic neurotransmission, such as tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs), have been shown to be effective in the treatment of not only affective disorders but also GI disorders such as irritable bowel syndrome (IBS) 314, 315, 316.
The immune system plays an important intermediary role in the dynamic equilibrium that exists between the brain and the gut 317. It is well known that the HPA axis, ANS, and ENS all have direct interactions with the immune system 318, 319, 320, 321, 322. The concept of the “leaky gut” again underpins the important bidirectional communication between the periphery and the CNS and the significant role the immune system plays. This phenomenon is thought to underlie disorders of the microbiota–gut–brain axis as well as CNS disorders such as major depressive disorder (MDD) 323 and alcohol addiction 324. It is thought that stress can lead to alterations in epithelial barrier integrity, which can become compromised, thus increasing intestinal permeability and consequently translocation of Gram‐negative bacteria across the mucosal lining. This allows humoral and cellular mediators to directly interact with immune cells and the ENS 325 leading to the activation of an immune response characterized by increased production of inflammatory mediators such as IL‐6 and IFN‐γ 326. Moreover, Toll‐like receptors have been shown to play a key role in IBS pathophysiology both in preclinical 327, 328 and in clinical studies 329. Microglia, the immune cells of the CNS, have also been shown to be altered by the gut microbiota, in particular microglia maturation and function 330.
Taken together, several routes of communication have been proposed to understand the communication between the intestine, including the microbiota, and the brain, some of which have been summarized here. The high comorbidity between gastrointestinal disorders including IBS and stress‐related psychiatric symptoms such as anxiety 331, 332, 333 are further evidence that perturbation of the microbiota–gut–brain axis leads to alterations in the stress response and overall behavior including pain sensitivity 334, 335.
Summary and Future Directions
In this review, we have attempted to highlight for the reader the mechanisms that facilitate both stress and nociception. Furthermore, we have provided evidence from the literature for the microbiota–gut–brain axis and the role that it plays in visceral nociception. An important key message is the need for more research focusing on the overlapping mechanisms linking stress and visceral hypersensitivity with alterations in the gut microbiome (Figure 2). The exact routes by which the microbiota can exert direct effects on visceral pain and vice versa remain to be explored, but potential targets include altered cognitive processes and epigenetic mechanisms 288, 336. We anticipate that future research will likely lead to novel therapeutic compounds targeting the microbiota–gut–brain axis to treat patients with functional bowel disorders such as IBS whose visceral pain is exacerbated during periods of stress. The complex and multilayered communication between the gut microbial population and the CNS has far‐reaching implications not least in the area of visceral pain and comorbid stress disorders. Being able to target one element of the axis in order to alleviate symptoms at both ends is a huge therapeutic leap. Studies on the modulation of the gut microbiota via prebiotic/probiotic treatment have shown a very positive light on the potential for this therapy in comorbid visceral pain and stress disorders 235, 236, 237, 337. Also of potential therapeutic value is the possibility of fecal transplantation. Studies have shown that condition and behavior are transferrable via transplantation of feces 291, 338, 339. Future wide‐scale clinical validation of such interventions in visceral pain disorders is now warranted.
Figure 2.
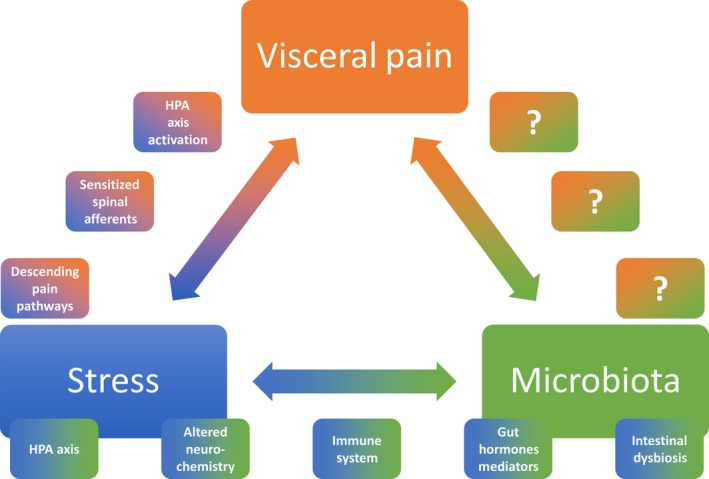
Summary Figure. The mechanisms by which stress can lead to heightened pain perception are varied and primarily occur through 3 distinct routes: (1) hypothalamic–pituitary–adrenal (HPA) axis activation, (2) sensitized spinal afferents, and (3) altered descending pain pathways. Stress and the gut microbiota are also known to interact bidirectionally, with stress causing intestinal dysbiosis, which subsequently alters HPA axis functioning. Many systems and mediators are involved in this complex network including altered neurochemistry of the central, peripheral, and enteric nervous systems, altered immune system functioning, and perturbed local production of gut hormones and mediators. What remains to be fully investigated are the exact pathways by which the microbiota can exert direct effects on visceral pain processes and vice versa.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
The Alimentary Pharmabiotic Centre is a research centre funded by Science Foundation Ireland (SFI) (grant numbers SFI/12/RC/2273, 02/CE/B124, and 07/CE/B1368), through the Irish Government's National Development Plan. TGD also has grant support from the Health Research Board of Ireland (HRA_POR/2011/23, HRA_POR/2012/32, and HRA‐POR‐2014‐647) and European FP7. TGD has been an invited speaker at meetings organized by Servier, Lundbeck, Janssen, and AstraZeneca. JFC has received grant support from the Health Research Board of Ireland (grant number HRA_POR/2012/32), Science Foundation Ireland (Investigator Award grant no. 12/IA/1537), and European Community's Seventh Framework Programme (grant number FP7/2007‐2013 under Grant Agreement No. 278948 (TACTICS‐Translational Adolescent and Childhood Therapeutic Interventions in Compulsive Syndrome)). JFC has been an invited speaker at meetings organized by Mead Johnson and Yakult. BG‐VM acknowledges the generous funding support for her Research Career Scientist and Merit Review Awards from the Department of Veterans Affairs [I01BX002188].
