Alzheimer's disease (AD), a major neurodegenerative disorder, is a matter of health concern because of the lack of effective drugs to treat it. Thus, our group has recently identified N‐((5‐(3‐(1‐benzylpiperidin‐4‐yl) propoxy)‐1‐methyl‐1H‐indol‐2‐yl)methyl)‐N‐methylprop‐2‐yn‐1‐amine (ASS234, Figure 1), a new multitarget propargylamine for the potential treatment and prevention of AD, and described that this compound is able to act simultaneously both as a reversible inhibitor of human acetylcholinesterase (AChE)/butyrylcholinesterase (BuChE) and as an irreversible inhibitor of human monoamine oxidase A/B 1. In addition, ASS234 has been reported to inhibit in vitro β‐amyloid (Αβ)1–42 aggregation induced by AChE and Αβ 1–40 self‐aggregation, thus limiting the formation of fibrillar and oligomeric species and reducing Αβ 1–42‐mediated toxicity 2. However, the mechanism underlying its neuroprotective effect remains unclear still.
Figure 1.
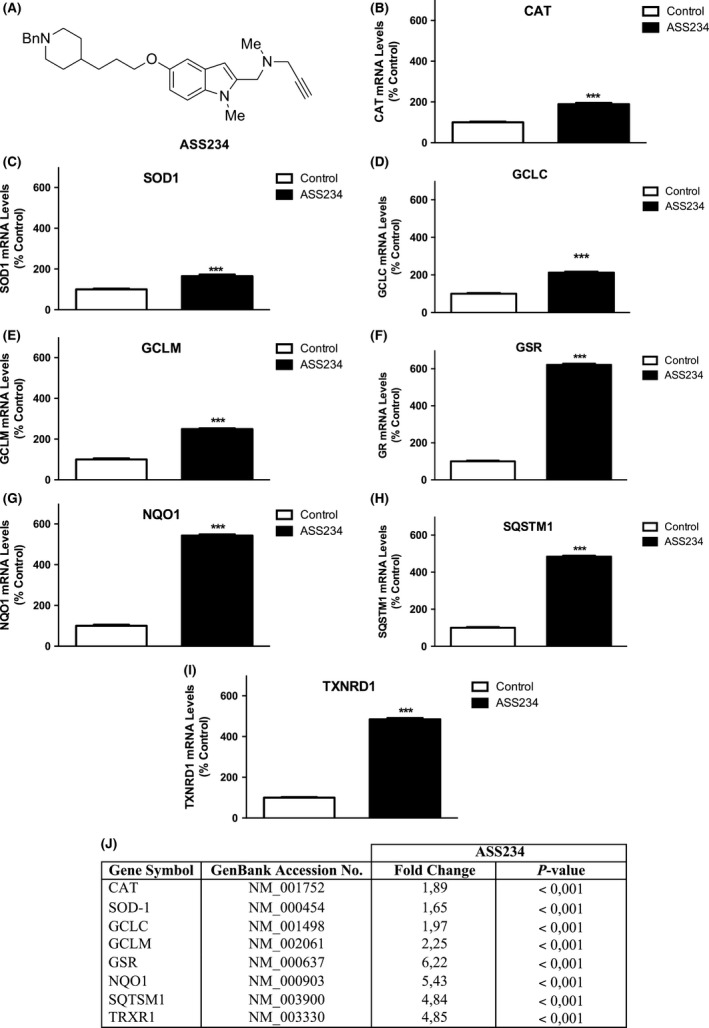
(A) Represents chemical structure of ASS234. (B–I) Shows results from real‐time PCR arrays targeting select genes after ASS234 (5 μM) treatment. (J) Representative table of data, in which specific gene expression was compared with controls [cells treated with DMSO (0.1%) were the negative control]. Each bar represents mean ± SEM of six independent experiments. ACTB was used as an internal control. ***P < 0.001, significantly different from controls.
