Summary
Aims
We evaluate safety and efficacy of autologous bone marrow‐derived mesenchymal stem cells (MSCs) as a potential treatment for neuromyelitis optica spectrum disorder (NMOSD).
Methods
Fifteen patients with NMOSD were recruited. All patients received a single intravenous infusion of 1.0 × 108 autologous MSC within 3–4 generations derived from bone marrow. The primary endpoints of the study were efficacy as reflected by reduction in annualized relapse rates (ARRs) and inflammatory lesions observed by MRI.
Results
At 12 months after MSC infusion, the mean ARR was reduced (1.1 vs. 0.3, P = 0.002), and the T2 or gadolinium‐enhancing T1 lesions decreased in the optic nerve and spinal cord. Disability in these patients was reduced (EDSS, 4.3 vs. 4.9, P = 0.021; visual acuity, 0.4 vs. 0.5, P = 0.007). The patients had an increase in retinal nerve fiber layer thickness, optic nerve diameters and upper cervical cord area. We did not identify any serious MSC‐related adverse events. At 24 months of MSC infusion, of 15 patients, 13 patients (87%) remained relapse‐free, the mean ARR decreased to 0.1; the disability of 6 patients (40%) was improved, and the mean EDSS decreased to 4.0.
Conclusions
This pilot trial demonstrates that MSC infusion is safe, reduces the relapse frequency, and mitigates neurological disability with neural structures in the optic nerve and spinal cord recover in patients with NMOSD. The beneficial effect of MSC infusion on NMOSD was maintained, at least to some degree, throughout a 2‐year observational period.
Keywords: Mesenchymal stem cells, Neuromyelitis optica spectrum disorder, Recovery, Relapse, Treatment
Introduction
Neuromyelitis optica (NMO), and its spectrum disorders, are severe inflammatory central nervous system (CNS) disorders that clinically manifests with attacks of optic neuritis and (or) myelitis. Neuromyelitis optica spectrum disorders (NMOSDs) are characterized by autoantibodies against aquaporin 4 (AQP4) and perhaps other neuronal antigens that activate the classical complement cascade, which elicits inflammatory responses with marked granulocyte and macrophage infiltration. In turn, these inflammatory responses cause secondary oligodendrocyte damage, demyelination, as well as poor remyelination and disease recovery 1, 2. Within active NMOSD lesions, both antibody‐ and cell‐mediated immunopathology can be visualized 1, 2, 3, suggesting diverse immune effector mechanisms. Damage to the optic nerve and spinal cord are usually prominent, especially during the early stage of the disease. As a result of accumulating damage during acute attacks, NMOSD runs a fast course by progressing to a partial or complete loss of vision, and paraplegia around 5 years after disease onset in the vast majority of these patients 4, 5.
Halting damage accumulation in NMOSD poses significant challenges. Some effective MS therapies, such as interferons, natalizumab, and fingolimod, are not effective in NMOSD and, in some individuals, even exacerbate disease 6, 7, 8. Case studies and pilot trials have suggested that B‐cell depletion with rituximab, or complement inhibition with eculizumab, are promising treatment strategies 9, 10. For complex diseases such as NMOSD, immune‐mediated tissue damage is orchestrated by multiple cell types and cytokines. Targeting multiple aspects of the pathogenic mechanisms may mitigate immune damage and promote endogenous and exogenous repair mechanisms 11.
Bone marrow‐derived mesenchymal stem cells (MSCs) are adult stromal progenitor cells of mesodermal origin. MSC can differentiate into other cell types, which makes them attractive for cell replacement therapy. Additionally, these cells also exert paracrine effects by modulating the plasticity of damaged host tissues, secreting neurotrophic and survival‐promoting growth factors, restoring synaptic transmitter release, integrating into existing neural and synaptic networks, and re‐establishing functional afferent and efferent connections 12, 13. Moreover, MSCs possess strong immunomodulatory properties that inhibit the release of pro‐inflammatory cytokines from both innate and adaptive immune cells 14, 15. These potent properties of MSC have been tested in preclinical and clinical studies of several inflammatory and autoimmune diseases 16, 17, 18. The effects of intravenous autologous MSC have been investigated in pilot trials with MS patients 19, 20, 21. Of particular relevance to the present work is that MSC markedly improved visual impairments in some patients with secondary progressive MS 22. The potential dual effect of MSC on tissue repair and modulation of immune functions prompted us to initiate the current study on NMOSD.
Materials and Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol and informed consent procedures were approved by the institutional review board at Tianjin Medical University General Hospital. This study is registered with ClinicalTrials.gov, number: NCT02249676.
Study Participants
Between September 2013 and January 2015, participants were consecutively recruited into an open‐label trial at Tianjin Medical University General Hospital, Tianjin, China. Inclusion criteria were as follows: clinically definite NMO, as defined by 2006 criteria 23, or NMOSD with positive AQP4 antibody [either recurrent optic neuritis (rON) or recurrent longitudinally extensive transverse myelitis (rLETM) 24, being in remission period; expanded disability status scale (EDSS) score ≥2.5; and age ranged from 16 to 65 years. Exclusion criteria included pregnancy, breastfeeding, or plans to conceive during the course of the study (women only); history of splenectomy or asplenia (potential increased risk of meningococcal infection); and combined blood disorders, cancer, active or chronic infection within at least 3 months. Written informed consent was provided by the patients.