References
- 1. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 2. Lackner JM, Ma CX, Keefer L, et al. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Folks DG. The interface of psychiatry and irritable bowel syndrome. Curr Psychiatry Rep 2004;6:210–215. [DOI] [PubMed] [Google Scholar]
- 4. Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome‐gut‐brain axis disorder? World J Gastroenterol 2014;20:14105–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota–gut–brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol 2014;592:2989–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayer EA, Tillisch K. The brain‐gut axis in abdominal pain syndromes. Annu Rev Med 2011;62:381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lackner JM, Brasel AM, Quigley BM, et al. The ties that bind: perceived social support, stress, and IBS in severely affected patients. Neurogastroenterol Motil 2010;22:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut 1998;43:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Venkova K, Johnson AC, Myers B, Greenwood‐Van Meerveld B. Exposure of the amygdala to elevated levels of corticosterone alters colonic motility in response to acute psychological stress. Neuropharmacology 2010;58:1161–1167. [DOI] [PubMed] [Google Scholar]
- 10. Hyland NP, Quigley EM, Brint E. Microbiota‐host interactions in irritable bowel syndrome: epithelial barrier, immune regulation and brain‐gut interactions. World J Gastroenterol 2014;20:8859–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vicario M, Alonso C, Guilarte M, et al. Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin‐releasing factor receptor type‐1 upregulation in the rat intestine and IBS‐like gut dysfunction. Psychoneuroendocrinology 2012;37:65–77. [DOI] [PubMed] [Google Scholar]
- 12. Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2009;58:196–201. [DOI] [PubMed] [Google Scholar]
- 13. Moloney RD, O'Leary OF, Felice D, Bettler B, Dinan TG, Cryan JF. Early‐life stress induces visceral hypersensitivity in mice. Neurosci Lett 2012;512:99–102. [DOI] [PubMed] [Google Scholar]
- 14. Tramullas M, Dinan TG, Cryan JF. Chronic psychosocial stress induces visceral hyperalgesia in mice. Stress 2012;15:281–292. [DOI] [PubMed] [Google Scholar]
- 15. Cryan JF, Dinan TG. Mind‐altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–712. [DOI] [PubMed] [Google Scholar]
- 16. Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012;10:735–742. [DOI] [PubMed] [Google Scholar]
- 17. Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol 1999;35:146–155. [PubMed] [Google Scholar]
- 18. Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsy Clin Neurosci 1998;10:230–231. [DOI] [PubMed] [Google Scholar]
- 19. Cannon WB. Bodily changes in pain, hunger, fear, and rage; an account of recent researches into the function of emotional excitement. New York, London: D. Appleton and Company, 1915. xiii, 311. [Google Scholar]
- 20. Cannon WB. The wisdom of the body. New York: W.W. Norton & Company, 1932. xv p., 1 l., 19–312. [Google Scholar]
- 21. Smith SM, Vale WW. The role of the hypothalamic‐pituitary‐adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 2006;8:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 1984;5:25–44. [DOI] [PubMed] [Google Scholar]
- 23. Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 1996;17:245–261. [DOI] [PubMed] [Google Scholar]
- 24. Sapolsky RM, McEwen BS, Rainbow TC. Quantitative autoradiography of [3H]corticosterone receptors in rat brain. Brain Res 1983;271:331–334. [DOI] [PubMed] [Google Scholar]
- 25. Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 1985;117:2505–2511. [DOI] [PubMed] [Google Scholar]
- 26. Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo‐pituitary‐adrenocortical axis. Trends Neurosci 1997;20:78–84. [DOI] [PubMed] [Google Scholar]
- 27. Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin‐releasing factor mRNA in the central amygdaloid nucleus and anxiety‐like behavior. Brain Res 2000;861:288–295. [DOI] [PubMed] [Google Scholar]
- 28. Schulkin J, Gold PW, McEwen BS. Induction of corticotropin‐releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology 1998;23:219–243. [DOI] [PubMed] [Google Scholar]
- 29. McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA 2012;109(Suppl 2):17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nutt DJ, Malizia AL. Structural and functional brain changes in posttraumatic stress disorder. J Clin Psychiatry 2004;65(Suppl 1):11–17. [PubMed] [Google Scholar]
- 31. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009;10:434–445. [DOI] [PubMed] [Google Scholar]
- 32. Ulrich‐Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 2009;10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cervero F, Laird JM. Visceral pain. Lancet 1999;353:2145–2148. [DOI] [PubMed] [Google Scholar]
- 34. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377–1390. [DOI] [PubMed] [Google Scholar]
- 35. Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol 2009;194:31–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mayer EA, Raybould HE. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology 1990;99:1688–1704. [DOI] [PubMed] [Google Scholar]
- 37. Randich A, Thurston‐Stanfield C. Vagal Input and Descending Modulation In: Gebhart G, Schmidt R, editors. Encyclopedia of Pain. Berlin Heidelberg: Springer, 2013;4145–4149. [Google Scholar]
- 38. Wang EM, Li WT, Yan XJ, et al. Vagal afferent‐dependent cholecystokinin modulation of visceral pain requires central amygdala NMDA‐NR2B receptors in rats. Neurogastroenterol Motil 2015;27:1333–1343. [DOI] [PubMed] [Google Scholar]
- 39. Yan XJ, Feng CC, Liu Q, et al. Vagal afferents mediate antinociception of estrogen in a rat model of visceral pain: the involvement of intestinal mucosal mast cells and 5‐hydroxytryptamine 3 signaling. J Pain 2014;15:204–217. [DOI] [PubMed] [Google Scholar]
- 40. Chen SL, Wu XY, Cao ZJ, et al. Subdiaphragmatic vagal afferent nerves modulate visceral pain. Am J Physiol Gastrointest Liver Physiol 2008;294:G1441–G1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lowen MB, Mayer E, Tillisch K, et al. Deficient habituation to repeated rectal distensions in irritable bowel syndrome patients with visceral hypersensitivity. Neurogastroenterol Motil 2015;27:646–655. [DOI] [PubMed] [Google Scholar]
- 42. Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995;109:40–52. [DOI] [PubMed] [Google Scholar]
- 43. Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res 1988;450:153–169. [DOI] [PubMed] [Google Scholar]
- 44. Kamp EH, Jones RC 3rd, Tillman SR, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol 2003;284:G434–G444. [DOI] [PubMed] [Google Scholar]
- 45. O'Mahony SM, Tramullas M, Fitzgerald P, Cryan JF. Rodent models of colorectal distension. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al. ]. 2012;Chapter 9:Unit 9 40. [DOI] [PubMed]
- 46. Larauche M, Gourcerol G, Million M, Adelson DW, Tache Y. Repeated psychological stress‐induced alterations of visceral sensitivity and colonic motor functions in mice: influence of surgery and postoperative single housing on visceromotor responses. Stress 2010;13:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Larauche M, Mulak A, Tache Y. Stress‐related alterations of visceral sensation: animal models for irritable bowel syndrome study. J Neurogastroenterol Motil 2011;17:213–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Greenwood‐Van MB, Prusator DK, Johnson AC. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 2015;308:G885–G903. [DOI] [PubMed] [Google Scholar]
- 49. Moloney RD, O'Mahony SM, Dinan TG, Cryan JF. Stress‐induced visceral pain: toward animal models of irritable‐bowel syndrome and associated comorbidities. Front Psychiatry 2015;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen J, Winston JH, Fu Y, et al. Genesis of anxiety, depression, and ongoing abdominal discomfort in ulcerative colitis‐like colon inflammation. Am J Physiol Regul Integr Comp Physiol 2015;308:R18–R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol 2012;233:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Malley D, Quigley EM, Dinan TG, Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav Immun 2011;25:1333–1341. [DOI] [PubMed] [Google Scholar]
