Summary
Introduction
Presence of white matter hyperintensity (WMH) on MRI is a marker of cerebral small vessel disease and is associated with increased small vessel stroke and increased risk of hemorrhagic transformation (HT) after thrombolysis.
Aim
We sought to determine whether white matter hypoperfusion (WMHP) on perfusion CT (CTP) was related to WMH, and if WMHP predisposed to acute lacunar stroke subtype and HT after thrombolysis.
Methods
Acute ischemic stroke patients within 12 h of symptom onset at 2 centers were prospectively recruited between 2011 and 2013 for the International Stroke Perfusion Imaging Registry. Participants routinely underwent baseline CT imaging, including CTP, and follow‐up imaging with MRI at 24 h.
Results
Of 229 ischemic stroke patients, 108 were Caucasians and 121 Chinese. In the contralateral white matter, patients with acute lacunar stroke had lower cerebral blood flow (CBF) and cerebral blood volume (CBV), compared to those with other stroke subtypes (P = 0.041). There were 46 patients with HT, and WMHP was associated with increased risk of HT (R 2 = 0.417, P = 0.002). Compared to previously reported predictors of HT, WMHP performed better than infarct core volume (R 2 = 0.341, P = 0.034), very low CBV volume (R 2 = 0.249, P = 0.026), and severely delayed perfusion (Tmax>14 second R 2 = 0.372, P = 0.011). Patients with WMHP also had larger acute infarcts and increased infarct growth compared to those without WMHP (mean 28 mL vs. 13 mL P < 0.001).
Conclusion
White matter hypoperfusion remote to the acutely ischemic region on CTP is a marker of small vessel disease and was associated with increased HT, larger acute infarct cores, and greater infarct growth.
Keywords: CTP, Intracerebral Hemorrhage, Small vessel disease, White matter disease, White matter hyper intensity
Introduction
White matter hyperintensity (WMH) of presumed vascular origin is frequently found in older adults on MR imaging (MRI). It is thought that WMH is a marker of cerebral small vessel disease (SVD) as a result of chronic ischemia leading to vascular endothelial damage 1. WMH burden is independently associated with an increased risk of stroke 2, 3, stroke recurrence 4, 5, and worse outcome 6, 7. Increased WMH burden has also been shown to be associated with an increased risk of lacunar stroke compared to other pathogenic ischemic stroke subtypes, both in population‐based case control studies 8 and hospital‐based stroke cohorts 9, 10. Moreover, studies have shown that moderate‐to‐severe WMH indicates an increased risk of hemorrhagic transformation (HT) after stroke thrombolysis 11, 12, although this is still controversial 13. Currently, WMH can only be assessed in the acute setting using MRI; however, a practical surrogate marker on perfusion CT would provide clinically meaningful data to physicians when treating acute stroke patients.
Reduced white matter perfusion in patients with WMH has been previously demonstrated using xenon‐CT 14, 15, PET 16, SPECT 17, and MRI 18, 19. CT perfusion (CTP)‐derived hemodynamic parameters were also found to be correlated with WMH severity in a small study 20. However, it is unknown whether generalized white matter hypoperfusion (WMHP), presumably as a marker of chronic small vessel disease, is similarly associated with increased acute small vessel stroke, nor whether WMHP is associated with HT after reperfusion therapy. If WMHP does reflect chronic small vessel ischemia, it is also possible that poorer blood flow in chronically ischemic white matter in patients with acute occlusions might be associated with larger acute ischemic cores, smaller penumbral volumes, and greater subsequent infarct growth.
The hypotheses tested were that WMHP on CTP will be more common in patients with WMH on MRI, and that WMHP remote to the acutely ischemic region will be associated with increased acute lacunar stroke, and increased risk of HT after reperfusion therapy. We also hypothesized that those with large volumes of WMHP with acute occlusions would have larger acute ischemic cores, smaller penumbral volume, and greater subsequent infarct growth than those without large WHMP volumes. We aimed to test these hypotheses in a cohort of acute ischemic stroke patients assessed for eligibility for reperfusion therapy using advanced CT imaging at baseline and MRI at 24 h poststroke onset.