Procedures
Generation of Autologous MSC and Infusion
Production of autologous MSC is illustrated in Figure 1A. The phenotype of the cells was assessed by flow cytometry to confirm the expression of CD73, CD90, and CD105 surface molecules (>95%) and the absence of CD34, CD45, CD14, and CD3 (<2%), and the ability of the cells to differentiate into adipocytes and osteocytes in culture was confirmed in vitro following the 2006 International Society of Cellular Therapy's criteria 25. Viability was greater than 95% for infusion and tested negative for endotoxin, hepatitis C virus, hepatitis B virus, HIV, syphilis, fungi, Mycoplasma species, and chromosomal aberrations in the final cellular product 26. Confluent autologous MSCs at passages 3– 4 were collected in M199 culture media containing 1% human serum albumin and stored for up to 1 h at 4°C. MSC suspensions of 5 × 105/mL were transferred into 200‐mL syringes for intravenous infusion over 45 min. Each participant received single infusion of autologous MSC administered intravenously at a dose of 1 × 108 cells which had been used and shown efficacy for patients with primary progressive multiple sclerosis 20. To reduce type I hypersensitivity reactions, premedication with 10 mg chlorpheniramine, 100 mg hydrocortisone, and 10 mg metoclopramide was given 30 min before administration of the cells. After administration of cell suspensions, we infused normal saline (500 mL) over 4 h. Participants were monitored clinically for evidence of adverse reactions over a minimum of 24 h.
Figure 1.
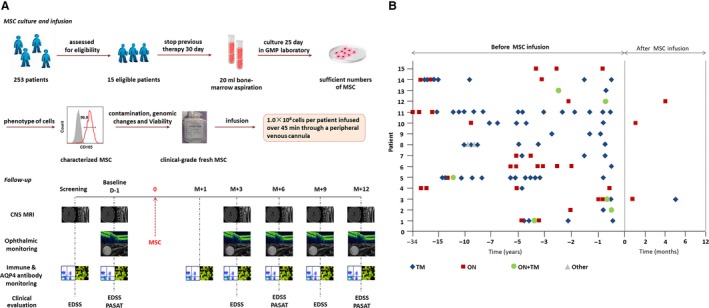
Study design and effects of MSC infusion on relapse of NMOSD. (A) Study design: Fifteen eligible patients with NMOSD were enrolled. Prior to bone marrow aspiration, all treatments with corticosteroids and other systemic immunosuppression therapies were discontinued for 30 days. Bone marrow cell aspirates (20 mL) were obtained while patients were under local anesthesia from the posterior iliac crest. Following current good manufacturing practices, mononuclear bone marrow cells were isolated by Percoll (1.073 g/mL) centrifugation and allowed to adhere to a flask for 72 h in low‐glucose Dulbecco's modified Eagle's medium (GibcoInvitrogen), and the culture medium was changed every 3 days. The phenotype of the cells was assessed by flow cytometry to confirm the expression of CD73, CD90, and CD105 surface molecules (>95%) and absence of CD34, CD45, CD14, and CD3 (<2%), and the ability of the cells to differentiate into adipocytes and osteocytes in culture was confirmed in vitro following the 2006 International Society of Cellular Therapy's criteria.25 At 70–80% confluence, cells were detached and re‐plated at 1 × 106/175 cm2 culture to process for infusion. Cell viability was determined by trypan blue staining at the end of the harvest. Viability was greater than 95% for infusion and tested negative for endotoxin, hepatitis C virus, hepatitis B virus, HIV, syphilis, fungi, Mycoplasma species, and Chlamydia before infusion. G‐banding karyotype analysis was performed to confirm the absence of chromosomal aberrations in the final cellular product.26 After MSCs were characterized in accordance with the International Society of Cellular Therapy (ISCT) recommendations,25 108 MSC of 5 × 105 cells/mL were transferred into 200‐mL syringes for intravenous infusion over a 45‐minute time period for each patient. All participants were assessed at 1 day (D‐1, baseline) before treatment and at 1 month (M + 1), 3 months (M + 3), 6 months (M + 6), 9 months (M + 9), and 12 months (M + 12) after treatment. Assessments included clinical assessment (Expanded Disability Status Scale (EDSS) and Paced Auditory Serial Addition Test [PASAT]); optical nerve, brain, and spinal cord MRI; visual evoked potential, optical coherence tomography (OCT), and ophthalmological assessments (visual acuity, visual field); serum anti‐AQP4 antibody concentrations; and lymphocyte phenotyping. (B) Frequency of relapses before and after mesenchymal stem cell (MSC) infusion, TM = transverse myelitis, ON = optic neuritis.