- 53. McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med 1993;153:2093–2101. [PubMed] [Google Scholar]
- 54. Scarinci IC, McDonald‐Haile J, Bradley LA, Richter JE. Altered pain perception and psychosocial features among women with gastrointestinal disorders and history of abuse: a preliminary model. Am J Med 1994;97:108–118. [DOI] [PubMed] [Google Scholar]
- 55. Lampe A, Doering S, Rumpold G, et al. Chronic pain syndromes and their relation to childhood abuse and stressful life events. J Psychosom Res 2003;54:361–367. [DOI] [PubMed] [Google Scholar]
- 56. Maizels M, Aurora S, Heinricher M. Beyond neurovascular: migraine as a dysfunctional neurolimbic pain network. Headache 2012;52:1553–1565. [DOI] [PubMed] [Google Scholar]
- 57. Racine M, Tousignant‐Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and pain perception ‐ part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 2012;153:619–635. [DOI] [PubMed] [Google Scholar]
- 58. Al OY, Aziz Q. Functional brain imaging in gastroenterology: to new beginnings. Nat Rev Gastroenterol Hepatol 2014;11:565–576. [DOI] [PubMed] [Google Scholar]
- 59. Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology 1997;112:64–72. [DOI] [PubMed] [Google Scholar]
- 60. Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology 2000;118:842–848. [DOI] [PubMed] [Google Scholar]
- 61. Bernstein CN, Frankenstein UN, Rawsthorne P, Pitz M, Summers R, McIntyre MC. Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. Am J Gastroenterol 2002;97:319–327. [DOI] [PubMed] [Google Scholar]
- 62. Bonaz B, Baciu M, Papillon E, et al. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol 2002;97:654–661. [DOI] [PubMed] [Google Scholar]
- 63. Sidhu H, Kern M, Shaker R. Absence of increasing cortical fMRI activity volume in response to increasing visceral stimulation in IBS patients. Am J Physiol Gastrointest Liver Physiol 2004;287:G425–G435. [DOI] [PubMed] [Google Scholar]
- 64. Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology 2006;130:26–33. [DOI] [PubMed] [Google Scholar]
- 65. Berman S, Munakata J, Naliboff BD, et al. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain 2000;4:157–172. [DOI] [PubMed] [Google Scholar]
- 66. Verne GN, Himes NC, Robinson ME, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain 2003;103:99–110. [DOI] [PubMed] [Google Scholar]
- 67. Yuan YZ, Tao RJ, Xu B, et al. Functional brain imaging in irritable bowel syndrome with rectal balloon‐distention by using fMRI. World J Gastroenterol 2003;9:1356–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ringel Y, Drossman DA, Turkington TG, et al. Regional brain activation in response to rectal distension in patients with irritable bowel syndrome and the effect of a history of abuse. Dig Dis Sci 2003;48:1774–1781. [DOI] [PubMed] [Google Scholar]
- 69. Wilder‐Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004;53:1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Naliboff BD, Berman S, Chang L, et al. Sex‐related differences in IBS patients: central processing of visceral stimuli. Gastroenterology 2003;124:1738–1747. [DOI] [PubMed] [Google Scholar]
- 71. Labus JS, Van Horn JD, Gupta A, et al. Multivariate morphological brain signatures predict patients with chronic abdominal pain from healthy control subjects. Pain 2015;156:1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Irimia A, Labus JS, Torgerson CM, Van Horn JD, Mayer EA. Altered viscerotopic cortical innervation in patients with irritable bowel syndrome. Neurogastroenterol Motil 2015;27:1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gupta A, Rapkin AJ, Gill Z, et al. Disease‐related differences in resting‐state networks: a comparison between localized provoked vulvodynia, irritable bowel syndrome, and healthy control subjects. Pain 2015;156:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urol 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Labus JS, Dinov ID, Jiang Z, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain 2014;155:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ellingson BM, Mayer E, Harris RJ, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain 2013;154:1528–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tillisch K, Mayer EA, Labus JS. Quantitative meta‐analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011;140:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lelic D, Nissen TD, Brock C, Aziz Q, Drewes AM. Rapid balloon distension as a tool to study cortical processing of visceral sensations and pain. Neurogastroenterol Motil 2015;27:832–840. [DOI] [PubMed] [Google Scholar]
- 79. Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 2015;12:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Keefer L, Mandal S. The potential role of behavioral therapies in the management of centrally mediated abdominal pain. Neurogastroenterol Motil 2015;27:313–323. [DOI] [PubMed] [Google Scholar]
- 81. Zernicke KA, Campbell TS, Blustein PK, et al. Mindfulness‐based stress reduction for the treatment of irritable bowel syndrome symptoms: a randomized wait‐list controlled trial. Int J Behav Med 2013;20:385–396. [DOI] [PubMed] [Google Scholar]
- 82. Ljotsson B, Andreewitch S, Hedman E, Ruck C, Andersson G, Lindefors N. Exposure and mindfulness based therapy for irritable bowel syndrome–an open pilot study. J Behav Ther Exp Psychiatry 2010;41:185–190. [DOI] [PubMed] [Google Scholar]
- 83. Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas MJ, Whitehead WE. Therapeutic mechanisms of a mindfulness‐based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med 2012;35:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ljotsson B, Falk L, Vesterlund AW, et al. Internet‐delivered exposure and mindfulness based therapy for irritable bowel syndrome–a randomized controlled trial. Behav Res Ther 2010;48:531–539. [DOI] [PubMed] [Google Scholar]
- 85. Lackner JM, Keefer L, Jaccard J, et al. The Irritable Bowel Syndrome Outcome Study (IBSOS): rationale and design of a randomized, placebo‐controlled trial with 12 month follow up of self‐ versus clinician‐administered CBT for moderate to severe irritable bowel syndrome. Contemp Clin Trials 2012;33:1293–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tang QL, Lin GY, Zhang MQ. Cognitive‐behavioral therapy for the management of irritable bowel syndrome. World J Gastroenterol 2013;19:8605–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li L, Xiong L, Zhang S, Yu Q, Chen M. Cognitive‐behavioral therapy for irritable bowel syndrome: a meta‐analysis. J Psychosom Res 2014;77:1–12. [DOI] [PubMed] [Google Scholar]
- 88. Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stress‐induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol 2002;282:G307–G316. [DOI] [PubMed] [Google Scholar]
- 89. Eutamene H, Lamine F, Chabo C, et al. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress‐induced gut permeability and sensitivity increase in rats. J Nutr 2007;137:1901–1907. [DOI] [PubMed] [Google Scholar]
- 90. O'Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 2009;65:263–267. [DOI] [PubMed] [Google Scholar]
- 91. Ren TH, Wu J, Yew D, et al. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2007;292:G849–G856. [DOI] [PubMed] [Google Scholar]
- 92. Schwetz I, Bradesi S, McRoberts JA, et al. Delayed stress‐induced colonic hypersensitivity in male Wistar rats: role of neurokinin‐1 and corticotropin‐releasing factor‐1 receptors. Am J Physiol Gastrointest Liver Physiol 2004;286:G683–G691. [DOI] [PubMed] [Google Scholar]
- 93. Wouters MM, Van WS, Casteels C, et al. Altered brain activation to colorectal distention in visceral hypersensitive maternal‐separated rats. Neurogastroenterol Motil 2012;24:678–685, e297. [DOI] [PubMed] [Google Scholar]
- 94. Barreau F, Ferrier L, Fioramonti J, Bueno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr Res 2007;62:240–245. [DOI] [PubMed] [Google Scholar]
- 95. O'Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain‐gut axis dysfunction. Psychopharmacology 2011;214:71–88. [DOI] [PubMed] [Google Scholar]
- 96. Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin‐releasing factor (CRF) mRNA, median eminence CRF content and stress‐induced release in adult rats. Brain Res Mol Brain Res 1993;18:195–200. [DOI] [PubMed] [Google Scholar]