On the other hand, oxidative stress has been implicated as a possible mechanism in the etiology and progression of AD 3. In this regard, our previous studies showed that ASS234 is a potent antioxidant due to its free radical scavenging capacity, preventing, also, the Aβ 1–42‐induced depletion of antioxidant enzymes such as catalase and superoxide dismutase‐1 (SOD‐1) 2, which are mainly regulated by the nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2) pathway 4. Using Ingenuity pathways analysis (IPA) (Ingenuity H Systems, Redwood City, CA, USA) to evaluate the Nrf2 pathway, we found that this molecule regulates many antioxidant enzymes (Figure 2), such as NAD(P)H quinone oxidoreductase 1 (NQO1), glutathione reductase (GSR), thioredoxin reductase 1 (TRXR1), sequestosome 1 (SQSTM1), which have been described to be affected in AD disease 3, 5, 6, 7, 8. According to this information, we tested whether ASS234 induced the gene expression of the Nrf2‐related antioxidant enzymes CAT, SOD‐1, NQO1, GCLC, GCLM, GSR, TRXR1 and SQSTM1 in SH‐SY5Y cells as a mechanism of neuroprotection. Relative changes in gene expression were calculated using the Ct (cycle threshold) method. The expression data are shown as actual change multiples. At least six replicates for each experimental condition were carried out, and the presented results were representative of these replicates. Data are represented as means ± standard error of the mean (SEM). Comparisons between experimental and control groups were performed by Student's t‐test. Statistical difference was accepted when P ≤ 0.05. Statistical analysis of data was carried out by computer using GraphPad Prism software v. 5.02 (La Jolla, CA, USA).
Figure 2.
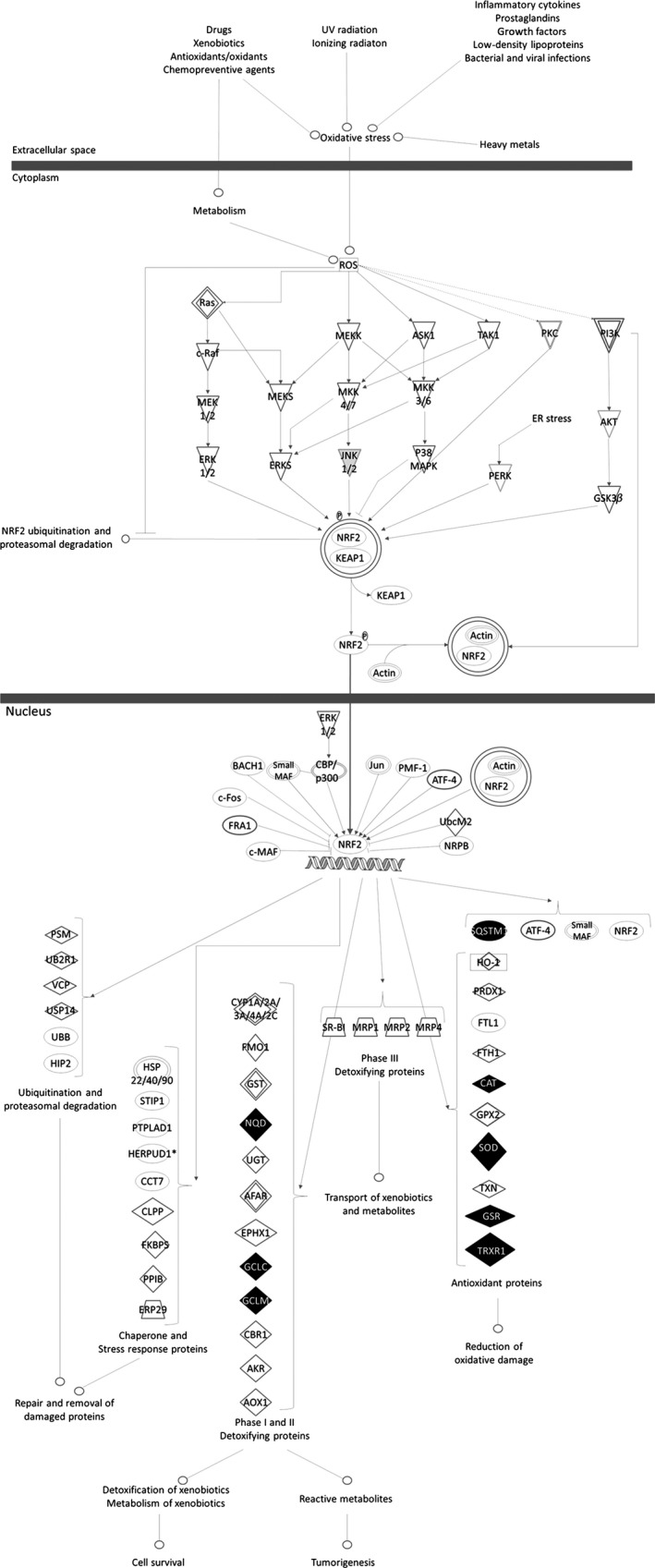
Ingenuity pathways analysis (IPA) of ASS234 (5 μM) treatment. Nrf2‐mediated oxidative stress response pathway. The genes that are shaded were determined to be significant from the statistical analysis. A solid line represents a direct interaction between the two gene products and a dotted line means there is an indirect interaction. Green represents downregulation, while red depicts upregulation. White represents no tested.
