Summary
Aims
Transgenic TGR(ASrAOGEN)680 (TGR) rats with specific downregulation of glial angiotensinogen (AOGEN) synthesis develop cardiovascular deficits, anxiety, altered response to stress, and depression. Here, we evaluated whether these deficits are associated with alteration of the integrity of the noradrenergic system originating from locus coeruleus (LC) neurons.
Methods
Adult TGR rats were compared to control Sprague Dawley rats in terms of the following: tissue levels of transcripts encoding noradrenergic markers, tissue tyrosine hydroxylase (TH) protein level, in vivo TH activity, density of TH‐containing fibers, behavioral response to novelty, locomotor activity, and polysomnography.
Results
TH expression was increased in the LC of TGR rats compared to controls. In LC terminal fields, there was an increase in density of TH‐containing fibers in TGR rats that was associated with an elevation of in vivo TH activity. TGR rats also displayed locomotor hyperactivity in response to novelty. Moreover, polysomnographic studies indicated that daily paradoxical sleep duration was increased in TGR rats and that the paradoxical sleep rebound triggered by total sleep deprivation was blunted in these rats.
Conclusions
Altogether, these results suggest that disruption of astroglial AOGEN synthesis leads to cardiovascular, cognitive, behavioral, and sleep disorders that might be partly due to LC dysfunction.
Keywords: Angiotensin, Astrocytes, Brainstem, Transgenic rats, Tyrosine hydroxylase
Introduction
There is growing evidence that the brain renin–angiotensin system (RAS) plays a critical role in hypertension, cardiac hypertrophy, heart failure, Alzheimer's disease, stroke, alcoholism, and depression 1, 2. Thus, the brain RAS could be a versatile therapeutic target. Therefore, it is essential to identify how alterations of specific brain RAS components can affect the function of other brain systems.
Several rodent models have been engineered to discriminate the role of brain‐derived angiotensins from that of their circulating counterparts, and to determine how a specific perturbation in brain RAS function results in disease 3, 4, 5, 6, 7, 8. One of the most characterized models, the TGR(ASrAOGEN)680 (TGR) rat, was developed based on the observation that the main source of brain angiotensinogen (AOGEN), the only known precursor of brain‐derived angiotensins, is provided by astrocytes 9, 10. In adult TGR rats, brain levels of AOGEN and of two of its bioactive metabolites, angiotensin (Ang)‐II and Ang‐(1‐7), are reduced by ~90% compared to control rats 8, 11, without any alteration in plasma AOGEN, Ang‐I, Ang‐II and Ang‐(1‐7) levels, due to the expression of an AOGEN antisense RNA (AS RNA) which is under the control of the glial fibrillary acidic protein (GFAP) gene promoter 8. The AS RNA is highly expressed in the brain of TGR rats and its expression pattern is similar to that of the endogenous GFAP promoter. Adult TGR rats display obvious signs of brain RAS dysfunction, including reduced behavioral response to intracerebroventricular infusions of renin and Ang‐II, and decreased plasma level of vasopressin 8, 12. These rats also develop profound cardiovascular 8, 13, 14, 15, 16 and metabolic 16 dysfunctions, and exhibit behavioral deficits, that is, increased sensitivity to stress and anxiety 17, 18, 19. Although these behavioral deficits could be related to brainstem noradrenergic dysfunction 20, 21, 22, the neurophysiological and neurochemical integrity of locus coeruleus (LC) neurons, the main source of noradrenaline (NA) to the brain 23, has not been investigated in TGR rats, so far.
It has long been suggested that the brain RAS exerts its functions by targeting hypothalamic and brainstem noradrenergic neurons 24, 25, 26, 27, 28. Accordingly, our laboratory presented data suggesting that noradrenergic neurons of the nucleus tractus solitarius are targeted by the brain RAS 29. Indeed, we demonstrated in TGR that chronic glial AOGEN depletion in rats results in alteration of the ontogeny of these neurons. Brainstem LC neurons might also be important mediators of the effects of the brain RAS. Indeed, initial studies performed 30 years ago demonstrated that intracerebroventricular injection of Ang‐II elicits an increase in NA turnover within the LC of adult rats 27, 30. Subsequently, in vitro studies revealed that exogenously applied Ang‐II depresses glutamate depolarizations and postsynaptic potentials in LC neurons 31, 32. In addition, more recent in vivo studies demonstrated that peripheral administration of angiotensin receptor antagonists alters the expression of tyrosine hydroxylase (TH), the rate‐limiting enzyme in catecholamine biosynthesis 33, within the LC of normotensive and of spontaneously hypertensive rats 34, 35, 36. However, there is no direct evidence, to date, that the endogenous brain RAS controls the catecholaminergic activity and function of LC neurons.
In this study, we used adult TGR rats to determine whether phenotypic attributes of brainstem noradrenergic LC neurons are altered when astrocytic AOGEN synthesis is specifically disrupted. These rats are a particularly relevant study model to address this question, as astrocytes are the only source of AOGEN in the LC region 37, 38.
Materials and Methods
Animals
The animal protocols used in this study were approved by the Claude Bernard University Animal Care and Use Committee (protocol # BH2013‐02) and were in accordance with the guidelines of the European Union (directive 2010/63/UE) for the care and use of laboratory animals, in compliance with the French law (decree 2013‐118). All adult (12‐week‐old males) TGR and age‐matched Hannover Sprague Dawley rats (SD; parent strain originally used for the generation of TGR rats) were obtained from our breeding colony. Breeders were originally obtained from the Max‐Delbrück Center for Molecular Medicine (MDC), Berlin‐Buch, Germany. The animals were kept at room temperature (25 ± 1°C) with a 12 h/12 h darkness (19:00–07:00 h)/light (07:00–19:00 h) cycle. They received food and water ad libitum.
Experimental Design and Tissue Preparation
In a first set of experiments, nine Sprague Dawley and nine TGR rats were anesthetized with sodium pentobarbital, then their brain was removed and quickly frozen in isopentane. Five hundred‐micrometer‐thick slices were cut throughout the pontine region using a freezing microtome and LC was micropunched bilaterally, as previously described 29. For each animal, tissue punches were pooled and homogenized in distilled water containing a cocktail of proteinase inhibitors. These LC samples were used to quantify tissue TH levels using the dot blot technique.