- 97. Rosztoczy A, Fioramonti J, Jarmay K, Barreau F, Wittmann T, Bueno L. Influence of sex and experimental protocol on the effect of maternal deprivation on rectal sensitivity to distension in the adult rat. Neurogastroenterol Motil 2003;15:679–686. [DOI] [PubMed] [Google Scholar]
- 98. Chaloner A, Greenwood‐Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J Pain 2013;14:270–280. [DOI] [PubMed] [Google Scholar]
- 99. Prusator DK, Greenwood‐Van Meerveld B. Gender specific effects of neonatal limited nesting on viscerosomatic sensitivity and anxiety‐like behavior in adult rats. Neurogastroenterol Motil 2015;27:72–81. [DOI] [PubMed] [Google Scholar]
- 100. Moloney RD, Stilling RM, Dinan TG, Cryan JF. Early‐life stress‐induced visceral hypersensitivity and anxiety behavior is reversed by histone deacetylase inhibition. Neurogastroenterol Motil 2015;27:1831–1836. [DOI] [PubMed] [Google Scholar]
- 101. Schwetz I, McRoberts JA, Coutinho SV, et al. Corticotropin‐releasing factor receptor 1 mediates acute and delayed stress‐induced visceral hyperalgesia in maternally separated Long‐Evans rats. Am J Physiol Gastrointest Liver Physiol 2005;289:G704–G712. [DOI] [PubMed] [Google Scholar]
- 102. Zhang M, Leung FP, Huang Y, Bian ZX. Increased colonic motility in a rat model of irritable bowel syndrome is associated with up‐regulation of L‐type calcium channels in colonic smooth muscle cells. Neurogastroenterol Motil 2010;22:e162–e170. [DOI] [PubMed] [Google Scholar]
- 103. Oines E, Murison R, Mrdalj J, Gronli J, Milde AM. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol Behav 2012;105:1058–1066. [DOI] [PubMed] [Google Scholar]
- 104. Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res 2006;59:83–88. [DOI] [PubMed] [Google Scholar]
- 105. Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol 2007;293:G198–G203. [DOI] [PubMed] [Google Scholar]
- 106. Al‐Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 2000;119:1276–1285. [DOI] [PubMed] [Google Scholar]
- 107. Lin C, Al‐Chaer ED. Long‐term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res 2003;971:73–82. [DOI] [PubMed] [Google Scholar]
- 108. Wang J, Gu C, Al‐Chaer ED. Altered behavior and digestive outcomes in adult male rats primed with minimal colon pain as neonates. Behav Brain Funct 2008;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen ZY, Zhang XW, Yu L, et al. Spinal toll‐like receptor 4‐mediated signalling pathway contributes to visceral hypersensitivity induced by neonatal colonic irritation in rats. Eur J Pain 2015;19:176–186. [DOI] [PubMed] [Google Scholar]
- 110. Saab CY, Park YC, Al‐Chaer ED. Thalamic modulation of visceral nociceptive processing in adult rats with neonatal colon irritation. Brain Res 2004;1008:186–192. [DOI] [PubMed] [Google Scholar]
- 111. Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain 2010;151:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schwaller F, Fitzgerald M. The consequences of pain in early life: injury‐induced plasticity in developing pain pathways. Eur J Neuorsci 2014;39:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tran L, Chaloner A, Sawalha AH, Greenwood Van‐Meerveld B. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology 2013;38:898–906. [DOI] [PubMed] [Google Scholar]
- 114. Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 2009;58:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hong S, Zheng G, Wiley JW. Epigenetic regulation of genes that modulate chronic stress‐induced visceral pain in the peripheral nervous system. Gastroenterology 2015;148:148–157, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bonaz B, Tache Y. Water‐avoidance stress‐induced c‐fos expression in the rat brain and stimulation of fecal output: role of corticotropin‐releasing factor. Brain Res 1994;641:21–28. [DOI] [PubMed] [Google Scholar]
- 117. Enck P, Merlin V, Erckenbrecht JF, Wienbeck M. Stress effects on gastrointestinal transit in the rat. Gut 1989;30:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gue M, Del Rio‐Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Stress‐induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil 1997;9:271–279. [DOI] [PubMed] [Google Scholar]
- 119. Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther 2004;20(Suppl 7):31–39. [DOI] [PubMed] [Google Scholar]
- 120. Choung RS, Locke GR 3rd, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol 2009;104:1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro‐immune mechanisms. Brain Behav Immun 2011;25:386–394. [DOI] [PubMed] [Google Scholar]
- 122. Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 2005;289:G42–G53. [DOI] [PubMed] [Google Scholar]
- 123. Larsson MH, Miketa A, Martinez V. Lack of interaction between psychological stress and DSS‐induced colitis affecting colonic sensitivity during colorectal distension in mice. Stress 2009;12:434–444. [DOI] [PubMed] [Google Scholar]
- 124. Traub RJ, Cao DY, Karpowicz J, et al. A clinically relevant animal model of temporomandibular disorder and irritable bowel syndrome comorbidity. J Pain 2014;15:956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci 2003;23:3908–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS‐induced colitis and impairs regeneration. Endocrinology 2006;147:4968–4976. [DOI] [PubMed] [Google Scholar]
- 127. Finger BC, Dinan TG, Cryan JF. High‐fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience 2011;192:351–360. [DOI] [PubMed] [Google Scholar]
- 128. Myers B, Greenwood‐Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety‐like behavior and pain sensitivity. Behav Brain Res 2010;214:465–469. [DOI] [PubMed] [Google Scholar]
- 129. Bercik P, Wang L, Verdu EF, et al. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology 2004;127:179–187. [DOI] [PubMed] [Google Scholar]
- 130. Long Y, Liu Y, Tong J, Qian W, Hou X. Effectiveness of trimebutine maleate on modulating intestinal hypercontractility in a mouse model of postinfectious irritable bowel syndrome. Eur J Pharmacol 2010;636:159–165. [DOI] [PubMed] [Google Scholar]
- 131. McLean PG, Picard C, Garcia‐Villar R, More J, Fioramonti J, Bueno L. Effects of nematode infection on sensitivity to intestinal distension: role of tachykinin NK2 receptors. Eur J Pharmacol 1997;337:279–282. [DOI] [PubMed] [Google Scholar]
- 132. Coelho AM, Fioramonti J, Bueno L. Systemic lipopolysaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and vagus nerve. Am J Physiol Gastrointest Liver Physiol 2000;279:G781–G790. [DOI] [PubMed] [Google Scholar]
- 133. Sm OM, Clarke G, McKernan DP, Bravo JA, Dinan TG, Cryan JF. Differential visceral nociceptive, behavioural and neurochemical responses to an immune challenge in the stress‐sensitive Wistar Kyoto rat strain. Behav Brain Res 2013;253:310–317. [DOI] [PubMed] [Google Scholar]
- 134. Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 2010;7:163–173. [DOI] [PubMed] [Google Scholar]
- 135. Akiho H, Ihara E, Nakamura K. Low‐grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol 2010;1:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut 2002;51(Suppl 1):i41–i44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Collins SM, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut 2001;49:743–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Burton MB, Gebhart GF. Effects of intracolonic acetic acid on responses to colorectal distension in the rat. Brain Res 1995;672:77–82. [DOI] [PubMed] [Google Scholar]
- 139. Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain 2005;117:433–442. [DOI] [PubMed] [Google Scholar]
- 140. Palecek J, Willis WD. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rats. Pain 2003;104:501–507. [DOI] [PubMed] [Google Scholar]
- 141. Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and non‐NMDA receptors. Brain Res 1996;736:7–15. [DOI] [PubMed] [Google Scholar]
- 142. Traub RJ, Murphy A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain 2002;95:93–102. [DOI] [PubMed] [Google Scholar]
- 143. Adam B, Liebregts T, Gschossmann JM, et al. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain 2006;123:179–186. [DOI] [PubMed] [Google Scholar]
- 144. Gschossmann JM, Liebregts T, Adam B, et al. Long‐term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig Dis Sci 2004;49:96–101. [DOI] [PubMed] [Google Scholar]