Materials and Methods
Population
All consecutive acute ischemic stroke patients undergoing acute whole brain CTP within 12 h of symptom onset as routine at 2 centers (John Hunter Hospital, NSW, Australia and Huashan Hospital, Shanghai, China) were prospectively recruited between 2011 and 2013 for the International Stroke Perfusion Imaging Registry (INSPIRE). Ethics approval was acquired from the two relevant ethics committees. Written informed consent was obtained from all participants. All included patients underwent baseline multimodal CT imaging with noncontrast CT, CT perfusion, and follow‐up imaging with MRI at 24 h poststroke onset. Clinical stroke severity was assessed at the two imaging time points using the National Institutes of Health Stroke Scale (NIHSS). Eligible patients were treated with intravenous thrombolysis according to local guidelines. Detailed clinical history was prospectively collected, and TOAST stroke subtyping 21 was assigned to each case by stroke neurologists independently at each of the respective study sites, blinded to quantification of WMH and white matter perfusion. At 90 days after stroke admission, modified Rankin scale (mRS) scores were recorded. Patient exclusion criteria from this study included incomplete follow‐up data.
Imaging
Whole brain CTP and CTA were acquired simultaneously in a single acquisition. Subjects recruited at John Hunter Hospital were scanned using a Toshiba Aquilion One 320‐slice CT scanner (Toshiba Medical Systems; Tokyo, Japan). A total of 19 acquisitions occurred in 60 s. Forty milliliters of contrast agent (Ultravist 370; Bayer HealthCare; Berlin, Germany) was injected at 6 mL/second, followed by 30 mL of saline. Participants recruited at Huashan Hospital were scanned using a Brilliance iCT 256‐slice CT scanner (Philips Medical Systems, Cleveland, OH, USA). A total of 23 acquisitions occurred in 60 second. Forty milliliters of the same contrast was injected at 5 mL/second, followed by 20 mL saline. Images were formatted as 5 mm slices.
All patients underwent MRI at 24 h after symptom onset, using 1.5 T or 3 T scanners (Siemens Avanto or Verio). MRI sequences included T1‐weighted imaging, T2‐weighted imaging, diffusion‐weighted imaging (DWI), a gradient echo sequence, and flow‐attenuated inversion recovery (FLAIR).
Image Postprocessing
All perfusion imaging was centrally postprocessed on commercial software MIStar (Apollo Medical Imaging Technology, Melbourne, Vic., Australia). Acute perfusion imaging was processed using single value deconvolution with delay and dispersion correction 22. An arterial input function and venous outflow function were semi‐automatically selected from the nonstroke hemisphere MCA/ACA and sagittal sinus, respectively. Previously validated thresholds were applied to measure the volume of the acute perfusion lesion (relative delay time, DT >3 seconds) and acute infarct core (relative CBF <30%) 23. Penumbral volume was calculated from the volume of the perfusion lesion (DT threshold >3 seconds) minus the volume of the infarct core (relative CBF threshold <30% within the DT >3 second lesion).
Assessment of white matter hypoperfusion was performed retrospectively using acute CTP. White matter hypoperfusion (WMHP) was defined in white matter, contralateral to the acute ischemic hemisphere, where the white matter CBF is <50% of the hemispheric CBF with an area affected of larger than 20 mL 14, 16, 24. Contralateral to acute ischemic stroke, hemisphere regions were segmented into gray and white matter using a semi‐automated approach based on a Hounsfield unit change with manual adjustment where required. Perfusion measurements of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and delay time (Delay Time) were recorded in these regions of interest (ROIs) on CTP from the contralateral to ischemic hemisphere ROIs.
Lacunar infarction was defined using the 24‐h MRI DWI as infarction restricted to the deep white matter. For hemorrhagic transformation (HT), two stroke neurologists independently assessed the 24‐h MRI and then reached consensus using the European Cooperative Acute Stroke Study (ECASS) rating system 25. Other acute CTP variables known to predict HT were also measured: acute infarct core volume >50 mL 26, 27, ischemic hemisphere very low cerebral blood volume <2 mL (very low defined as <2.5% compared to contralateral hemisphere)28, and volume of severe delay within the acute perfusion lesion (large volume of Tmax 14 second) 29.