Follow‐up
Participants were assessed at 1 day before treatment as baseline, and at 1, 3, 6, 9, and 12 months after treatment (Figure 1A). Assessment at each time point was within an interval of less than 1 week. Assessments included three parts: functional outcomes [Expanded Disability Status Scale (EDSS), visual acuity, visual field, visual evoked potential, and Paced Auditory Serial Addition Test (PASAT)], structural outcomes [optic nerve, brain and spinal cord MRs, and optical coherence tomography (OCT)], and potential information on the mechanism (serum AQP4 antibody and immune phenotyping). Details of these assessments are described in the Data S1. Relapses were defined as new or recurrent neurological symptoms not associated with fever or infection, lasting at least 24 h, accompanied by new objective neurological findings and separated from the onset of other confirmed relapses by at least 30 days.
Safety was determined by immediate or delayed adverse events. Immediate reactions included allergic reactions (tachycardia, fever), respiratory failure, local complications (dizziness, lumbago, headache), and systemic complications (systemic infections). Delayed reactions included tumor formation.
During the second year after MSC infusion, only clinical outcomes were assessed at each visit. Participants underwent clinical ophthalmology, neurological function evaluations every 6 months.
Outcomes
The primary outcomes measure was efficacy as reflected by reduction in annual relapse rate and the number or volume of T2 and enhancing lesions over 12 months after MSC treatment. The secondary outcome was efficacy for accelerating recovery included neural function improvement (e.g., EDSS and visual acuity) and tissue restoration (e.g., retinal nerve fiber layer thickness, optic nerve area diameter, brain atrophy, and upper cervical cord area). The third outcome was safety and feasibility. The images analysis was performed by someone blinded to patient and sequence of the studies.
Statistical Analysis
Descriptive summaries are reported as means ± SE for continuous variables, mean (SD, range) for abnormal distribution variables and as frequencies (%) for categorical variables. Paired comparisons of continuous outcomes of interest before and after treatment were performed with paired t‐test, discontinuous outcomes or abnormal distribution with Wilcoxon signed rank test and categorical variables with chi‐squared test. All tests were two‐sided, and P values of less than 0.05 were considered to be significant. SPSS for Windows version 17.0 software (SPSS, Inc, Chicago, IL, USA) was used for the analysis.
Results
Study Subject Characterization
We enrolled 15 patients (NMO: rON: rLETM, 12: 1:2) with a mean age of 47 years and all patients completed follow‐up and data collecting. Baseline characteristics for patients are shown in Table 1. The mean disease duration of patients was 9 years, and 13 of the 15 patients (87%) were positive for AQP4 antibodies. In the 1–34 years before recruitment, 13 patients (25 eyes) had clinical optic neuritis and 14 patients had longitudinally extensive spinal cord lesions (≥3 vertebral segments) (Table S1). Seven patients received prednisolone for preventing relapses before MSC treatment, 6 patients received azathioprine or azathioprine plus prednisolone, and 3 patients received cyclophosphamide treatment (Table 1). All patients failed previous treatment (≥1 attack when receiving treatment) despite treatment for over 90 days of duration.
Table 1.
Baseline characteristics of patients
| Number of participants | 15 |
| Sex ratio (men: women) | 1:14 |
| Age at enrollment (years) | 47 (14; 19–63) |
| Age at disease onset | 38 (15; 15–58) |
| Duration of disease (years) | 9 (10; 1–34) |
| Diagnosis | |
| Neuromyelitis optica | 12 |
| Relapsing optic neuritis | 1 |
| Relapsing transverse myelitis | 2 |
| Aquaporin 4 antibody positivity | 13 |
| Coexisting autoimmune diseases | |
| Connective tissue disease | 2 |
| Hypothyroidism | 1 |
| Acute inflammatory demyelinating polyneuropathy | 1 |
| Annualized relapse rates | 1.1 (0.9; 0.3–4.0) |
| Expanded disability status scale score | 4.9 (2.1; 2.5–8.5) |
Data are n or mean (SD; range).
Safety
We successfully isolated and cultured MSC to the target dose from the bone marrow aspirates of all patients (1.0 × 108 cells per patient). Mean culture duration was 25 days (20–30 days). We did not record any adverse events during infusion. Only one patient developed a low‐grade fever (37.5°C) and knee pain at about 3 h after the start of infusion that resolved spontaneously over 2 h. Results of weekly blood testing of clinical chemistry and hematology during the 4 weeks after infusion were unremarkable. We did not identify tumor formation during the follow‐up phase.
Effects of MSC Infusion on Reducing Annual Relapse Rate and Inflammatory Activity
Twelve patients remained completely relapse‐free, and three patients (20%) had at least 1 relapse, with a total of 4 mild relapses among them after 12 months of MSC therapy. There was no further worsening of EDSS scores during these relapses and the new CNS symptoms and signs completely disappeared after 1 month. Among the relapses, 2 occurred within 1 month after treatment. The mean annualized relapse rates before treatment were 1.1 [0.9; 0.3–4.0 (SD, range)], whereas the mean annualized relapse rates after MSC therapy were 0.3 [0.6; 0–2.0 (SD, range)]. The mean annualized relapse rates in the 12 months before initiating BMSC therapy were 1.3 (a total of 19 relapse), and after 12 months of BMSC therapy, these decreased to 0.3, a reduction of 76.9% (Figure 1B).