In a second set of experiments, five Sprague Dawley and five TGR rats were used to prepare LC tissue extracts as described above. LC punches were homogenized in RNAse‐free water, and the resulting homogenate was used to measure TH, dopamine‐β‐hydroxylase (DBH) and NA transporter (NET) gene transcript levels using RT‐qPCR.
In a third set of experiments, three rats of each experimental group were perfused transcardially with 4% paraformaldehyde. Their brain was dissected, postfixed at 4°C for 2 h, cryoprotected at 4°C in 25% sucrose and frozen in isopentane. Twenty‐five‐micrometer‐thick coronal sections were performed using a cryomicrotome (Leica) and processed free‐floating for TH immunohistological detection in the brainstem and in the forebrain.
In a fourth set of experiments, in vivo TH activity was assessed in the LC, hippocampus and frontal cortex of five TGR rats and five controls. Rats received a single i.p. injection of the aromatic amino acid decarboxylase inhibitor NSD‐1015 (100 mg/kg; Sigma‐Aldrich, St. Quentin Fallavier, France) and were decapitated 20 min later. Then, their brain was extracted and frozen in isopentane. Five hundred‐micrometer‐thick slices were cut throughout the pontine and forebrain regions of each brain using a freezing microtome, and the LC, hippocampus and frontal cortex were subsequently microdissected. Brain tissues were homogenized and processed for determination of tissue L‐DOPA concentration using high‐pressure liquid chromatography.
In a last set of experiments, we measured anxiety‐induced locomotor activity as well as daily locomotor activity in five Sprague Dawley and five TGR rats using an open‐field test. Subsequently, rats were prepared for polysomnographic recordings.
In Vitro Protocols
Tissue TH Protein Levels
Tyrosine hydroxylase protein level was determined in LC samples using an immunoautoradiographic technique, as previously described 29, 39.
Quantification of Transcript Levels
Total RNA was extracted from LC punches and the messenger RNAs (mRNAs) contained in 1 μg of purified RNA extracts were then reverse transcribed as previously described 40. smRNA was used to normalize the RT step (Morales and Bezin, patent WO2004.092414). The cDNAs obtained after RT were quantified by real‐time PCR using the 1.2 LightCycler System (Roche Diagnostics, Indianapolis, IN, USA) and the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA, USA). Results obtained for the targeted mRNAs were normalized against those obtained for the smRNA. Sequences of the different primer pairs used are listed in Table 1. All primer pairs were designed with the Primer 3 software (Whitehead Institute for Biomedical Research; http://primer3.ut.ee).
Table 1.
Sequences of the primer pairs used for qPCR
| Target cDNA | GenBank reference # | Forward primer sequence | Reverse primer sequence | Product size (base pairs) |
|---|---|---|---|---|
| DBH | NM_013158.2 | 5′‐CGA AAT CTG GAA TCC GCA TCT‐3′ | 5′‐TCA TTG CCG AAC CGG TTT ACT‐3′ | 376 |
| NET | NM_031343.1 | 5′‐TTG CTA TGC CTG ATG GTC GTT‐3′ | 5′‐AAA TCC AGC TCC CAG GGA AAA‐3′ | 258 |
| TH | NM_012740.3 | 5′‐ACT GTC CGC CCG TGA TTT TC‐3′ | 5′‐TCC CCA TTC TGT TTA CAT AGC CC‐3′ | 273 |
Tissue L‐DOPA Concentration
Catecholamines were extracted and L‐DOPA concentration measured by high‐pressure liquid chromatography followed by electrochemical detection, as previously described 29.
TH Immunohistochemistry
Tyrosine hydroxylase protein was detected by immunohistochemistry using a mouse monoclonal anti‐TH antibody (1:1000; Millipore MAB5280) and a biotinylated horse anti‐mouse IgG secondary antibody (1:1000; Vector BA 2001). At the end of the staining protocol, sections were digitized and the density of TH‐immunolabeled processes was quantified in the LC, dentate gyrus and frontal cortex using the Leica QWin™ software (Leica Microsystems, Nanterre, France), as described previously 29. Measurement boxes with constant size and location were drawn onto digitized pictures. The density of pixels which intensity was above background level was measured inside the boxes. Results were expressed as the percent surface occupied by positive pixels in the measurement boxes.
In Vivo Protocols
Locomotor Activity
An open‐field test was used to measure the novelty stress‐induced locomotor activity and daily locomotor activity of Sprague Dawley and TGR rats (n = 5 per strain). Locomotor activity was monitored in a polycarbonate cage (W260 × L410 × H200 mm; Imetronic, Pessac, France) equipped with an array of four pairs of infrared beams. The lower beams were positioned 40 mm above the floor of the cage. The activity cage was connected to a computer interface that counted photocell beam breaks. A locomotor event was recorded when two successive horizontal or vertical breaks occurred. A first set of experiments was performed to measure the locomotor activity of rats facing a new environment, which constitutes a stressful condition. For this purpose, rats were recorded for 20 min after their first introduction inside the cage; the number of movements was pooled and collected every minute during this protocol. Two days after the novelty stress test, rats were recorded continuously for 26 h in the same cage to determine whether TGR rats exhibit motor deficits; the number of movements was pooled and collected every 30 min during this protocol.
Polysomnographic Recordings
Rats were implanted for chronic sleep recordings (EMG, EcoG) and cerebellar temperature measurement (Tbr) under deep anesthesia (sodium pentobarbital, 50 mg/kg i.p.), as previously described 41. After 10 days of recovery and habituation to the recording conditions, baseline sleep–wake activity was recorded for three consecutive days using a 16‐channel digital recorder (SomnologicaTM; Flaga Group hf., Reykjavik, Iceland) and data were stored online on the hard drive of a computer. Animals were then placed in a water tank on top of a small platform and subjected to a 10‐h total sleep deprivation (TSD) 41. At the end of the TSD, rats were put back to their home cages and recorded continuously for the next 24 h. Polygraphic recordings were visually scored by 30‐second epochs, according to the classical criteria of the vigilance stages, that is, slow wave sleep (SWS), paradoxical sleep (PS) and wakefulness (Wake). Baseline Wake, SWS and PS durations were calculated from the data collected during the 72 h preceding TSD.