WMH was categorized on MRI FLAIR imaging 24 h after ischemic stroke using semi‐automated quantification from a region of interest tool with manual adjustments by a reader blind to the acute CTP.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics (version 20; IBM Corp, Armonk, NY, USA). Kruskal–Wallis and Fisher's exact tests were used to explore differences in clinical and neuroimaging characteristics. Univariate analyze was performed to assess for an association between stroke subtype and WMH (as a dichotomous variable—a volumetric threshold of >10 ml was used to define the presence or absence of WMH). Other analyses included infarct growth from acute to 24‐h time points, volume of the acute penumbra, reperfusion, and other clinical measures such as the acute and 24‐h NIHSS.
Next, we assessed the odds ratio of having any HT and specifically PH in patients with both WMH and WMHP using linear regression. We then used logistic regression likelihood ratios to determine the optimal perfusion thresholds at predicting any HT and PH. This threshold was then compared to previously described predictors (infarct core >50 mL, VLCBV and Tmax 14 second lesion volumes).
Finally, to test the hypothesis that chronic WMHP might predispose patients to more severe acute white matter hypoperfusion resulting in larger acute cores and smaller penumbras, we compared volumes of the acute CTP defined penumbra and ischemic core to patients with and without large volumes of WMHP (in unaffected hemisphere). Similarly, we compared infarct core growth from acute CTP to 24‐h DWI in patients with and without large volumes of WMHP.
Results
During the study period, 229 patients with acute ischemic stroke were included in the analysis, 121 Chinese and 108 Caucasians. The mean age was 68 years (range 28–95 years), median baseline NIHSS was 13 (IQR 7–19), and median WMH volume was 10 mL (IQR 1.51–19.83, Figure 1). There were 127 patients treated with intravenous thrombolysis. Fifty‐nine patients (25.8%) had cardioembolic stroke, 93 (40.6%) intracranial large artery stroke, 63 (27.5%) small vessel stroke, and 15 (6.1%) were cryptogenic. Of the stroke subtypes, cardio embolic and small vessel disease patients had the highest rate of WMH (72% and 81%, respectively) compared to large vessel occlusions (63%) and cryptogenic strokes (51%). There were no significant differences between Chinese and Caucasian populations regarding age, gender, risk factors, baseline NIHSS, stroke etiology, and outcome (Table 1).
Figure 1.
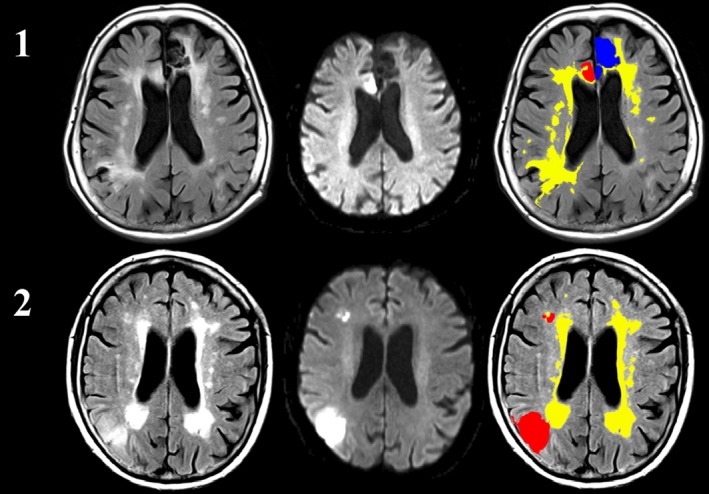
A 24‐h MRI in a patient with extensive WMH (1) and a patient with extensive WMH (yellow) and a relatively small anterior cerebral artery stroke (red) that subsequently hemorrhaged (blue, 2).
Table 1.