- 145. Verma‐Gandhu M, Verdu EF, Bercik P, et al. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut 2007;56:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Buckley MM, O'Halloran KD, Rae MG, Dinan TG, O'Malley D. Modulation of enteric neurons by interleukin‐6 and corticotropin‐releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol 2014;592:5235–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Greenwood‐Van MB, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin‐releasing factor 1 receptor‐mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol Motil 2005;17:415–422. [DOI] [PubMed] [Google Scholar]
- 148. Trimble N, Johnson AC, Foster A, Greenwood‐van Meerveld B. Corticotropin‐releasing factor receptor 1‐deficient mice show decreased anxiety and colonic sensitivity. Neurogastroenterol Motil 2007;19:754–760. [DOI] [PubMed] [Google Scholar]
- 149. Tran L, Schulkin J, Greenwood‐Van MB. Importance of CRF receptor‐mediated mechanisms of the bed nucleus of the stria terminalis in the processing of anxiety and pain. Neuropsychopharmacology 2014;39:2633–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tache Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress‐related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol 2004;141:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Tache Y, Martinez V, Million M, Maillot C. Role of corticotropin releasing factor receptor subtype 1 in stress‐related functional colonic alterations: implications in irritable bowel syndrome. Eur J Surg Suppl 2002;587:16–22. [PubMed] [Google Scholar]
- 152. Million M, Wang L, Wang Y, et al. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut 2006;55:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tache Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin‐releasing factor pathways in stress‐related alterations of colonic motor function and viscerosensibility in female rodents. Gend Med 2005;2:146–154. [DOI] [PubMed] [Google Scholar]
- 154. Arvidsson S, Larsson M, Larsson H, Lindstrom E, Martinez V. Assessment of visceral pain‐related pseudo‐affective responses to colorectal distension in mice by intracolonic manometric recordings. J Pain 2006;7:108–118. [DOI] [PubMed] [Google Scholar]
- 155. Larauche M, Gourcerol G, Wang L, et al. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol 2009;297:G215–G227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tache Y. Corticotrophin‐releasing factor 1 activation in the central amygdale and visceral hyperalgesia. Neurogastroenterol Motil 2015;27:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Myers DA, Gibson M, Schulkin J, Greenwood Van‐Meerveld B. Corticosterone implants to the amygdala and type 1 CRH receptor regulation: effects on behavior and colonic sensitivity. Behav Brain Res 2005;161:39–44. [DOI] [PubMed] [Google Scholar]
- 158. Larauche M. Novel insights in the role of peripheral corticotropin‐releasing factor and mast cells in stress‐induced visceral hypersensitivity. Neurogastroenterol Motil 2012;24:201–205. [DOI] [PubMed] [Google Scholar]
- 159. Nozu T, Okumura T. Corticotropin‐releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J Gastroenterol 2015;50:819–830. [DOI] [PubMed] [Google Scholar]
- 160. Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci 1999;885:312–328. [DOI] [PubMed] [Google Scholar]
- 161. Grammatopoulos DK. Insights into mechanisms of corticotropin‐releasing hormone receptor signal transduction. Br J Pharmacol 2012;166:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Nijsen M, Ongenae N, Meulemans A, Coulie B. Divergent role for CRF1 and CRF2 receptors in the modulation of visceral pain. Neurogastroenterol Motil 2005;17:423–432. [DOI] [PubMed] [Google Scholar]
- 163. Sinniger V, Porcher C, Mouchet P, Juhem A, Bonaz B. c‐fos and CRF receptor gene transcription in the brain of acetic acid‐induced somato‐visceral pain in rats. Pain 2004;110:738–750. [DOI] [PubMed] [Google Scholar]
- 164. Wang L, Martinez V, Larauche M, Tache Y. Proximal colon distension induces Fos expression in oxytocin‐, vasopressin‐, CRF‐ and catecholamines‐containing neurons in rat brain. Brain Res 2009;1247:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tjong YW, Ip SP, Lao L, et al. Neonatal maternal separation elevates thalamic corticotrophin releasing factor type 1 receptor expression response to colonic distension in rat. Neuro Endocrinol Lett 2010;31:215–220. [PubMed] [Google Scholar]
- 166. Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol 2011;14:666–683. [DOI] [PubMed] [Google Scholar]
- 167. Kim SH, Han JE, Hwang S, Oh DH. The expression of corticotropin‐releasing factor in the central nucleus of the amygdala, induced by colorectal distension, is attenuated by general anesthesia. J Korean Med Sci 2010;25:1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ouelaa W, Ghouzali I, Langlois L, et al. Gastric electrical stimulation decreases gastric distension‐induced central nociception response through direct action on primary afferents. PLoS ONE 2012;7:e47849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Johnson AC, Tran L, Greenwood‐Van MB. Knockdown of corticotropin‐releasing factor in the central amygdala reverses persistent viscerosomatic hyperalgesia. Transl Psychiatry 2015;5:e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Johnson AC, Tran L, Schulkin J, Greenwood‐Van Meerveld B. Importance of stress receptor‐mediated mechanisms in the amygdala on visceral pain perception in an intrinsically anxious rat. Neurogastroenterol Motil 2012;24:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Su J, Tanaka Y, Muratsubaki T, Kano M, Kanazawa M, Fukudo S. Injection of corticotropin‐releasing hormone into the amygdala aggravates visceral nociception and induces noradrenaline release in rats. Neurogastroenterol Motil 2015;27:30–39. [DOI] [PubMed] [Google Scholar]
- 172. Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin‐releasing hormone receptor 1 antagonist blocks brain‐gut activation induced by colonic distention in rats. Gastroenterology 2005;129:1533–1543. [DOI] [PubMed] [Google Scholar]
- 173. Moran CP, Shanahan F. Gut microbiota and obesity: role in aetiology and potential therapeutic target. Best Pract Res Clin Gastroenterol 2014;28:585–597. [DOI] [PubMed] [Google Scholar]
- 174. Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry 2015;28:1–6. [DOI] [PubMed] [Google Scholar]
- 175. Nicholson JK, Holmes E, Kinross J, et al. Host‐gut microbiota metabolic interactions. Science 2012;336:1262–1267. [DOI] [PubMed] [Google Scholar]
- 176. De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature 2011;473:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science 2005;308:1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Blaut M, Collins MD, Welling GW, Dore J, van Loo J, de Vos W. Molecular biological methods for studying the gut microbiota: the EU human gut flora project. Br J Nutr 2002;87(Suppl 2):S203–S211. [DOI] [PubMed] [Google Scholar]
- 180. Weinstock GM. Genomic approaches to studying the human microbiota. Nature 2012;489:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Inglis GD, Thomas MC, Thomas DK, Kalmokoff ML, Brooks SP, Selinger LB. Molecular methods to measure intestinal bacteria: a review. J AOAC Int 2012;95:5–23. [DOI] [PubMed] [Google Scholar]
- 182. Fraher MH, O'Toole PW, Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol 2012;9:312–322. [DOI] [PubMed] [Google Scholar]
- 183. Sommer F, Backhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol 2013;11:227–238. [DOI] [PubMed] [Google Scholar]
- 184. Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 2014;20:509–518. [DOI] [PubMed] [Google Scholar]
- 185. Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol 2015;50:495–507. [DOI] [PubMed] [Google Scholar]
- 186. Collado MC, Calabuig M, Sanz Y. Differences between the fecal microbiota of coeliac infants and healthy controls. Curr Issues Intest Microbiol 2007;8:9–14. [PubMed] [Google Scholar]
- 187. McLean MH, Dieguez D Jr, Miller LM, Young HA. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut 2015;64:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 2015;26:26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cryan JF, O'Mahony SM. The microbiome‐gut‐brain axis: from bowel to behavior. Neurogastroenterol Motil 2011;23:187–192. [DOI] [PubMed] [Google Scholar]