As shown in Figure 2, after receiving an MSC infusion, patients had a reduced mean cumulative number of optic nerve and spinal cord lesions on T2 images or gadolinium‐enhancing T1 lesions at 12 months relative to baseline (0.2 vs. 0, P = 0.031; 0.13 vs. 0, P = 0.043, respectively, for optic nerve, and 10 vs. 7 segments, P < 0.001; 0.6 vs. 0.1, P = 0.015, respectively, for spinal cord). Although the number of T2 lesions in the brain was not significantly decreased (Figure 2G), the volume of T2 lesions in the brain was reduced (2.6 vs. 1.8 mL, P = 0.035) (Figure 2H). There were no gadolinium‐enhancing lesions observed in the brain at baseline and during the follow‐up period.
Figure 2.
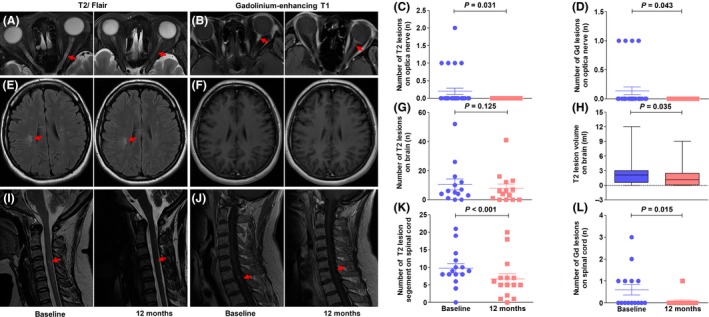
Impact of MSC infusion on inflammatory activity in the optical nerve, brain and spinal cord. Optical nerve active lesions (red arrow) were monitored on fat‐suppressed T2 (A) and gadolinium‐enhancing T1 sequences (B); MSC infusion reduced the number of T2 (C) and gadolinium‐enhancing lesions (D) in the optic nerve. Brain active lesions (red arrow) were measured on fluid‐attenuated inversion recovery (FLAIR) (E) and gadolinium‐enhancing T1 sequences (F); patients had unchanged numbers of T2 brain lesions (G) but decreased volume of T2 brain lesions after MSC treatment (H). No hyperintense signals appeared on gadolinium‐enhancing T1 imaging between the baseline and follow‐up period. Spinal cord active lesions (red arrows) were assessed on T2 (I) and gadolinium‐enhancing T1 (J) sequences. MSC reduced the number of segments of T2 lesions (K) and the number of gadolinium‐enhancing lesions in the spinal cord (L). All statistical analyses were performed by Wilcoxon signed rank test.
Evidence of Disease Recovery
We observed a reduction after treatment in general disability measured by EDSS (4.9 vs. 4.3, P = 0.021), which was prominently reflected in pyramidal and sensory function, although we did not identify a change in bowel or bladder function (Figure 3A–D). The EDSS scores were improved in 7 (47%) patients and were stabilized in 8 patients, with an average 0.6 EDSS score decrease at 12 months after MSC treatment. In contrast, EDSS scores worsened in 9 patients, were stable in 4 patients, and mildly improved in 2 patients (P < 0. 001), with an average 0.4 EDSS score increase (P = 0.002) during the 12‐month period before MSC treatment. After treatment, there was an improvement in visual acuity (0.4 vs. 0.5, P = 0.007) (Figure 3E). No significant changes were evident in visual fields (Figure 3F) and visual evoked response latency. We also identified an improvement in cognition as measured by PASAT (42 vs. 46, P = 0.003).
Figure 3.
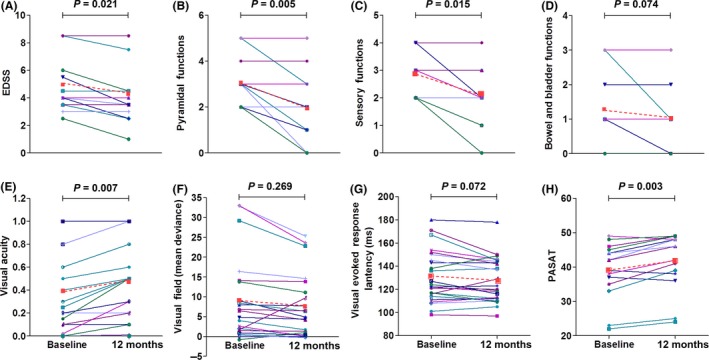
Assessment of visual and cognition functions in MSC‐treated NMOSD patients. Paired changes in EDSS and functional systems scores (A–D, n = 15 patients), visual acuity (E, n = 30 eyes), whole‐field visual (F, n = 26 eyes; 4 eyes were excluded for inadequate quality for analysis), evoked response latency (G, n = 26; 4 eyes were excluded for inadequate quality for analysis), and PASAT (H) are shown for individual patients at baseline and at 12 months after MSC treatment (solid lines). The mean changes are also shown (red dashed line). Statistical analyses were performed by Wilcoxon signed rank test. PASAT, the Paced Auditory Serial Addition Task, was used to evaluate auditory processing speed, attention, and working memory.