Baseline characteristics of controls and stroke subtypes
| Cardio embolic | Large Artery | Other/Cryptogenic | SVD | WMHP patients | |
|---|---|---|---|---|---|
| Patients | 59 | 93 | 14 | 63 | 89 |
| Age | 71 | 70 | 63 | 60 | 69 |
| Median NIHSS | 10 | 16 | 11 | 9 | 12 |
| Median 90 day mRS | 2 | 3 | 2 | 1 | 2 |
| Hypertension | 61% | 66% | 62% | 57% | 68% |
| AF (past or acutely) | 38% | 41% | 18% | 3% | 39% |
| Hypercholesterolemia | 33% | 30% | 26% | 30% | 35% |
| DM | 15% | 19% | 22% | 26% | 29% |
| WMH volume | 8.72 mL (IQR 4.21–32.97) | 7.51 mL (IQR 3.19–28.13) | 7.91 mL (IQR 3.1–19.4) | 14.11 mL (IQR 9.71–28.51) | 18.37 mL (IQR 11.13– 25.61) |
Statistical comparison is performed using Fisher's exact test for categorical variables and Kruskal–Wallis test for continuous variables.
White matter hyperintensity (WMH) was defined as a MRI FLAIR lesion >5 mL.
White matter hypoperfusion (WMHP) was defined as CBF <50% of median stroke baseline values to define contralateral.
In univariate analysis, individuals with acute lacunar stroke had larger WMH volumes (lacunar stroke 14.1 mL, IQR 9.7–28.5, cardioembolic 8.7 mL, IQR 4.2–32.0; large artery 7.5 mL, IQR 3.2–28.1; other 7.9 mL, IQR 3.1–19.4, Table 1). In multivariate logistic regression analysis, patients with WMH were more likely to have lacunar stroke than cardioembolic (OR = 1.97), large artery (OR = 2.09), or other (OR = 2.87) stroke subtypes (P = 0.007). Age was the only other significant predictor of WMH (OR 1.89, P = 0.003), and Chinese descent was not significantly associated with the presence of WMH (OR 1.27, P = 0.193).
Patients with WMH on FLAIR had reduced CBF in the hemisphere contralateral to the acutely ischemic region compared to patients without WMH (12.9 vs. 17.1/100 g/min P < 0.001) and CBV (2.9 vs. 3.4 mL/100 g P < 0.001). Patients with WMHP were significantly more likely to have WMH (OR 7.61, P < 0.001).
Patients with acute large artery occlusion with significant WMHP had reduced penumbral volume (mean 12 mL vs. 39 mL P < 0.001) and larger infarct cores compared to patients without WMHP (mean 23 mL vs. 11 mL P < 0.001). Patients with WMHP also had more infarct growth from acute to 24‐h imaging (mean growth 13 mL vs. 28 mL, P < 0.001).
There were 46 patients with any HT hemorrhage on follow‐up MR, 7 hemorrhagic infarction 1 (HI1), 11 hemorrhagic infarction 2 (HI2), 13 parenchymal hemorrhage 1 (PH1), and 15 parenchymal hemorrhage 2 (PH2). Overall, 14 (77%) the HT patients and 25 (89%) of the PH patients received IV thrombolysis. Table 2 shows the clinical and imaging characteristics in the stroke patients with and without HT. There was no significant difference in the rate of HI or PH between Caucasians and Chinese (17% vs. 24% P = 0.191). HI and PH patients had higher WMH volumes (18.3 mL vs. 7.4 mL P < 0.001) and contralateral WMHP (8.4 mL vs. 21 mL, P < 0.001). WMHP lesion volume was significantly associated with PH (r 2 = 0.417, 95% p 0.002).
Table 2.
Clinical and neuroimaging characteristics between patients with and without haemorrhagic transformation
| Haemorrhage | Non Haemorrhage | |
|---|---|---|
| Patients | 46 | 183 |
| Age | 68 | 68 |
| Median NIHSS | 14 | 9 |
| Median 90 day mRS | 3 | 2 |
| Reperfusion therapy | 39 (84%) | 90 (49%) |
| Hypertension | 67% | 52% |
| AF | 32% | 28% |
| Cholesterol | 30% | 26% |
| DM | 17% | 17% |
| Mean 24 h infarct | 35 mL | 29 mL |
| Mean WMH volume | 21 mL (IQR 12.24–31.19) | 8.42 mLa (IQR 1.51–17.83) |
| Mean CBF | 12.9 ± 6.1 | 17.1 ± 6.6 |
| Mean CBV | 1.8 ± 0.9 | 2.8 ± 0.5 |
Statistical comparison is performed using Fisher's exact test for categorical variables and Kruskal–Wallis test for continuous variables.