Statistical Analysis
Differences between control and TGR rats for TH protein concentration, the density of TH‐immunolabeled processes, mRNA levels, L‐DOPA concentration, stress‐induced locomotor activity and duration of sleep–wake stages were tested using the ANOVA I followed by a post hoc Newman–Keuls' test. Differences between control and TGR rats for the daily locomotor activity were tested using a two‐way repeated‐measures ANOVA (RM ANOVA II; time = factor 1 and strain = factor 2).
Results
TH Expression is Altered in LC Neurons of TGR Rats
Tyrosine hydroxylase protein concentration within the LC of TGR rats was 126 ± 7% of control rats (Figure 1A; P < 0.05, ANOVA I). This was associated with a + 62 ± 20% increase in TH‐mRNA level in the LC of TGR rats (Figure 1B; P < 0.05, ANOVA I). This increase seemed rather specific to TH as we could not detect any alteration of the levels of DBH and NET‐mRNAs in TGR rats compared to controls (Figure 1B). Although TH expression was increased in TGR rats, in situ distribution of the enzyme within the LC region of these rats was similar to that of controls (Figure 1C–E).
Figure 1.
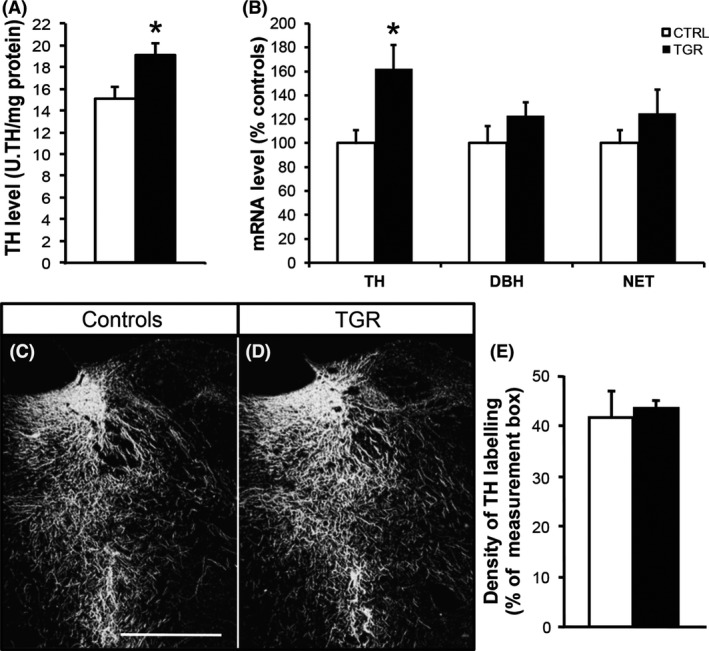
Tyrosine hydroxylase expression is altered in the locus coeruleus of TGR rats. (A) TH level is increased in the LC of TGR rats (black bars) compared to controls (white bars). Results are the mean ± SEM (n = 9). *P < 0.05, ANOVA I. (B) TH‐mRNA expression is concomitantly increased in the LC of TGR rats; however, the concentration of the mRNA encoding dopamine beta‐hydroxylase (DBH) and the NA transporter (NET) in TGR rats is similar to controls. Results are the mean ± SEM (n = 5). *P < 0.05, ANOVA I. (C and D) TH protein is detected by immunohistochemistry in the LC region in both control (C) and TGR rats (D). Scale bar: 500 μm. (E) Density of TH immunolabeling is similar in the LC of control and TGR rats. Results are the mean ± SEM (n = 3).
TH Protein Distribution is Altered in the Hippocampus and Cortex of TGR Rats
As we detected abnormally high levels of TH protein and mRNA within the LC of TGR rats, we next compared the distribution of TH protein in the hippocampus and cortex between control and TGR rats using immunohistochemistry. We specifically focused on the subgranular zone of the dentate gyrus (SGZ) and the molecular layer of the frontal cortex (MLFC), which receive dense noradrenergic innervation arising exclusively from LC neurons and are devoid of dopaminergic terminals 42, 43. We detected dense networks of TH‐immunopositive processes in the SGZ and MLFC, both in control and TGR rats (Figure 2A–D). However, the density of TH‐containing axons in these forebrain regions was dramatically increased in TGR rats compared to controls, by 101 ± 12% in the SGZ and 74 ± 14% in the MLFC (Figure 2E; P < 0.001, ANOVA I).
Figure 2.
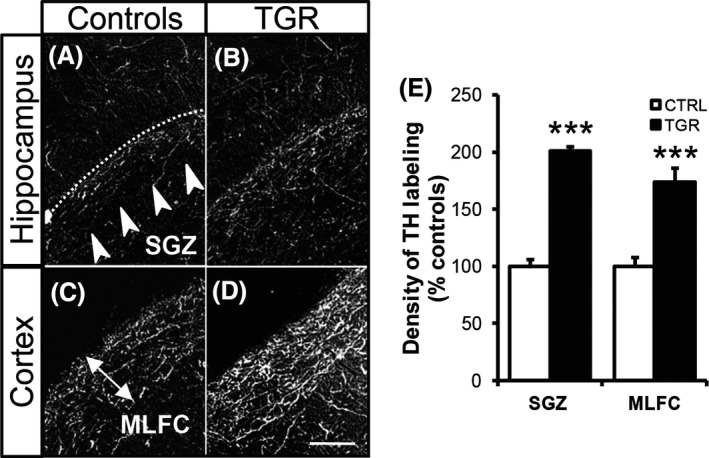
Tyrosine hydroxylase concentration and distribution are altered in the terminal fields of locus coeruleus neurons of TGR rats. TH protein is detected by immunohistochemistry within the subgranular layer of the dentate gyrus (SGZ; A,B) and the molecular layer of the frontal cortex (MLFC; C,D) in both control (A and C) and TGR rats (B and D). Scale bar: 100 μm. (E) Density of TH immunolabeling is increased in the SGZ and MLFC of TGR rats, as compared to controls. Results are the mean ± SEM (n = 3). ***P < 0.001, ANOVA I.