Because acute swelling temporarily increases the tissue volume during disease relapses, patients with acute ON or myelitis at 3 months before baseline assessment or during the follow‐up period were excluded. Imaging measures showed an increase in retinal nerve fiber layer thickness from baseline to 12 months (73 vs. 81 μm, P < 0.001), optic nerve diameter (2.3 vs. 2.6 mm, P < 0.001), and upper cervical cord area (69 vs. 73 mm2, P < 0.001) after MSC treatment (Figure 4). No changes were evident in macular volume, total cerebral volume, cerebral gray matter volume and cerebral white volume after treatment.
Figure 4.
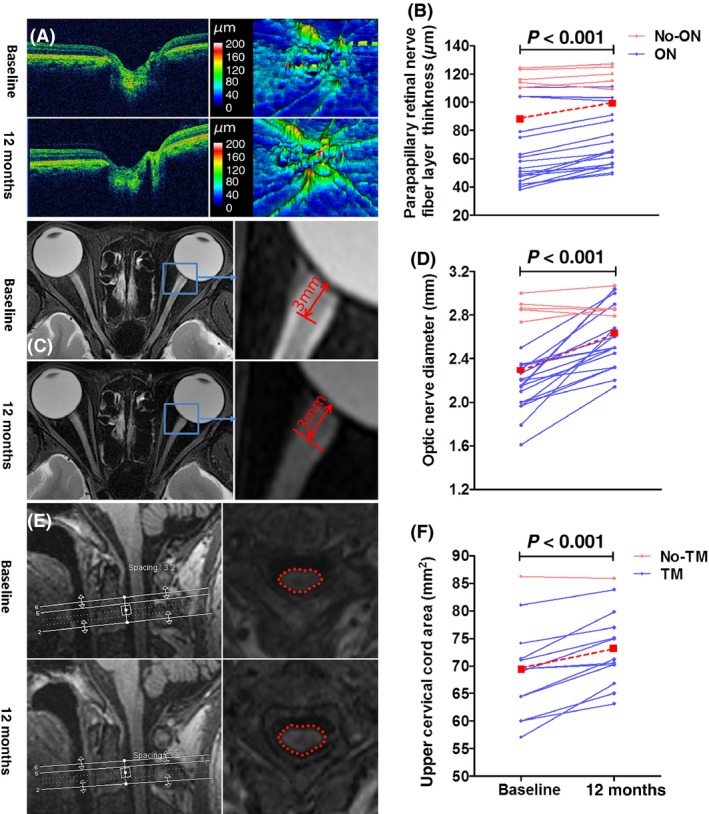
Evidence of disease recovery in MSC‐treated NMOSD patients. Color‐scale raw optical coherence tomography scan (OCT) in the papillary area (A, left) and pseudocolor thickness map generated from cube scan based on the thickness of the layers evaluated (A, right). Pseudocolor thickness map images of an eye with history of optic neuritis showed that the retinal nerve fiber layer in the nasal inferior and temporal quadrants exhibited severe thinness at baseline, whereas at 12 months after MSC treatment, the retinal nerve fiber layer in these areas had increased thickness. Compared with baseline, the retinal nerve fiber layer thickness significantly increased at 12 months (B). Statistical analysis was performed with paired t‐test, with n = 23 eyes (6 eyes were excluded due to optical neuritis relapse within the 12‐month follow‐up period, and one eye was excluded for inadequate signal strength on OCT scan), including 18 eyes with a history of optical neuritis and 5 eyes without a history of optical neuritis. The axial fat‐suppressed T2 sequence (C, left) was used to measure optic nerve diameter in an axis perpendicular to the optic nerve 3 mm (C, right). The optic nerve was severely atrophied and the optic nerve sheath appeared as a high signal surrounding a region of low signal corresponding to atrophy of the optic nerve at baseline. At 12 months after MSC treatment, optic nerve atrophy improved and the high signal of the optic nerve sheath mostly disappeared. Compared with baseline, the optic nerve diameter had significantly widened at 12 months after treatment (D). Statistical analysis was performed with paired t‐test, with n = 24 eyes (6 eyes were excluded due to optical neuritis relapse during the follow‐up phase), including 19 eyes with a history of optical neuritis and 5 eyes without a history of optical neuritis. Three‐D T1 images showing mid‐sagittal images of a NMO patient (E, right). White lines show the location of the upper cervical cord assessment. Reformatted axial slices at cervical level C2 (E, left). Note the marked reduction in the upper cervical area at baseline. At 12 months after MSC treatment, upper cervical cord atrophy had improved. Compared with baseline, the upper cervical cord area was significantly enlarged at 12 months after treatment (F). Statistical analysis was performed with paired t‐test, with n = 13 (one patient was excluded due to myelitis relapse during the follow‐up phase, and another patient was excluded for inadequate signal strength on MRI scans).