P < 0.001.
Next, we compared WMHP volume to other predictors of HT or PH, such as acute infarct core volume (r 2 = 0.341, P = 0.034, Figure 2), very low ischemic hemisphere CBV volume (r 2 = 0.249, P = 0.026), severely delayed perfusion lesion volume (Tmax>14 second, r 2 = 0.372, P = 0.011). In a backwards multivariate analysis including the significant univariate predictors of PH, only contralateral WMHP volume (P < 0.001) and ischemic hemisphere Tmax>14 second lesion volume (P < 0.014) remained significant predictors (overall R 2 = 0.488 P < 0.001).
Figure 2.
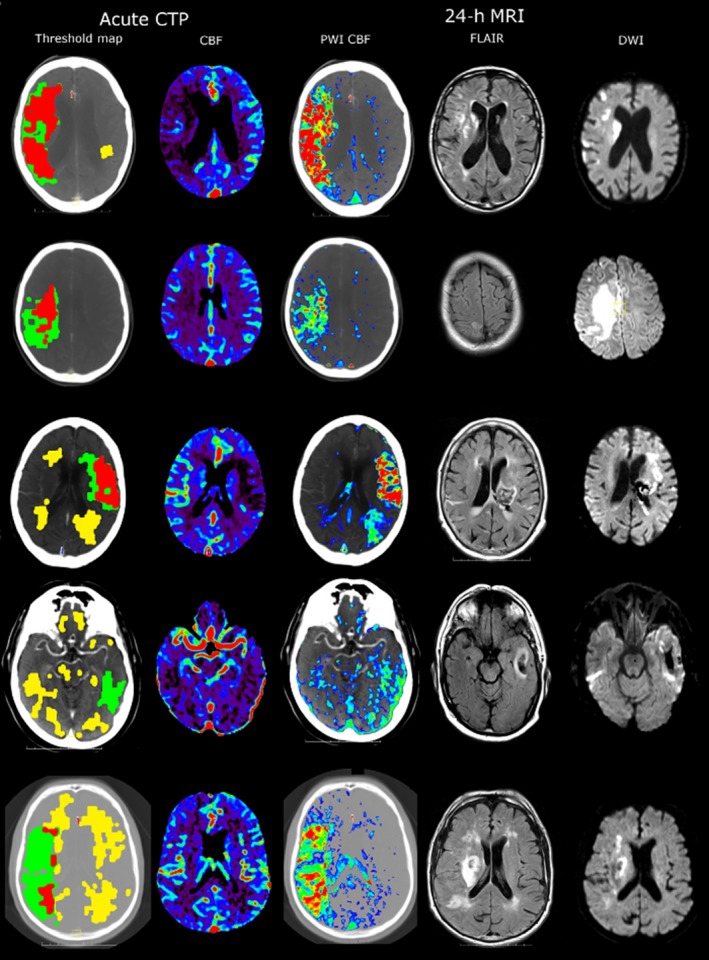
Five patients scanned with acute CTP showing the penumbra map (red is ischemic core, green is penumbra, and yellow is white matter hypoperfusion) and CBF. Patients also underwent a 24‐h MRI with perfusion (CBF map shown) a FLAIR and DWI scans. Patients with more extensive white matter hypoperfusion (first column, yellow areas for patients 3–5) have HT.
Discussion
Our data support the hypothesis that white matter hypoperfusion (WMHP) was associated with WMH on MRI and, like WHM, was associated with acute lacunar infarction, and a higher risk of HT. We also found, for the first time, that WMHP, remote to the acutely ischemic region, was associated with larger acute infarct core and smaller penumbral volumes, as well as greater infarct growth. These results suggest that WMHP is a marker of chronic small vessel disease, which may make patients more susceptible to acute small vessel infarction, more vulnerable to accelerated infarct expansion with acute occlusion, and to reperfusion complications such as HT. Finally, this has implications for clinical practice as it is possible to assess for chronic small vessel disease in the hyperacute stroke situation using the much more practical CTP modality rather than MRI.