In Vivo TH Activity is Increased in the Hippocampus and Cortex of TGR Rats
We further investigated whether increased TH protein concentration was associated with enhanced activity of the enzyme in the LC, hippocampus and cortex of TGR rats. Although TH activity (tissue L‐DOPA accumulation after inhibition of the aromatic amino acid decarboxylase) within the LC tissue was up by 23 ± 12% in TGR rats compared to controls (controls vs. TGR: 1.65 ± 0.15 vs. 2.03 ± 0.20 ng L‐DOPA/mg tissue), which is in the range of the increase in TH protein concentration measured in this brain region (see Figure 1A), our data could not reach statistical significance (Figure 3A). In contrast, TH activity was significantly increased (P < 0.05, ANOVA I) by 55% in both the hippocampus and the cortex of TGR rats compared to controls: hippocampus (Figure 3B), 0.049 ± 0.005 (controls) versus 0.077 ± 0.006 (TGR) ng L‐DOPA/mg tissue; cortex (Figure 3C), 0.074 ± 0.005 (controls) versus 0.115 ± 0.009 (TGR) ng L‐DOPA/mg tissue.
Figure 3.
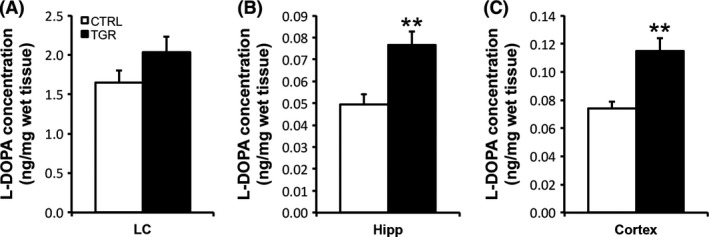
In vivo TH activity is increased in the hippocampus and cortex of TGR rats. In vivo TH activity was estimated by measuring the regional accumulation of L‐DOPA in LC (A), hippocampal (B) and cortical (C) tissues extracted from control (white bars) and TGR rats (black bars) 20 min after the injection of the DOPA decarboxylase blocking agent NSD‐1015. Although TH activity tends to be increased in the LC region of TGR rats compared to controls, there is no significant difference between the two groups of rats. In contrast, TH activity is significantly increased in the hippocampus and frontal cortex of TGR rats compared to controls. Results are the mean concentration of L‐DOPA ± SEM (n = 5). **P < 0.01, ANOVA I.
TGR Rats Exhibit Hyperactivity in Response to Novelty
Abnormal LC neurotransmission may lead to behavioral deficits related to stress adaptation 44. Indeed, release of NA within the cortex is a strong modulator of the response to stressful events. It is particularly important for adaptation to novelty 45, 46, 47. Thus, we exposed rats to a novel environment and measured their locomotor activity in response to novelty. TGR rats exhibited hyperactivity during the first 5 min of a 20‐min open‐field test, compared to controls (Figure 4A). Within this period of time, we measured abnormally high numbers of horizontal movements (locomotion) and vertical movements (rearing) in TGR rats. This was not associated with an alteration of the daily locomotor activity of TGR rats, as compared to controls (Figure 4B,C).
Figure 4.
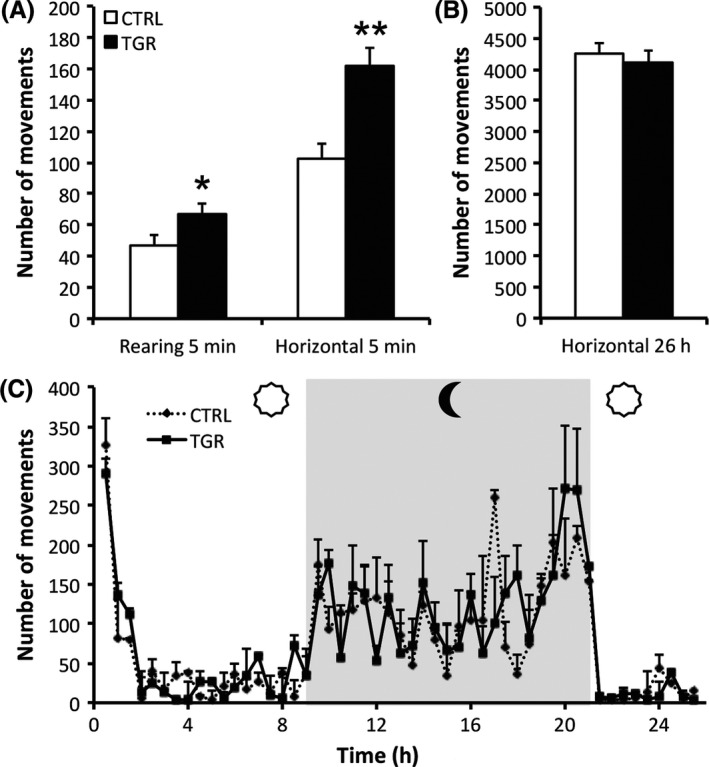
TGR rats exhibit locomotor hyperactivity in response to novelty. (A) The number of horizontal and vertical movements counted during the first 5 min of the 20‐min open‐field test is greater in TGR rats (black bars) than in controls (white bars). (B,C) The daily locomotor activity profile is not different between TGR and control rats, suggesting that the hyperactivity displayed by TGR rats in response to novelty is not the consequence of locomotor dysfunction. Results are the mean ± SEM (n = 5). *P < 0.05, **P < 0.01, ANOVA I.