- 190. De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota‐gut‐brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol 2014;592:2989–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. O'Mahony SM, Felice VD, Nally K, et al. Disturbance of the gut microbiota in early‐life selectively affects visceral pain in adulthood without impacting cognitive or anxiety‐related behaviors in male rats. Neuroscience 2014;277:885–901. [DOI] [PubMed] [Google Scholar]
- 192. Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. J Physiol 2004;558:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun 1974;9:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tannock GW, Smith JM. The effect of food and water deprivation (stress) on Salmonella‐carrier mice. J Med Microbiol 1972;5:283–289. [DOI] [PubMed] [Google Scholar]
- 195. O'Mahony SM, Clarke G, Dinan TG, Cryan JF. Early‐life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience 2015; doi: 10.1016/j.neuroscience.2015.09.068 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 196. Amaral FA, Sachs D, Costa VV, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci USA 2008;105:2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor‐induced immunomodulation. Brain Behav Immun 2011;25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Powell ND, Sloan EK, Bailey MT, et al. Social stress up‐regulates inflammatory gene expression in the leukocyte transcriptome via beta‐adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA 2013;110:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun 2010;78:1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Campos‐Rodriguez R, Godinez‐Victoria M, Abarca‐Rojano E, et al. Stress modulates intestinal secretory immunoglobulin A. Front Integr Neurosci 2013;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa‐bacteria interactions. Cell Tissue Res 2011;343:23–32. [DOI] [PubMed] [Google Scholar]
- 202. Barouei J, Moussavi M, Hodgson DM. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS ONE 2012;7:e46051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Garcia‐Rodenas CL, Bergonzelli GE, Nutten S, et al. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr 2006;43:16–24. [DOI] [PubMed] [Google Scholar]
- 204. Eutamene H, Bueno L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut 2007;56:1495–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010;170:1179–1188. [DOI] [PubMed] [Google Scholar]
- 206. De Palma G, Blennerhassett P, Lu J, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun 2015;6:7735. [DOI] [PubMed] [Google Scholar]
- 207. O'Malley D, Julio‐Pieper M, Gibney SM, Dinan TG, Cryan JF. Distinct alterations in colonic morphology and physiology in two rat models of enhanced stress‐induced anxiety and depression‐like behaviour. Stress 2010;13:114–122. [DOI] [PubMed] [Google Scholar]
- 208. Jeffery IB, O'Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species‐specific alterations in faecal microbiota. Gut 2012;61:997–1006. [DOI] [PubMed] [Google Scholar]
- 209. Matto J, Maunuksela L, Kajander K, et al. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome–a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol 2005;43:213–222. [DOI] [PubMed] [Google Scholar]
- 210. Malinen E, Rinttila T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real‐time PCR. Am J Gastroenterol 2005;100:373–382. [DOI] [PubMed] [Google Scholar]
- 211. Kassinen A, Krogius‐Kurikka L, Makivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007;133:24–33. [DOI] [PubMed] [Google Scholar]
- 212. Lyra A, Rinttila T, Nikkila J, et al. Diarrhoea‐predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol 2009;15:5936–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 2010;22:512–519. [DOI] [PubMed] [Google Scholar]
- 214. Shankar V, Homer D, Rigsbee L, et al. The networks of human gut microbe‐metabolite associations are different between health and irritable bowel syndrome. ISME J 2015;9:1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mayer EA, Savidge T, Shulman RJ. Brain‐gut microbiome interactions and functional bowel disorders. Gastroenterology 2014;146:1500–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol 2014;11:497–505. [DOI] [PubMed] [Google Scholar]
- 217. Bennet SM, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liv 2015;9:318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Noor SO, Ridgway K, Scovell L, et al. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol 2010;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Maccaferri S, Candela M, Turroni S, et al. IBS‐associated phylogenetic unbalances of the intestinal microbiota are not reverted by probiotic supplementation. Gut Microbes 2012;3:406–413. [DOI] [PubMed] [Google Scholar]
- 220. Ng SC, Lam EF, Lam TT, et al. Effect of probiotic bacteria on the intestinal microbiota in irritable bowel syndrome. J Gastroenterol Hepatol 2013;28:1624–1631. [DOI] [PubMed] [Google Scholar]
- 221. Jalanka‐Tuovinen J, Salojarvi J, Salonen A, et al. Faecal microbiota composition and host‐microbe cross‐talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014;63:1737–1745. [DOI] [PubMed] [Google Scholar]
- 222. Theodorou V, Ait Belgnaoui A, Agostini S, Eutamene H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes 2014;5:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Urita Y, Goto M, Watanabe T, et al. Continuous consumption of fermented milk containing Bifidobacterium bifidum YIT 10347 improves gastrointestinal and psychological symptoms in patients with functional gastrointestinal disorders. Biosci Microbiota Food Health 2015;34:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double‐blind, placebo‐controlled trial. J Gastroenterol Hepatol 2014;29:52–59. [DOI] [PubMed] [Google Scholar]
- 225. Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: a liquid multi‐strain probiotic vs. placebo in the irritable bowel syndrome–a 12 week double‐blind study. Aliment Pharmacol Ther 2014;40:51–62. [DOI] [PubMed] [Google Scholar]
- 226. Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–a double‐blind, placebo‐controlled study. Aliment Pharmacol Ther 2011;33:1123–1132. [DOI] [PubMed] [Google Scholar]
- 227. Michail S, Kenche H. Gut microbiota is not modified by Randomized, Double‐blind, Placebo‐controlled Trial of VSL#3 in Diarrhea‐predominant Irritable Bowel Syndrome. Probiotics Antimicrob Proteins 2011;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Farup PG, Jacobsen M, Ligaarden SC, Rudi K. Probiotics, symptoms, and gut microbiota: what are the relations? A randomized controlled trial in subjects with irritable bowel syndrome. Gastroenterol Res Pract 2012;2012:214102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Charbonneau D, Gibb RD, Quigley EM. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes 2013;4:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Desbonnet L, Clarke G, Traplin A, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun 2015;48:165–173. [DOI] [PubMed] [Google Scholar]
- 231. Clarke G, Grenham S, Scully P, et al. The microbiome‐gut‐brain axis during early life regulates the hippocampal serotonergic system in a sex‐dependent manner. Mol Psychiatry 2013;18:666–673. [DOI] [PubMed] [Google Scholar]
- 232. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 2011;108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Cervero F. Visceral pain: mechanisms of peripheral and central sensitization. Ann Med 1995;27:235–239. [DOI] [PubMed] [Google Scholar]
- 234. Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS ONE 2013;8:e63893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Dai C, Guandalini S, Zhao DH, Jiang M. Antinociceptive effect of VSL#3 on visceral hypersensitivity in a rat model of irritable bowel syndrome: a possible action through nitric oxide pathway and enhance barrier function. Mol Cell Biochem 2012;362:43–53. [DOI] [PubMed] [Google Scholar]
- 236. McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil 2010;22:1029–1035, e268. [DOI] [PubMed] [Google Scholar]
- 237. Johnson AC, Greenwood‐Van Meerveld B, McRorie J. Effects of Bifidobacterium infantis 35624 on post‐inflammatory visceral hypersensitivity in the rat. Dig Dis Sci 2011;56:3179–3186. [DOI] [PubMed] [Google Scholar]