Impact of MSC Infusion on Lymphocyte Subpopulations and Serum AQP4 Antibody Concentrations
Immunological analyses indicated a decrease in CD19+ B‐cell counts and T follicular helper (Tfh)‐cell counts at 1 month after treatment, and at 3 months CD19+ B‐cell and Tfh‐cell counts returned to baseline levels. Compared with baseline, no significant changes were observed during the follow‐up period in CD4, Th1, Th17, Treg, natural killer (NK), and natural killer T (NKT)‐cell counts (Figure S1A‐C).
Compared with baseline levels, fluctuations of anti‐AQP4 antibody titers were observed starting 1 month after MSC treatment. A decrease at 3 months (40 vs. 54 FU, P = 0.035) was evident, but levels returned close to baseline at 6–12 months (Figure S1D). Interestingly, the serum levels of IL‐21 and IL‐6 were also decreased after 3 months of MSC treatment (Figure S1E,F).
The Second‐Year Follow‐Up of NMOSD Patients in Annualized Relapse Rate and Disability after MSC Infusion
Of the 15 patients, 13 showed (87%) remained relapse‐free during the second year after MSC infusion. The mean ARR was 1.1 (0.9) before treatment, and the mean (SD) ARR at second year was 0.1 (0.3) (P < 0.001) after treatment. Among all patients, the EDSS scores improved in 6 patients and stabilized in 9 during the second year after MSC treatment. The mean EDSS score was 4.9 (SD, 2.1) before MSC treatment and 4.0 (SD, 2.3) after treatment (P < 0.001). So, a follow‐up analysis during the second year of this study revealed significant improvements in relapse rates and disability which suggests that the effect of MSC therapy can last more than 1 year (Table 2).
Table 2.
A 2‐Year follow‐up on NMOSD patients after MSC infusion
| Before MSC | 1 year after infusion | 2 years after infusion | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| ARR, mean (SD; range) | 1.1 (0.9; 0.3–4.0) | 0.3 (0.6; 0–2.0) | 0.1 (0.3; 0–1.0) | 0.002 | <0.0001 | 0.894 |
| Relapse free, n (%) | 5* (33%) | 12 (80%) | 13 (87%) | 0.025 | 0.008 | 1.000 |
| EDSS, mean (SD; range) | 4.9 (2.1; 2.5–8.5) | 4.3 (2.2, 1.0–8.5) | 4.0 (2.3, 1.0–8.5) | 0.021 | <0.0001 | 0.861 |
| EDSS score change, n (%) | 0.001 | 0.002 | 0.713 | |||
| Decreased EDSS score | 2* (13%) | 7 (47%) | 6 (40%) | |||
| Stable EDSS score | 4* (27%) | 8 (53%) | 9 (60%) | |||
| Increased EDSS score | 9* (60%) | 0 | 0 |
Wilcoxon signed rank test (ARR, ESS); chi‐square test (Relapse free, EDSS score change);
*the last year before autologous mesenchymal stem cell infusion; P1 = (before MSC) versus (1 year after infusion); P2 = (before MSC) versus (2 years after infusion);
P3 = (1 year after infusion) versus (2 years after infusion).
Discussion
Challenges to the management of patients with NMOSD are related to its rapid disease progression and the limited efficacy of disease‐modifying drugs employed for MS. Development of therapies for NMOSD is impeded by the lack of sensitive and specific clinical assessment modalities designed specifically for NMOSD. Further, the low incidence of NMOSD particularly among Caucasians and wide variations in the rate of disease progression between individuals complicate clinical studies on NMOSD. In an attempt to overcome these hurdles, we and several previous studies focused on a sentinel lesion that included the retrobulbar optical nerve and the upper cervical cord and retina, which has little variability in patients and is sensitive to examination of clinical outcomes 27, 28, 29. With these approaches, we found that a single infusion of autologous MSC slows the progression, or stabilizes or improves neurological functions in patients with NMOSD over 1 year. These patients did not respond to previous treatment with cyclophosphamide or azathioprine with or without prednisone for reducing relapses. After receiving MSC, 12 of 15 patients were completely free from relapses during the 12‐month observation period. Three patients together developed 4 mild relapses without a decrease in EDSS. The new CNS symptoms and signs in the latter patients completely recovered 1 month after the relapse. In accordance with these clinical observations, MRI studies revealed reduction in spinal cord and optic lesions. None of the 15 patients reported severe adverse events during the 12‐month follow‐up period. Therefore, these preliminary results provide evidence that autologous MSC infusion is a safe and effective treatment for NMOSD.