Basal Paradoxical Sleep Duration and Sleep Rebound Following Total Sleep Deprivation are Altered in TGR Rats
Gonzalez et al. 48, 49 showed that the duration of the PS phase is increased in rats during the day following a 10‐h TSD. This PS rebound is, at least in part, modulated by the LC noradrenergic system. Thus, we measured the duration of wakefulness, SWS and PS during 24 h in control and TGR rats in basal conditions and one day after TSD. We found that duration of the PS phase was increased in TGR rats compared to controls in basal conditions (Figure 5A; P < 0.01, ANOVA I). TSD increased the duration of the PS phase in both control and TGR rats (Figure 5B); however, the amplitude of the post‐TSD PS rebound was blunted in TGR rats (Figure 5C; P < 0.05, ANOVA I).
Figure 5.
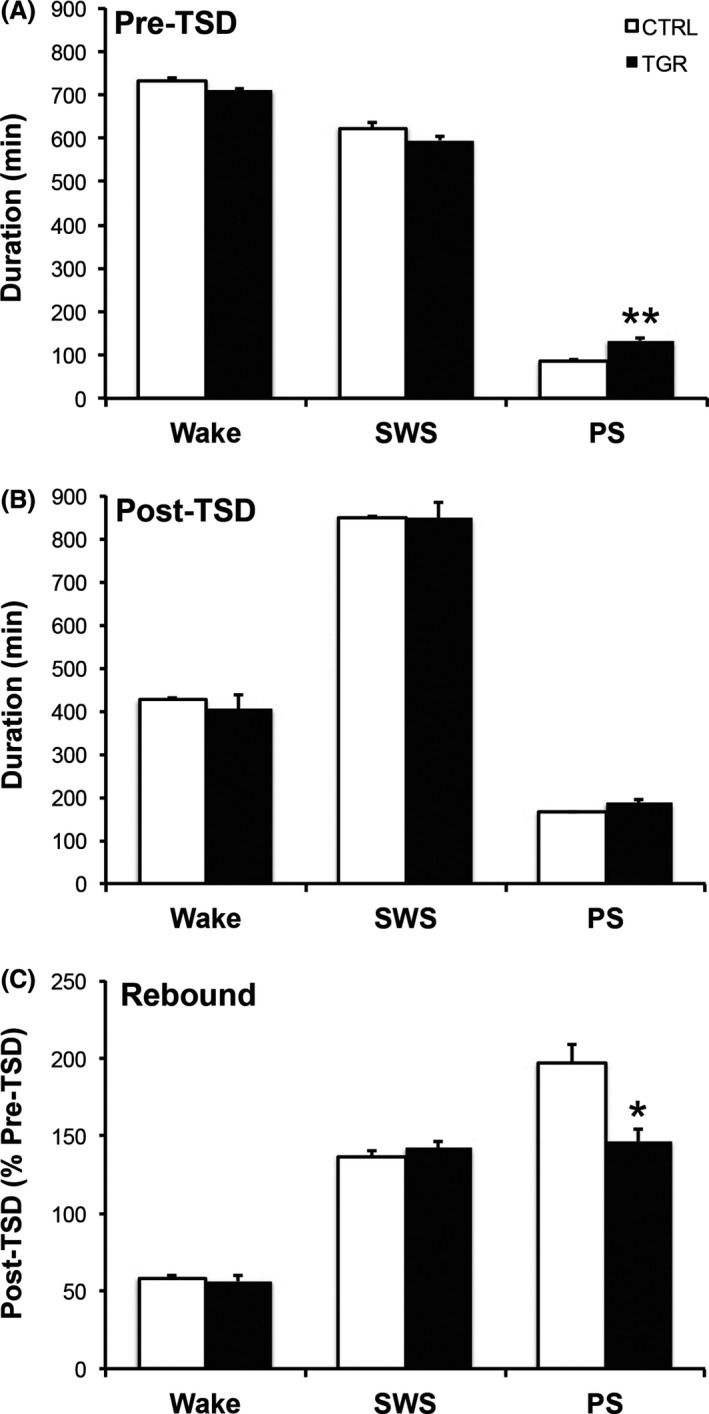
Daily paradoxical sleep duration and sleep rebound following total sleep deprivation (TSD) are altered in TGR rats. (A) Daily duration of wakefulness (Wake), slow wave sleep (SWS) and paradoxical sleep (PS) was recorded in basal conditions (baseline) in control (white bars) and TGR (black bars) rats. Baseline PS duration was significantly increased in TGR rats compared to controls. (B) There was no significant difference in the duration of Wake, SWS and PS between TGR rats and their controls during the 24 h following TSD. (C) Consequently, the ratio between PS duration after and before TSD, called PS rebound, is reduced in TGR rats compared to controls. Results are the mean ± SEM (n = 5). *P < 0.05, **P < 0.01, ANOVA I.
Discussion
The data presented here reinforce the hypothesis that LC noradrenergic neurons are a main target for the brain RAS in vivo. In addition, our results suggest that chronic brain RAS disturbances might lead to long‐term LC dysfunction and associated behavioral and cognitive alterations.
In this study, we used the TGR(ASrAOGEN) rat line 680 to explore the functional relationship between the brain RAS and the NA neurotransmitter system. Originally, two transgenic lines expressing the AS RNA transgene were analyzed in detail, the TGR680 and TGR1 8. Heterozygous animals of these lines were characterized with regard to the expression pattern of the AS RNA and pathophysiological alterations. In TGR1 rats, the transgene is transmitted exclusively to male offspring, indicating that it is most likely integrated into the Y chromosome. By contrast, transgene‐positive rats of both genders were obtained in the 680 line, proving the existence of alternative integration sites. The pathophysiological alterations observed in male TGR1 and TGR680 are similar, therefore, it is likely that they are caused by the expression of AS RNA rather than by insertional mutations.