- 238. Agostini S, Goubern M, Tondereau V, et al. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I‐2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol Motil 2012;24:376–e172. [DOI] [PubMed] [Google Scholar]
- 239. Verdu EF, Bercik P, Verma‐Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006;55:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague‐Dawley rats. Gut 2006;55:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 2007;13:35–37. [DOI] [PubMed] [Google Scholar]
- 242. Laval L, Martin R, Natividad JN, et al. Lactobacillus rhamnosus CNCM I‐3690 and the commensal bacterium Faecalibacterium prausnitzii A2‐165 exhibit similar protective effects to induced barrier hyper‐permeability in mice. Gut Microbes 2015;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res 2013;69:42–51. [DOI] [PubMed] [Google Scholar]
- 244. Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol 2012;46(Suppl):S52–S55. [DOI] [PubMed] [Google Scholar]
- 245. Aguilera M, Cerda‐Cuellar M, Martinez V. Antibiotic‐induced dysbiosis alters host‐bacterial interactions and leads to colonic sensory and motor changes in mice. Gut Microbes 2015;6:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Saadi M, McCallum RW. Rifaximin in irritable bowel syndrome: rationale, evidence and clinical use. Ther Adv Chronic Dis 2013;4:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Schoenfeld P, Pimentel M, Chang L, et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double‐blind, placebo‐controlled trials. Aliment Pharmacol Ther 2014;39:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Schmulson M, Bielsa MV, Carmona‐Sanchez R, et al. Microbiota, gastrointestinal infections, low‐grade inflammation, and antibiotic therapy in irritable bowel syndrome: an evidence‐based review. Rev Gastroenterol Mex 2014;79:96–134. [DOI] [PubMed] [Google Scholar]
- 249. Pimentel M, Morales W, Chua K, et al. Effects of rifaximin treatment and retreatment in nonconstipated IBS subjects. Dig Dis Sci 2011;56:2067–2072. [DOI] [PubMed] [Google Scholar]
- 250. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011;364:22–32. [DOI] [PubMed] [Google Scholar]
- 251. Moraru IG, Portincasa P, Moraru AG, Diculescu M, Dumitrascu DL. Small intestinal bacterial overgrowth produces symptoms in irritable bowel syndrome which are improved by rifaximin. A pilot study. Rom J Intern Med 2013;4:143–147. [PubMed] [Google Scholar]
- 252. Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta‐analysis. Am J Gastroenterol 2012;107:28–35; quiz 6. [DOI] [PubMed] [Google Scholar]
- 253. Jolley J. High‐dose rifaximin treatment alleviates global symptoms of irritable bowel syndrome. Clin Exp Gastroenterol 2011;4:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Boltin D, Perets TT, Shporn E, et al. Rifaximin for small intestinal bacterial overgrowth in patients without irritable bowel syndrome. Ann Clin Microbiol Antimicrob 2014;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil 2015;27:19–29. [DOI] [PubMed] [Google Scholar]
- 256. Sha S, Liang J, Chen M, et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther 2014;39:1003–1032. [DOI] [PubMed] [Google Scholar]
- 257. Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol 2012;107:1452–1459. [DOI] [PubMed] [Google Scholar]
- 258. Crouzet L, Gaultier E, Del'Homme C, et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil 2013;25:e272–e282. [DOI] [PubMed] [Google Scholar]
- 259. Le Gall G, Noor SO, Ridgway K, et al. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J Proteome Res 2011;10:4208–4218. [DOI] [PubMed] [Google Scholar]
- 260. Shankar V, Homer D, Rigsbee L, et al. The networks of human gut microbe‐metabolite associations are different between health and irritable bowel syndrome. ISME J 2015;9:1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Chumpitazi BP, Hollister EB, Oezguen N, et al. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes 2014;5:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol 2011;60(Pt 6):817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Annahazi A, Gecse K, Dabek M, et al. Fecal proteases from diarrheic‐IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain 2009;144:209–217. [DOI] [PubMed] [Google Scholar]
- 264. Ahmed I, Greenwood R, de Costello BL, Ratcliffe NM, Probert CS. An investigation of fecal volatile organic metabolites in irritable bowel syndrome. PLoS ONE 2013;8:e58204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated‐irritable bowel syndrome. Aliment Pharmacol Ther 2012;35:828–838. [DOI] [PubMed] [Google Scholar]
- 266. Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea‐predominant irritable bowel syndrome. Neurogastroenterol Motil 2012;24:513–520, e246‐7. [DOI] [PubMed] [Google Scholar]
- 267. Cenac N, Bautzova T, Le FP, et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 2015;149:433–444, e7. [DOI] [PubMed] [Google Scholar]
- 268. Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short‐chain fatty acids in patients with diarrhea‐predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr 1996;23:280–286. [DOI] [PubMed] [Google Scholar]
- 269. Husebye E, Hellstrom PM, Midtvedt T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig Dis Sci 1994;39:946–956. [DOI] [PubMed] [Google Scholar]
- 270. Ochoa‐Cortes F, Ramos‐Lomas T, Miranda‐Morales M, et al. Bacterial cell products signal to mouse colonic nociceptive dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol 2010;299:G723–G732. [DOI] [PubMed] [Google Scholar]
- 271. Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid‐4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 2008;135:937–946, 46 e1‐2. [DOI] [PubMed] [Google Scholar]
- 272. Cenac N, Altier C, Motta JP, et al. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut 2010;59:481–488. [DOI] [PubMed] [Google Scholar]
- 273. Annahazi A, Ferrier L, Bezirard V, et al. Luminal cysteine‐proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation‐predominant IBS. Am J Gastroenterol 2013;108:1322–1331. [DOI] [PubMed] [Google Scholar]
- 274. Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010;59:1213–1221. [DOI] [PubMed] [Google Scholar]
- 275. Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Investig 2007;117:636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Hyland NP, Julio‐Pieper M, O'Mahony SM, et al. A distinct subset of submucosal mast cells undergoes hyperplasia following neonatal maternal separation: a role in visceral hypersensitivity? Gut 2009;58:1029–1030; author reply 30‐1. [DOI] [PubMed] [Google Scholar]
- 277. Carroll SY, O'Mahony SM, Grenham S, Cryan JF, Hyland NP. Disodium cromoglycate reverses colonic visceral hypersensitivity and influences colonic ion transport in a stress‐sensitive rat strain. PLoS ONE 2013;8:e84718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Heuston S, Hyland NP. Chymase inhibition as a pharmacological target: a role in inflammatory and functional gastrointestinal disorders? Br J Pharmacol 2012;167:732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Vergnolle N. Protease‐activated receptors as drug targets in inflammation and pain. Pharmacol Ther 2009;123:292–309. [DOI] [PubMed] [Google Scholar]
- 280. O'Sullivan M. Therapeutic potential of ketotifen in irritable bowel syndrome (IBS) may involve changes in mast cells at sites beyond the rectum. Gut 2011;60:423; author rpely. [DOI] [PubMed] [Google Scholar]
- 281. De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut 2015; doi: 10.1136/gutjnl-2015-309757. [DOI] [PubMed] [Google Scholar]
- 282. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015;64:93–100. [DOI] [PubMed] [Google Scholar]
- 283. Austin GL, Dalton CB, Hu Y, et al. A very low‐carbohydrate diet improves symptoms and quality of life in diarrhea‐predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2009;7:706–708, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014;146:67–75, e5. [DOI] [PubMed] [Google Scholar]
- 285. Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther 2015;42:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet 2011;24:487–495. [DOI] [PubMed] [Google Scholar]
- 287. Staudacher HM, Irving PM, Lomer MC, Whelan K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat Rev Gastroenterol Hepatol 2014;11:256–266. [DOI] [PubMed] [Google Scholar]