WMH lesions on MRI are associated pathologically with small vessel disease 30. It is thought that chronic hypoperfusion of the tissue and disruption of the blood–brain barrier leads to chronic leakage of plasma into the white matter 18, 31. Although the relationship between WMH and WMHP is unclear, we suggest the increased risk of hemorrhagic transformation seen in patients with a large WMHP burden may also be due to a combination of more intense acute endothelial damage additional to chronic small vessel injury. It was particularly interesting that increased WMHP volume, which appears to be a chronic marker of small vessel disease, was a stronger predictor of acute HT than a number of acute imaging predictors of HT such as very low CBV 28, 29 and acute infarct core volume. In a similar manner to the mechanism of HT in WMHP patients, our data also suggest patients with large areas of WMHP may suffer more intense white matter ischemia, which leads to large acute and subsequent infarcts.
Some study limitations should be acknowledged. Firstly, CTP does not give truly quantitative measures of CBF. Additionally, although we assume the contralateral to acute stroke hemisphere WMHP is chronic, we cannot exclude that there is some acute change in contralateral white matter perfusion, although the fact that contralateral WM perfusion values were lower in patients with acute lacunar stroke (than those with larger artery occlusions) is against this and suggests a chronic process 32. Moreover, we use the term global to describe the extent of the white matter hypoperfusion beyond the hemisphere of the ischemic lesion. This was done to avoid white matter CBF calculation errors in the affected hemisphere where the white matter tissue may be hypoperfused which would bias our baseline value. Therefore, assessing the contralateral hemisphere allowed a much more accurate and specific assessment of the baseline perfusion status.
We have identified a large burden of cerebral white matter hypoperfusion remote to the acutely ischemic region is a marker of hemorrhagic transformation and infarct size in patients with ischemic stroke. These data strongly suggest that WMHP is a marker of chronic small vessel disease. In addition to this pathophysiologic observation, our results have significant clinical implications as the volume of WMHP on acute CTP could be quantified on hyperacute CTP prior to reperfusion treatment. This would give clinicians the ability to prospectively to assess the risk of hemorrhage before reperfusion therapy.
Conflict of Interest
The authors declare no conflict of interest.
The first two authors contributed equally to this work.
References
- 1. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 2002;288:67–74. [DOI] [PubMed] [Google Scholar]
- 3. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: The rotterdam scan study. Stroke 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 4. Van Swieten JC, Kappelle LJ, Algra A, van Latum JC, Koudstaal PJ, van Gijn J. Hypodensity of the cerebral white matter in patients with transient ischemic attack or minor stroke: Influence on the rate of subsequent stroke. Dutch tia trial study group. Ann Neurol 1992;32:177–183. [DOI] [PubMed] [Google Scholar]
- 5. Henon H, Vroylandt P, Durieu I, Pasquier F, Leys D. Leukoaraiosis more than dementia is a predictor of stroke recurrence. Stroke 2003;34:2935–2940. [DOI] [PubMed] [Google Scholar]
- 6. Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 2009;72:1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kissela B, Lindsell CJ, Kleindorfer D, et al. Clinical prediction of functional outcome after ischemic stroke: The surprising importance of periventricular white matter disease and race. Stroke 2009;40:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li L, Simoni M, Kuker W, et al. Population‐based case‐control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke 2013;44:3063–3070. [DOI] [PubMed] [Google Scholar]
- 9. Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R. White matter hyperintensities on MRI in the neurologically nondiseased elderly: Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995;26:1171–1177. [DOI] [PubMed] [Google Scholar]