TGR rats display behavioral deficits, that is, increased sensitivity to stress and anxiety, as well as indices of depression 17, 18, 19, 50. Furthermore, we report, here, that TGR rats exhibit sleep disorders associated with increased daily PS duration and blunted adaptation to sleep deprivation. So far, behavioral deficits detected in TGR rats have been attributed to dysfunction of the serotonin neurotransmitter system 19, 50. However, these behavioral deficits could be related to noradrenergic dysfunction as well. Indeed, the central NA neurotransmitter system is involved in stress adaptation and participates to the alternation of sleep–wake stages 20, 21, 22, 48, 49. It is also required for adequate regulation of the cardiovascular function (blood pressure and baroreflex) 51, which is disturbed in TGR rats 8, 13. Unfortunately, this particular neurotransmitter system has been largely underinvestigated in TGR rats 29. Our data show, for the first time, that TH expression is increased in the LC of TGR rats compared to controls. In addition, we detected abnormally high level of TH and increased tissue TH activity in the cortex and hippocampus of these rats, while in situ distribution and activity of the enzyme within the LC region was unaffected. Thus, our data suggest that, in TGR rats, an excess amount of TH is produced by LC neurons and preferentially targeted toward their axons, likely to support greater NA neurotransmission in remote brain areas. Although further in vivo studies are needed to support this hypothesis, enhanced release of NA in the forebrain regions innervated by LC neurons is likely to contribute to cardiovascular, behavioral and cognitive deficits exhibited by adult TGR rats (see below).
A number of studies have explored the effects of lesions or stimulation of LC neurons on stress, sleep and the cardiovascular function in rodents. 6‐OH‐DA‐induced lesions of the LC and subsequent brain NA depletion are associated with reduced exploratory behavior in novel environment paradigms 47 as well as exaggerated sleep rebound following total sleep deprivation 48, 49. Furthermore, microinjection of glutamate or homocysteic acid into the LC decreases both blood pressure and heart rate 51. Thus, it is plausible that increased brain NA neurotransmission may be associated with lower sleep rebound following sleep deprivation, diminished blood pressure and baroreflex activity as well as increased exploratory activity in novel environment tests, all of which are displayed by adult TGR rats. To further support a role for NA neurotransmission in behavioral and cardiovascular deficits developed by TGR rats, future experiments will aim at measuring if alleviation of these deficits can be achieved by treating TGR rats with NA depleting agents, downregulating TH synthesis within the LC using local siRNA injections, or by optogenetic inhibition of the activity of LC neurons.
Locus coeruleus neurons express relatively high levels of AT2 receptors 52, 53 in vivo while only limited AT1 receptor mRNA level and AT4 receptor binding sites have been detected 54, 55. However, little is known about how AT2 receptors influence the activity of LC neurons in vivo. Recent work has demonstrated that intracerebroventricular injection of the AT2 receptor agonist CGP42112 decreases the hypothalamic NA release triggered by electrical stimulation of the LC 56, but the underlying mechanisms have not been investigated. A series of in vitro studies have established that hypothalamic and brainstem noradrenergic neurons isolated from newborn rats coexpress AT1 and AT2 angiotensin receptor subtypes in vitro, both of which are activated following treatment with Ang‐II 57, 58. These studies revealed that chronic treatment with Ang‐II increases the expression of TH protein through AT1 receptor stimulation and subsequent activation of the MAP kinase intracellular pathway; on the other hand, activation of AT2 receptors by Ang‐II in these neurons has an inhibitory effect on the MAP kinase pathway. Thus, AT2 stimulation could potentially decrease TH expression in noradrenergic neurons, especially in LC neurons. The results presented here are in line with this hypothesis. Indeed, we found that drastic downregulation of brain AOGEN synthesis, associated with low levels of brain angiotensins 8, 11, results in increased TH expression within LC neurons and enhanced density of TH‐containing axons within the cortex and hippocampus of TGR rats. To determine if hypostimulation of AT2 receptors is directly responsible for such alterations, future studies might investigate whether (1) expression of AT2 receptors in the LC is maintained or downregulated in LC neurons of TGR rats and, if maintained, (2) systemic or local (intracerebral injection) treatment with agonists that are highly specific to AT2 receptors, and do not bind to AT1 receptors, can restore TH expression in adult TGR rats down to control level.
The authors acknowledge that the work presented here has few methodological and technical limitations. Indeed, as the transgenic line used has constitutive expression of the antisense transgene, it is difficult to determine whether the effects of brain AOGEN depletion on TH expression and distribution are set during development or are ongoing. Nevertheless, prior studies from our laboratory have demonstrated that TH expression and distribution are altered in the medulla oblongata of 4‐week‐old TGR rats 29. Thus, it is reasonable to assume that the phenotypic alterations of LC noradrenergic neurons detected in adult TGR rats may be due to both developmental and ongoing effects of brain AOGEN depletion.
Conclusion
Altogether, we provide strong evidence indicating that glia‐derived angiotensin peptides play a critical role in the regulation of TH gene expression in LC neurons. In addition, our results suggest that noradrenergic dysfunction associated with disruption of the brain RAS may be responsible for cognitive and stress response deficits developed by TGR rats.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by grants from the Procope program (Deutscher Akademischer Austauschdienst, French Ministry of Foreign and European Affairs and French Ministry of Higher Education and Research).