- 288. Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour ‐ epigenetic regulation of the gut‐brain axis. Genes Brain Behav 2014;13:69–86. [DOI] [PubMed] [Google Scholar]
- 289. Cao DY, Bai G, Ji Y, Traub RJ. Epigenetic upregulation of metabotropic glutamate receptor 2 in the spinal cord attenuates oestrogen‐induced visceral hypersensitivity. Gut 2015;64:1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Tran L, Schulkin J, Ligon CO, Greenwood‐Van Meerveld B. Epigenetic modulation of chronic anxiety and pain by histone deacetylation. Mol Psychiatry 2015;20:1219–1231. [DOI] [PubMed] [Google Scholar]
- 291. Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain‐derived neurotropic factor and behavior in mice. Gastroenterology 2011;141:599–609, e1‐3. [DOI] [PubMed] [Google Scholar]
- 292. Diaz HR, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety‐like behavior and central neurochemical change in germ‐free mice. Neurogastroenterol Motil 2011;23:255–264, e119. [DOI] [PubMed] [Google Scholar]
- 294. Matsumoto M, Kibe R, Ooga T, et al. Cerebral low‐molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci 2013;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome : official journal of the International Mammalian Genome Society 2014;25:49–74. [DOI] [PubMed] [Google Scholar]
- 296. Burokas A, Moloney RD, Dinan TG, Cryan JF. Microbiota regulation of the Mammalian gut‐brain axis. Adv Appl Microbiol 2015;91:1–62. [DOI] [PubMed] [Google Scholar]
- 297. Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res 2015;63:1–9. [DOI] [PubMed] [Google Scholar]
- 298. Akbar A, Walters JR, Ghosh S. Review article: visceral hypersensitivity in irritable bowel syndrome: molecular mechanisms and therapeutic agents. Aliment Pharmacol Ther 2009;30:423–435. [DOI] [PubMed] [Google Scholar]
- 299. Piche T. Tight junctions and IBS–the link between epithelial permeability, low‐grade inflammation, and symptom generation? Neurogastroenterol Motil 2014;26:296–302. [DOI] [PubMed] [Google Scholar]
- 300. de CRH, Dantas BP, Rolim TL, Costa BA, de Medeiros AC. Main ion channels and receptors associated with visceral hypersensitivity in irritable bowel syndrome. Ann Gastroenterol 2014;27:200–206. [PMC free article] [PubMed] [Google Scholar]
- 301. Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni . Brain Behav Immun 2005;19:334–344. [DOI] [PubMed] [Google Scholar]
- 302. Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun 2010;24:9–16. [DOI] [PubMed] [Google Scholar]
- 303. Perez‐Burgos A, Wang B, Mao YK, et al. Psychoactive bacteria Lactobacillus rhamnosus (JB‐1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol 2013;304:G211–G220. [DOI] [PubMed] [Google Scholar]
- 304. Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut‐brain communication. Neurogastroenterol Motil 2011;23:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. van der Schaar PJ, van Hoboken E, Ludidi S, Masclee AA. Effect of cholecystokinin on rectal motor and sensory function in patients with irritable bowel syndrome and healthy controls. Colorectal Dis 2013;15:e29–e34. [DOI] [PubMed] [Google Scholar]
- 306. Cao B, Zhang X, Yan N, Chen S, Li Y. Cholecystokinin enhances visceral pain‐related affective memory via vagal afferent pathway in rats. Mol Brain 2012;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Friedrich AE, Gebhart GF. Effects of spinal cholecystokinin receptor antagonists on morphine antinociception in a model of visceral pain in the rat. J Pharmacol Exp Ther 2000;292:538–544. [PubMed] [Google Scholar]
- 308. Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum 2007;50:376–388. [DOI] [PubMed] [Google Scholar]
- 309. McLean PG, Borman RA, Lee K. 5‐HT in the enteric nervous system: gut function and neuropharmacology. Trends Neurosci 2007;30:9–13. [DOI] [PubMed] [Google Scholar]
- 310. Wrase J, Reimold M, Puls I, Kienast T, Heinz A. Serotonergic dysfunction: brain imaging and behavioral correlates. Cogn Affect Behav Neurosci 2006;6:53–61. [DOI] [PubMed] [Google Scholar]
- 311. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Ridaura V, Belkaid Y. Gut microbiota: the link to your second brain. Cell 2015;161:193–194. [DOI] [PubMed] [Google Scholar]
- 313. O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain‐gut‐microbiome axis. Behav Brain Res 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 314. Tack J, Broekaert D, Fischler B, Van Oudenhove L, Gevers AM, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Weilburg JB. An overview of SSRI and SNRI therapies for depression. Manag Care 2004;13(6 Suppl Depression):25–33. [PubMed] [Google Scholar]
- 316. Creed F, Fernandes L, Guthrie E, et al. The cost‐effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303–317. [DOI] [PubMed] [Google Scholar]
- 317. Bengmark S. Gut microbiota, immune development and function. Pharmacol Res 2013;69:87–113. [DOI] [PubMed] [Google Scholar]
- 318. Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry 2005;20(Suppl 3):S302–S306. [DOI] [PubMed] [Google Scholar]
- 319. Bateman A, Singh A, Kral T, Solomon S. The immune‐hypothalamic‐pituitary‐adrenal axis. Endocr Rev 1989;10:92–112. [DOI] [PubMed] [Google Scholar]
- 320. Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg 2003;186:253–258. [DOI] [PubMed] [Google Scholar]
- 321. Hori T, Katafuchi T, Take S, Shimizu N, Niijima A. The autonomic nervous system as a communication channel between the brain and the immune system. NeuroImmunoModulation 1995;2:203–215. [DOI] [PubMed] [Google Scholar]
- 322. Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987‐2007). Brain Behav Immun 2007;21:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Maes M, Kubera M, Leunis JC. The gut‐brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 2008;29:117–124. [PubMed] [Google Scholar]
- 324. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut‐bacterial dysbiosis, and behavioral markers of alcohol‐dependence severity. Proc Natl Acad Sci USA 2014;111:E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress‐induced intestinal damage. Curr Mol Med 2008;8:274–281. [DOI] [PubMed] [Google Scholar]
- 326. Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome 2014;25:49–74. [DOI] [PubMed] [Google Scholar]
- 327. Tramullas M, Finger BC, Moloney RD, et al. Toll‐like receptor 4 regulates chronic stress‐induced visceral pain in mice. Biol Psychiatry 2014;76:340–348. [DOI] [PubMed] [Google Scholar]
- 328. McKernan DP, Nolan A, Brint EK, et al. Toll‐like receptor mRNA expression is selectively increased in the colonic mucosa of two animal models relevant to irritable bowel syndrome. PLoS ONE 2009;4:e8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll‐like receptor activation in irritable bowel syndrome. Front Pharmacol 2012;3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Camara RJ, Ziegler R, Begre S, Schoepfer AM, von KR, Swiss Inflammatory Bowel Disease Cohort Study g . The role of psychological stress in inflammatory bowel disease: quality assessment of methods of 18 prospective studies and suggestions for future research. Digestion 2009;80:129–139. [DOI] [PubMed] [Google Scholar]
- 332. Mawdsley JE, Rampton DS. The role of psychological stress in inflammatory bowel disease. NeuroImmunoModulation 2006;13:327–336. [DOI] [PubMed] [Google Scholar]
- 333. Bonaz BL, Bernstein CN. Brain‐gut interactions in inflammatory bowel disease. Gastroenterology 2013;144:36–49. [DOI] [PubMed] [Google Scholar]
- 334. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain‐gut‐enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Grenham S, Clarke G, Cryan JF, Dinan TG. Brain‐gut‐microbe communication in health and disease. Front Physiol 2011;2:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Kennedy PJ, Clarke G, Quigley EM, Groeger JA, Dinan TG, Cryan JF. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci Biobehav Rev 2012;36:310–340. [DOI] [PubMed] [Google Scholar]
- 337. Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome–focus on lactic acid bacteria. Aliment Pharmacol Ther 2012;35:403–413. [DOI] [PubMed] [Google Scholar]
- 338. Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet‐induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008;3:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031. [DOI] [PubMed] [Google Scholar]