Without adequate tools to follow the infused MSC in vivo in human and lack of in situ and pathological data, we do not know precisely how and at what level MSCs modulate disease activity. Whether replacement of injured cells by MSCs differentiated locally contributes to the recovery is not clear. Although MSC therapies were originally based on the possibility to restore the damaged tissues, MSCs have emerged as a potential therapy for an inflammatory demyelinating disease of the CNS based on other properties than tissue replacement, such as their ability to inhibit pathogenic T‐ and B‐cell responses and on the release of neuroprotective favoring tissue protection and repair 13, 14, 15, 16. Our study suggests that alterations in inflammatory and autoimmune responses occurred in the periphery and within the brain. In the circulation, we followed counts of most lymphocytes and, among them, only numbers of B cells were reduced upon MSC infusion. Although anti‐AQP4 antibody titers fluctuated during the follow‐up period, it is likely that alterations in antibody levels do not necessarily reflect changes in overall B‐cell numbers or subsets, as is also the case for rituximab therapy 30. Production of antibodies against AQP4 is dependent on T helper cell 3. Among several T helper cell subsets analyzed, we found that Tfh cells were reduced accompanied by reduction in IL‐6 and IL‐21. Tfh cells and cytokines of IL‐21 and IL‐6 support B‐cell activation, expansion, and differentiation and play a role in the generation of anti‐AQP4 antibodies 31. Our data suggested that MSC transplantation might exert neuroprotection on NMOSD at least in part through immune modulation of Tfh cells and cytokines of IL‐6 and IL‐21. The mechanisms of action for MSC require investigation. Within the CNS, patients had significantly fewer hyperintense lesions on T2 images and gadolinium‐enhancing lesions in the optic nerve and reduced myelitis after MSC treatment. There were no gadolinium‐enhancing lesions in the brains of NMOSD patients during the entire observation period. Thus, attenuation of inflammation occurred both in the periphery and in the CNS of patients who received MSC.
In conjunction with the improved visual function observed in patients who received MSC, we recorded significant retinal nerve fiber layer thickening, optic nerve diameter increases, and upper cervical area enlargement. These findings suggest that MSCs exert accelerated functional recovery and tissue restoration of the optic nerve, brain and spinal cord. This is consistent with previous findings that MSC therapy accelerates injury structure repair or protection and functional recovery in patients with multiple system atrophy, stroke, cerebral palsy, spinal cord injuries, and progressive MS 22, 32, 33, 34, 35. Collectively, our data imply that the modulation of immune responses as well as promotion of tissue recovery/repair might contribute to the observed beneficial effects of MSC on NMOSD patients.
We were able to harvest sufficient numbers of MSC from each patient (patients had stopped previous immunosuppressive therapy 1 month prior to the time of MSC harvest) that did not express any aberrant cell‐surface markers compared with MSC harvested from healthy donors. This report is the first to describe the successful isolation, ex vivo culture expansion, and intravenous infusion of autologous MSC into patients with NMOSD. Despite the large numbers of MSC and ex vivo expansion of these cells, there was neither immediate nor delayed infusion‐related toxicity associated with the infusion of 1 × 108 MSC. No tumors were found after 1 year of follow‐up. Thus, our findings indicate that this form of cell therapy is feasible in NMOSD patients with neurological disability.
Limitations of the study include small sample size and a single arm, nonrandomized design. EDSS, designed for assessing neurological function in MS patients, does not have sufficient modalities to assess vision and spinal cord function that are dominant signs in NMOSD. A limitation of the pretest–posttest approach is that changes in disease activity evident after treatment may not be attributed exclusively to the effects of MSC. In addition, the age range of recruited patients was 19–63 years. Deficiency in MSC due to age as well as aberrancy of bone marrow due to ongoing autoimmunity, as previously observed in systemic lupus erythematosus 36 and multiple myeloma 37, may impair the therapeutic potential of MSC. It is likely that a single infusion with the given numbers of cells was unable to sustain long‐lasting peripheral immune modulatory effects. In support of this notion, the impact of MSC on B cells as well as Tfh cells lasted only for several weeks after infusion. Consequently, titers of anti‐AQP4 antibodies returned to close to baseline levels (Figure S1). The ongoing inflammatory and autoimmune responses in the periphery and within the CNS are also expected to impair the potential repair capacity of MSC, if any. Optimal frequency of MSC treatment for NMOSD needs to explore. On the other hand, a 2‐year follow‐up observation demonstrated that a decrease in ARR along with reduced disability (Table 2) suggests the lasting effects of MSC in the current study.
Despite these limitations, we have provided evidence that NMOSD benefits from MSC therapy, possibly via immune modulation and promotion of neurorepair mechanisms. Combination of a powerful disease‐modifying drug for NMOSD such as rituximab with MSC adoptive therapy may create more favorable conditions to facilitate the efficacy of MSC for treatment of NMOSD. Therefore, our study encourages further investigations of MSC as a monotherapy or combination therapy with disease‐modifying drugs for treatment of NMOSD.
Conflict of Interest
F‐D Shi has received research support from the National Basic Research Program of China (2013CB966900), the National Science Foundation of China (81171183, 81230028, 81301044, 81471221 and 81471535), the US National Institutes of Health (R01NS092713), and the American Heart Association (16SDG27250236). Drs. Y. Fu, Y.‐P.Yan, Y. Qi, L. Yang, T. Li, N.‐N.Zhang, C.‐S.Yu, L. Su, Y. Shen, R. Zhang, S. Lin, Q. Liu, and Z.‐C. Han report no disclosures.
Supporting information
Data S1. Method.
Table S1. Clinical profile of patients treated with autologous mesenchymal stem cells.
Figure S1. lymphocyte phenotyping, anti‐AQP4 antibody and cytokine levels in MSC‐treated NMOSD patients.
Acknowledgment
We thank our patients for participating in this study, the clinical neuroimmunology team for recruiting the patients; M. Li for laboratory tests; and N. Sun and K. Shi for editorial assistance.