- 10. Rost NS, Rahman RM, Biffi A, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 2010;75:1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neumann‐Haefelin T, Hoelig S, Berkefeld J, et al. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke 2006;37:2463–2466. [DOI] [PubMed] [Google Scholar]
- 12. Palumbo V, Boulanger JM, Hill MD, Inzitari D, Buchan AM. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology 2007;68:1020–1024. [DOI] [PubMed] [Google Scholar]
- 13. Demchuk AM, Khan F, Hill MD, et al. Importance of leukoaraiosis on ct for tissue plasminogen activator decision making: Evaluation of the ninds rt‐pa stroke study. Cerebrovasc Dis 2008;26:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyazawa N, Satoh T, Hashizume K, Fukamachi A. Xenon contrast ct‐cbf measurements in high‐intensity foci on t2‐weighted mr images in centrum semiovale of asymptomatic individuals. Stroke 1997;28:984–987. [DOI] [PubMed] [Google Scholar]
- 15. Cao W, Cheng X, Li H, Wang L, Zhang X, Dong Q. Evaluation of cerebrovascular reserve using xenon‐enhanced ct scanning in patients with symptomatic middle cerebral artery stenosis. J Clin Neurosci 2014;21:293–297. [DOI] [PubMed] [Google Scholar]
- 16. Hatazawa J, Shimosegawa E, Satoh T, Toyoshima H, Okudera T. Subcortical hypoperfusion associated with asymptomatic white matter lesions on magnetic resonance imaging. Stroke 1997;28:1944–1947. [DOI] [PubMed] [Google Scholar]
- 17. De Cristofaro MT, Mascalchi M, Pupi A, et al. Subcortical arteriosclerotic encephalopathy: Single photon emission computed tomography‐magnetic resonance imaging correlation. Am J Physiol Imaging 1990;5:68–74. [PubMed] [Google Scholar]
- 18. O'Sullivan M, Lythgoe DJ, Pereira AC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 2002;59:321–326. [DOI] [PubMed] [Google Scholar]
- 19. Marstrand JR, Garde E, Rostrup E, et al. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke 2002;33:972–976. [DOI] [PubMed] [Google Scholar]
- 20. Huynh TJ, Murphy B, Pettersen JA, et al. Ct perfusion quantification of small‐vessel ischemic severity. AJNR Am J Neuroradiol 2008;29:1831–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 22. Bivard A, Levi C, Spratt N, Parsons M. Perfusion CT in acute stroke: A comprehensive analysis of infarct and penumbra. Radiology 2013;267:543–550. [DOI] [PubMed] [Google Scholar]
- 23. Bivard A, Levi C, Krishnamurthy V, et al. Defining acute ischemic stroke tissue pathophysiology with whole brain CT perfusion. J Neuroradiol 2014;41:307–315. [DOI] [PubMed] [Google Scholar]
- 24. Law M, Saindane AM, Ge Y, et al. Microvascular abnormality in relapsing–remitting multiple sclerosis: perfusion MR imaging findings in normal‐appearing white matter. Radiology 2004;231:645–652. [DOI] [PubMed] [Google Scholar]
- 25. Renou P, Sibon I, Tourdias T, et al. Reliability of the ecass radiological classification of postthrombolysis brain haemorrhage: A comparison of ct and three mri sequences. Cerebrovasc Dis 2010;29:597–604. [DOI] [PubMed] [Google Scholar]
- 26. Thomalla G, Hartmann F, Juettler E, et al. Clinical Trial Net of the German Competence Network Stroke. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann Neurol 2010;68:435–445. [DOI] [PubMed] [Google Scholar]
- 27. Singer OC, Humpich MC, Fiehler J, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion‐weighted magnetic resonance imaging. Ann Neurol 2008;63:52–60. [DOI] [PubMed] [Google Scholar]
- 28. Campbell BC, Christensen S, Butcher KS, et al. Regional very low cerebral blood volume predicts hemorrhagic transformation better than diffusion‐weighted imaging volume and thresholded apparent diffusion coefficient in acute ischemic stroke. Stroke 2010;41:82–88. [DOI] [PubMed] [Google Scholar]
- 29. Yassi N, Parsons MW, Christensen S, et al. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke 2013;44:3039–3043. [DOI] [PubMed] [Google Scholar]
- 30. Van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain 1991;114:761–774. [DOI] [PubMed] [Google Scholar]
- 31. Topakian R, Barrick TR, Howe FA, Markus HS. Blood‐brain barrier permeability is increased in normal‐appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 2010;81:192–197. [DOI] [PubMed] [Google Scholar]
- 32. Lin L, Bivard A, Levi CR, Parsons MW. Comparison of computed tomographic and magnetic resonance perfusion measurements in acute ischemic stroke. Stroke 2014;45:1727–1732. [DOI] [PubMed] [Google Scholar]