References
- 1. Campos LA, Bader M, Baltatu OC. Brain renin‐angiotensin system in hypertension, cardiac hypertrophy, and heart failure. Front Physiol 2011;2:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med (Berl) 2008;86:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grobe JL, Grobe CL, Beltz TG, et al. The brain renin‐angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 2010;12:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain‐specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res 2001;89:365–372. [DOI] [PubMed] [Google Scholar]
- 5. Morimoto S, Cassell MD, Sigmund CD. Glia‐ and neuron‐specific expression of the renin‐angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem 2002;277:33235–33241. [DOI] [PubMed] [Google Scholar]
- 6. Morimoto S, Cassell MD, Sigmund CD. The brain renin‐angiotensin system in transgenic mice carrying a highly regulated human renin transgene. Circ Res 2002;90:80–86. [DOI] [PubMed] [Google Scholar]
- 7. Sakai K, Chapleau MW, Morimoto S, Cassell MD, Sigmund CD. Differential modulation of baroreflex control of heart rate by neuron‐ vs. glia‐derived angiotensin II. Physiol Genomics 2004;20:66–72. [DOI] [PubMed] [Google Scholar]
- 8. Schinke M, Baltatu O, Bohm M, et al. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci USA 1999;96:3975–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sernia C. Location and secretion of brain angiotensinogen. Regul Pept 1995;57:1–18. [DOI] [PubMed] [Google Scholar]
- 10. Stornetta RL, Hawelu‐Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science 1988;242:1444–1446. [DOI] [PubMed] [Google Scholar]
- 11. Gomes da Silva AQ, Xavier CH, Campagnole‐Santos MJ, et al. Cardiovascular responses evoked by activation or blockade of GABA(A) receptors in the hypothalamic PVN are attenuated in transgenic rats with low brain angiotensinogen. Brain Res 2012;1448:101–110. [DOI] [PubMed] [Google Scholar]
- 12. Monti J, Schinke M, Bohm M, Ganten D, Bader M, Bricca G. Glial angiotensinogen regulates brain angiotensin II receptors in transgenic rats TGR(ASrAOGEN). Am J Physiol Regul Integr Comp Physiol 2001;280:R233–R240. [DOI] [PubMed] [Google Scholar]
- 13. Baltatu O, Janssen BJ, Bricca G, et al. Alterations in blood pressure and heart rate variability in transgenic rats with low brain angiotensinogen. Hypertension 2001;37:408–413. [DOI] [PubMed] [Google Scholar]
- 14. Caligiorne SM, Silva AQ, Fontes MA, et al. Baroreflex control of heart rate and renal sympathetic nerve activity in rats with low brain angiotensinogen. Neuropeptides 2008;42:159–168. [DOI] [PubMed] [Google Scholar]
- 15. Diz DI, Jessup JA, Westwood BM, et al. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin Exp Pharmacol Physiol 2002;29:473–482. [DOI] [PubMed] [Google Scholar]
- 16. Kasper SO, Carter CS, Ferrario CM, et al. Growth, metabolism, and blood pressure disturbances during aging in transgenic rats with altered brain renin‐angiotensin systems. Physiol Genomics 2005;23:311–317. [DOI] [PubMed] [Google Scholar]
- 17. Muller H, Kroger J, Johren O, et al. Stress sensitivity is increased in transgenic rats with low brain angiotensinogen. J Endocrinol 2010;204:85–92. [DOI] [PubMed] [Google Scholar]
- 18. Voigt JP, Bader M, Bert B, Fink H, Rex A. Anxiety‐related behaviour of low brain angiotensinogen transgenic rats in the canopy test. Pharmacol Biochem Behav 2010;96:429–437. [DOI] [PubMed] [Google Scholar]
- 19. Voigt JP, Hortnagl H, Rex A, van Hove L, Bader M, Fink H. Brain angiotensin and anxiety‐related behavior: The transgenic rat TGR(ASrAOGEN)680. Brain Res 2005;1046:145–156. [DOI] [PubMed] [Google Scholar]
- 20. Durant C, Christmas D, Nutt D. The pharmacology of anxiety. Curr Top Behav Neurosci 2010;2:303–330. [DOI] [PubMed] [Google Scholar]
- 21. Goddard AW, Ball SG, Martinez J, et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety 2010;27:339–350. [DOI] [PubMed] [Google Scholar]
- 22. Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol 2010;22:355–361. [DOI] [PubMed] [Google Scholar]
- 23. Berridge CW, Waterhouse BD. The locus coeruleus‐noradrenergic system: Modulation of behavioral state and state‐dependent cognitive processes. Brain Res Brain Res Rev 2003;42:33–84. [DOI] [PubMed] [Google Scholar]
- 24. Camacho A, Phillips MI. Separation of drinking and pressor responses to central angiotensin by monoamines. Am J Physiol 1981;240:R106–R113. [DOI] [PubMed] [Google Scholar]
- 25. Gelband CH, Sumners C, Lu D, Raizada MK. Angiotensin receptors and norepinephrine neuromodulation: Implications of functional coupling. Regul Pept 1997;72:139–145. [DOI] [PubMed] [Google Scholar]
- 26. Marques‐Lopes J, Pinho D, Albino‐Teixeira A, Tavares I. The hyperalgesic effects induced by the injection of angiotensin II into the caudal ventrolateral medulla are mediated by the pontine A5 noradrenergic cell group. Brain Res 2010;1325:41–52. [DOI] [PubMed] [Google Scholar]
- 27. Sumners C, Phillips MI. Central injection of angiotensin II alters catecholamine activity in rat brain. Am J Physiol 1983;244:R257–R263. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka J, Miyakubo H, Hayashi Y. Decreased monoamine release in the median preoptic area following ventricular treatment with the angiotensin II antagonist saralasin in normotensive and spontaneously hypertensive rats. Regul Pept 2002;107:71–77. [DOI] [PubMed] [Google Scholar]
- 29. Ogier M, Bezin L, Cottet‐Emard JM, et al. Delayed maturation of catecholamine phenotype in nucleus tractus solitarius of rats with glial angiotensinogen depletion. Hypertension 2003;42:978–984. [DOI] [PubMed] [Google Scholar]
- 30. Speth RC, Grove KL, Rowe BP. Angiotensin II and the locus coeruleus. Prog Brain Res 1991;88:217–226. [DOI] [PubMed] [Google Scholar]
- 31. Xiong H, Marshall KC. Angiotensin II modulation of glutamate excitation of locus coeruleus neurons. Neurosci Lett 1990;118:261–264. [DOI] [PubMed] [Google Scholar]