References
- 1. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012;11:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jarius S, Paul F, Franciotta D, et al. Mechanisms of disease: Aquaporin‐4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol 2008;4:202–214. [DOI] [PubMed] [Google Scholar]
- 3. Pohl M, Kawakami N, Kitic M, et al. T cell‐activation in neuromyelitis optica lesions plays a role in their formation. Acta Neuropathol Commun 2013;1:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: Clinical predictors of a relapsing course and survival. Neurology 2003;60:848–853. [DOI] [PubMed] [Google Scholar]
- 5. Collongues N, Marignier R, Zephir H, et al. Neuromyelitis optica in France: A multicenter study of 125 patients. Neurology 2010;74:736–742. [DOI] [PubMed] [Google Scholar]
- 6. Palace J, Leite MI, Nairne A, Vincent A. Interferon Beta treatment in neuromyelitis optica: Increase in relapses and aquaporin 4 antibody titers. Arch Neurol 2010;67:1016–1017. [DOI] [PubMed] [Google Scholar]
- 7. Kleiter I, Hellwig K, Berthele A, et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol 2012;69:239–245. [DOI] [PubMed] [Google Scholar]
- 8. Min JH, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler 2012;18:113–115. [DOI] [PubMed] [Google Scholar]
- 9. Yang CS, Yang L, Li T, et al. Responsiveness to reduced dosage of rituximab in Chinese patients with neuromyelitis optica. Neurology 2013;81:710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pittock SJ, Lennon VA, McKeon A, et al. Eculizumab in AQP4‐IgG‐positive relapsing neuromyelitis optica spectrum disorders: An open‐label pilot study. Lancet Neurol 2013;12:554–562. [DOI] [PubMed] [Google Scholar]
- 11. Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: State‐of‐the‐art and emerging therapies. Nat Rev Neurol 2014;10:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997;276:71–74. [DOI] [PubMed] [Google Scholar]
- 13. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 14. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 2012;12:383–396. [DOI] [PubMed] [Google Scholar]
- 15. Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross‐talk between neural stem cells and immune cells: The key to better brain repair? Nat Neurosci 2012;15:1078–1087. [DOI] [PubMed] [Google Scholar]
- 16. Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T‐cell anergy. Blood 2005;106:1755–1761. [DOI] [PubMed] [Google Scholar]
- 17. Liang J, Zhang H, Hua B, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann Rheum Dis 2010;69:1423–1429. [DOI] [PubMed] [Google Scholar]
- 18. Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol 2014;12:64–71. [DOI] [PubMed] [Google Scholar]
- 19. Llufriu S, Sepulveda M, Blanco Y, et al. Randomized placebo‐controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One 2014;9:e113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J Neuroimmunol 2010;227:185–189. [DOI] [PubMed] [Google Scholar]
- 21. Karussis D, Karageorgiou C, Vaknin‐Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010;67:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open‐label phase 2a proof‐of‐concept study. Lancet Neurol 2012;11:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 24. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 25. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 26. Gong W, Han Z, Zhao H, et al. Banking human umbilical cord‐derived mesenchymal stromal cells for clinical use. Cell Transplant 2012;21:207–216. [DOI] [PubMed] [Google Scholar]
- 27. Geeraerts T, Newcombe VF, Coles JP, et al. Use of T2‐weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care 2008;12:R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ciccarelli O, Altmann DR, McLean MA, et al. Spinal cord repair in MS: Does mitochondrial metabolism play a role? Neurology 2010;74:721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henderson AP, Altmann DR, Trip AS, et al. A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain 2010;133:2592–2602. [DOI] [PubMed] [Google Scholar]
- 30. Pellkofer HL, Krumbholz M, Berthele A, et al. Long‐term follow‐up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 2011;76:1310–1315. [DOI] [PubMed] [Google Scholar]
- 31. Li YJ, Zhang F, Qi Y, et al. Association of circulating follicular helper T cells with disease course of NMO spectrum disorders. J Neuroimmunol 2015;278:239–246. [DOI] [PubMed] [Google Scholar]
- 32. Lee PH, Lee JE, Kim HS, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol 2012;72:32–40. [DOI] [PubMed] [Google Scholar]
- 33. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005;57:874–882. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Cheng H, Hua R, et al. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: A preliminary clinical study. Cytotherapy 2013;15:1549–1562. [DOI] [PubMed] [Google Scholar]
- 35. Pal R, Venkataramana NK, Bansal A, et al. Ex vivo‐expanded autologous bone marrow‐derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009;11:897–911. [DOI] [PubMed] [Google Scholar]
- 36. Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW, Fan LM. Abnormality of bone marrow‐derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus 2007;16:121–128. [DOI] [PubMed] [Google Scholar]
- 37. Arnulf B, Lecourt S, Soulier J, et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia 2007;21:158–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Method.
Table S1. Clinical profile of patients treated with autologous mesenchymal stem cells.
Figure S1. lymphocyte phenotyping, anti‐AQP4 antibody and cytokine levels in MSC‐treated NMOSD patients.