The analysis performed after incubating ASS234 (5 μM) in SH‐SY5Y cells during a 24‐h period showed that this molecule was able to induce the gene expression of CAT, SOD1, GCLC, GCLM, GSR, QSTM1, TXNRD1 and NQO1 (Figure 1) in a significant manner.
The target genes studied have been involved in the development of AD. In this context, a decrease in GSH levels and GSR expression and subsequent GSSG production has been linked to neuronal loss in AD 3. The first and rate‐limiting step in GSH synthesis is catalyzed by glutamate cysteine ligase (GCL), which is a heterodimeric protein composed of catalytic (GCLC) and modifier (GCLM) subunits that are expressed from different genes 9. Moreover, immunohistochemical analysis of frontal cortex samples from patients with AD revealed an overall decrease in TXNRD1 levels in neurons, but an increase in TXNRD1 immunoreactivity in astrocytes when compared with age‐matched control brains 5. Exogenous addition of thioredoxin or TXNRD1 has been shown to protect in vitro cell cultures from Aβ toxicity 5. Furthermore, it was also reported that NQO1, a flavoenzyme that is important in maintaining the cellular redox state and regulating protein degradation 6, was increased in the latest phases of AD as a protection mechanism 6 and the examination of hippocampi from patients with AD revealed that in half of the cases examined the NQO1 protein level was undetectable, suggesting that the age‐dependent accumulation of NQO1 is impaired in certain patients with AD 7. Finally, the lack of SQSTM1 (P62) protein expression provokes the tau pathology in mouse brain and leads to biochemical and cognitive deficits that resemble AD 10. Recent studies have shown that the p62 gene expression and cytoplasmic p62 protein levels are significantly reduced in the frontal cortex of patients with AD 8.
In summary, these data support our previous reports showing an increase in the expression of catalase and SOD1 by compound ASS234, which could protect against AD. Moreover, ASS234 promoted induction of CAT, SOD1, GCLC, GCLM, GSR, TRXR1, NQO1 and SQTSM1 might be regulated through the Nrf2 pathway; this is likely one of the possible mechanisms by which the antioxidant effects observed on this compound are leaded. Lastly, according to the reports shown, the increase in the expression of these genes could confer resistance to oxidative stress and may well contribute to ASS234 neuroprotective properties, rendering the compound as potentially valuable for the treatment of neurodegenerative diseases such as AD.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
JMC thanks the Spanish Ministry of Economy and Competence grant SAF2009‐07271 and the UCJC (Project 2014‐35: “NEW MULTIMOL”) for continued support.
References
- 1. Bolea I, Juarez‐Jimenez J, de los Rıos C, et al. Synthesis, biological evaluation, and molecular modeling of donepezil and N‐[(5‐(benzyloxy)‐1‐methyl‐1H‐indol‐2‐yl) methyl]‐N‐methylprop‐2‐yn‐1‐amine hybrids as new multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer's disease. J Med Chem 2011;54:8251–8270. [DOI] [PubMed] [Google Scholar]
- 2. Bolea I, Gella A, Monjas L, et al. The multipotent, permeable drug ASS234 inhibits Ab aggregation, possesses antioxidant properties and protects from Ab‐induced apoptosis. Curr Alzheimer Res 2013;9:797–808. [DOI] [PubMed] [Google Scholar]
- 3. Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 2010;69:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar H, Kim IS, More SV, et al. Natural product‐derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep 2014;31:109–139. [DOI] [PubMed] [Google Scholar]
- 5. Akterin S, Cowburn RF, Miranda‐Vizuete A, et al. Involvement of glutaredoxin‐1 and thioredoxin‐1 in beta‐amyloid toxicity and Alzheimer's disease. Cell Death Differ 2006;13:1454–1465. [DOI] [PubMed] [Google Scholar]
- 6. SantaCruz KS, Yazlovitskaya E, Collins J, et al. Regional NAD(P)H:Quinoneoxidoreductase activity in Alzheimer's disease. Neurobiol Aging 2004;25:63–69. [DOI] [PubMed] [Google Scholar]
- 7. Tsvetkov P, Adamovich Y, Elliott E, et al. E3 ligase STUB1/CHIP regulates NAD(P)H: Quinoneoxidoreductase 1 (NQO1) accumulation in aged brain, a process impaired in certain Alzheimer disease patients. J Biol Chem 2011;286:8839–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du Y, Wooten MC, Gearing M, et al. Age‐associated oxidative damage to the p62 promoter: Implications for Alzheimer disease. Free Radic Biol Med 2009;46:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franklin CC, Backos DS, Mohar I, et al. Structure, function, and post‐translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med 2009;30:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramesh Babu J, Lamar Seibenhener M, Peng J, et al. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem 2008;106:107–120. [DOI] [PubMed] [Google Scholar]