- 32. Xiong H, Marshall KC. Angiotensin II depresses glutamate depolarizations and excitatory postsynaptic potentials in locus coeruleus through angiotensin II subtype 2 receptors. Neuroscience 1994;62:163–175. [DOI] [PubMed] [Google Scholar]
- 33. Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem 1964;239:2910–2917. [PubMed] [Google Scholar]
- 34. Bregonzio C, Seltzer A, Armando I, Pavel J, Saavedra JM. Angiotensin II AT(1) receptor blockade selectively enhances brain AT(2) receptor expression, and abolishes the cold‐restraint stress‐induced increase in tyrosine hydroxylase mRNA in the locus coeruleus of spontaneously hypertensive rats. Stress 2008;11:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Macova M, Pavel J, Saavedra JM. A peripherally administered, centrally acting angiotensin II AT2 antagonist selectively increases brain AT1 receptors and decreases brain tyrosine hydroxylase transcription, pituitary vasopressin and ACTH. Brain Res 2009;1250:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saavedra JM, Armando I, Bregonzio C, et al. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress‐induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology 2006;31:1123–1134. [DOI] [PubMed] [Google Scholar]
- 37. Chai CY, Hellmann W, Tseng CJ, Luft FC, Ganten D. Angiotensinogen mRNA and pressor reactions to angiotensin in brain stem areas of spontaneously hypertensive rats. Clin Exp Hypertens 1993;15:709–725. [DOI] [PubMed] [Google Scholar]
- 38. Mungall BA, Shinkel TA, Sernia C. Immunocytochemical localization of angiotensinogen in the fetal and neonatal rat brain. Neuroscience 1995;67:505–524. [DOI] [PubMed] [Google Scholar]
- 39. Bezin L, Diaz JJ, Marcel D, et al. Controlled targeting of tyrosine hydroxylase protein toward processes of locus coeruleus neurons during postnatal development. Brain Res Mol Brain Res 1997;50:23–32. [DOI] [PubMed] [Google Scholar]
- 40. Sanchez PE, Fares RP, Risso JJ, et al. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc Natl Acad Sci USA 2009;106:9848–9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Charifi C, Paut‐Pagano L, Debilly G, Cespuglio R, Jouvet M, Valatx JL. Effect of noradrenergic denervation of the amygdala upon recovery after sleep deprivation in the rat. Neurosci Lett 2000;287:41–44. [DOI] [PubMed] [Google Scholar]
- 42. Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol 1980;189:699–710. [DOI] [PubMed] [Google Scholar]
- 43. Morrison JH, Molliver ME, Grzanna R, Coyle JT. The intra‐cortical trajectory of the coeruleo‐cortical projection in the rat: A tangentially organized cortical afferent. Neuroscience 1981;6:139–158. [DOI] [PubMed] [Google Scholar]
- 44. Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 2008;583:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oknina NB. [The dynamic behavioral changes in rats after destruction of the locus coeruleus]. Zh Vyssh Nerv Deiat Im I P Pavlova 1991; 41: 543–550. [PubMed] [Google Scholar]
- 46. Pudovkina OL, Kawahara Y, de Vries J, Westerink BH. The release of noradrenaline in the locus coeruleus and prefrontal cortex studied with dual‐probe microdialysis. Brain Res 2001;906:38–45. [DOI] [PubMed] [Google Scholar]
- 47. Velley L, Mormede P, Kempf E. Neurochemical lesion of the nucleus locus coeruleus increases neophobia in a specific exploration task but does not modify endocrine response to moderate stress. Pharmacol Biochem Behav 1988;29:1–7. [DOI] [PubMed] [Google Scholar]
- 48. Gonzalez MM, Debilly G, Valatx JL. Noradrenaline neurotoxin DSP‐4 effects on sleep and brain temperature in the rat. Neurosci Lett 1998;248:93–96. [DOI] [PubMed] [Google Scholar]
- 49. Gonzalez MM, Valatx JL, Debilly G. Role of the locus coeruleus in the sleep rebound following two different sleep deprivation methods in the rat. Brain Res 1996;740:215–226. [DOI] [PubMed] [Google Scholar]
- 50. Kangussu LM, Almeida‐Santos AF, Bader M, et al. Angiotensin‐(1‐7) attenuates the anxiety and depression‐like behaviors in transgenic rats with low brain angiotensinogen. Behav Brain Res 2013;257:25–30. [DOI] [PubMed] [Google Scholar]
- 51. Murase S, Takayama M, Nosaka S. Chemical stimulation of the nucleus locus coeruleus: Cardiovascular responses and baroreflex modification. Neurosci Lett 1993;153:1–4. [DOI] [PubMed] [Google Scholar]
- 52. Lenkei Z, Palkovits M, Corvol P, Llorens‐Cortes C. Distribution of angiotensin II type‐2 receptor (AT2) mRNA expression in the adult rat brain. J Comp Neurol 1996;373:322–339. [DOI] [PubMed] [Google Scholar]
- 53. Nuyt AM, Lenkei Z, Palkovits M, Corvol P, Llorens‐Cortes C. Ontogeny of angiotensin II type 2 receptor mRNA expression in fetal and neonatal rat brain. J Comp Neurol 1999;407:193–206. [PubMed] [Google Scholar]
- 54. Lenkei Z, Palkovits M, Corvol P, Llorens‐Cortes C. Distribution of angiotensin type‐1 receptor messenger RNA expression in the adult rat brain. Neuroscience 1998;82:827–841. [DOI] [PubMed] [Google Scholar]
- 55. von Bohlen und Halbach O. Angiotensin IV in the central nervous system. Cell Tissue Res 2003;311:1–9. [DOI] [PubMed] [Google Scholar]
- 56. Gong WK, Lu J, Wang F, Wang B, Wang MY, Huang HP. Effects of angiotensin type 2 receptor on secretion of the locus coeruleus in stress‐induced hypertension rats. Brain Res Bull 2015;111:62–68. [DOI] [PubMed] [Google Scholar]
- 57. Huang XC, Richards EM, Sumners C. Mitogen‐activated protein kinases in rat brain neuronal cultures are activated by angiotensin II type 1 receptors and inhibited by angiotensin II type 2 receptors. J Biol Chem 1996;271:15635–15641. [DOI] [PubMed] [Google Scholar]
- 58. Yang H, Lu D, Yu K, Raizada MK. Regulation of neuromodulatory actions of angiotensin II in the brain neurons by the Ras‐dependent mitogen‐activated protein kinase pathway. J Neurosci 1996;16:4047–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]


