Summary
Adult neurogenesis is the process of generating new neurons throughout life in the olfactory bulb and hippocampus of most mammalian species, which is closely related to aging and disease. Nicotinamide phosphoribosyltransferase (NAMPT), also an adipokine known as visfatin, is the rate‐limiting enzyme for mammalian nicotinamide adenine dinucleotide (NAD) salvage synthesis by generating nicotinamide mononucleotide (NMN) from nicotinamide. Recent findings from our laboratory and other laboratories have provided much evidence that NAMPT might serve as a therapeutic target to restore adult neurogenesis. NAMPT‐mediated NAD biosynthesis in neural stem/progenitor cells is important for their proliferation, self‐renewal, and formation of oligodendrocytes in vivo and in vitro. Therapeutic interventions by the administration of NMN, NAD, or recombinant NAMPT are effective for restoring adult neurogenesis in several neurological diseases. We summarize adult neurogenesis in aging, ischemic stroke, traumatic brain injury, and neurodegenerative disease and review the advances of targeting NAMPT in restoring neurogenesis. Specifically, we provide emphasis on the P7C3 family, a class of proneurogenic compounds that are potential NAMPT activators, which might shed light on future drug development in neurogenesis restoration.
Keywords: Adult neurogenesis, Neurological disease, Nicotinamide adenine dinucleotide, Nicotinamide mononucleotide, Nicotinamide phosphoribosyltransferase
Adult Neurogenesis in Aging and Disease
With decades of effort, the existence of lifelong neurogenesis in animals and humans has been demonstrated 1, 2, 3. Adult neurogenesis in the mammalian brain is limited to certain areas rather than spread widely, as shown by evidence that new neurons in rodents are continuously generated by the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) of the lateral ventricles where new neurons migrate into the olfactory bulb and differentiate into olfactory neurons 4, 5. Although neurogenesis exists in the human SGZ of the hippocampal DG, there is very limited postnatal neurogenesis in the human olfactory bulb 6, 7, 8. The newborn neurons dividing from neural stem/progenitor cells (NSPCs) are important for replacing gradual neuron loss and maintaining specific function throughout life. How do new neurons exert their functions to maintain neurogenic niches and how is this process linked to human disorders? Considerable progress has been made in deciphering the role of neurogenesis in aging and disease.
Adult Neurogenesis in Aging
Aging leads to the physiological breakdown of health and significant functional decline. The integration of adult‐born neurons into the circuitry of the adult hippocampus plays a role in learning and memory. In mice, the number of proliferating NSPCs in the SGZ is nearly completely absent by 24 months of age 9, 10, 11. Aging attenuates the proliferation of the SVZ neural precursors, and the aged SVZ cells lose their capacity to proliferate and to be recruited by the lesion 12. The loss of active horizontal neural stem cells (NSCs) in aged mice correlates with reduced neurogenesis 11. In addition, a recent study reported that aging could increase microglial proliferation and neutrophil infiltration and inhibit SVZ cell proliferation and migration of neuroblasts in the cerebral ischemia model of middle‐aged mice, indicating that stroke‐induced neurogenesis is reduced in aged mice 13. Therefore, the regulation of adult neurogenesis as a therapeutic strategy to compensate for the defects of aging is very relevant.
Adult Neurogenesis in Cerebral Ischemia and Traumatic Brain Injury
Neurogenesis persists in the adult mammalian brain. Successive studies have shown that neurogenesis involves the body's intrinsic defense system to resist impairment by cerebral ischemia. After focal cerebral ischemia in rats, nascent neurons were increased in two neuroproliferative regions—the SGZ of the DG and the rostral SVZ 14. Later, a study found newborn neurons in the ischemic penumbra surrounding cerebral cortical infarcts in patients with stroke, suggesting that stroke‐induced compensatory neurogenesis may contribute to postischemic recovery and represent a target for stroke therapy 15. What is responsible for ischemia‐induced neurogenesis? A recent study found that early immature neuronal death in the DG following transient forebrain ischemia/reperfusion in mice could trigger cerebral ischemia‐induced neurogenesis in the DG through microglia‐derived insulin‐like growth factor‐1 16.
Similarly, traumatic brain injury (TBI) enhances neurogenesis in the rodent hippocampus and stimulates an increase in proliferation of endogenous NSPCs to heal itself after injury 17, 18, 19.
Adult Neurogenesis in Neurodegenerative Disease
Neurodegenerative disease is accompanied with the progressive loss of neuronal structure and function, which is associated with brain networks and chronic disease 20, 21, 22. In recent decades, it is widely accepted that alterations in neurogenesis are closely associated with monogenic and sporadic neurodegenerative diseases. Aggrieved adult neurogenesis in neurodegenerative diseases fails to retain existing neurons and loses the endogenous repair mechanism for cell renewal and differentiation into distinct functional neurons.
Substantial alterations of neurogenesis in human patients suffering from Alzheimer's disease (AD), Huntingdon's disease (HD), and/or other cognitive impaired diseases were observed in different studies 23, 24, 25. Patients with AD have a smaller hippocampal volume, which is associated with cognitive deficits 26. A reduction of dopaminergic neurons in the SVZ is the hallmark of Parkinson's disease (PD). The number of neural precursor cells in the SVZ and olfactory bulb is reduced in postmortem brains of patients with PD 27. Dopamine treatment could increase the proliferation of SVZ‐derived cells in in vitro adult SVZ cultures 28. In addition, α‐synuclein, a protein abundantly expressed in the human brain, has been reported as a key protein in PD, AD, Lewy body disease, and other neurodegenerative diseases 29, 30. α‐Synuclein knockdown mice showed increased new neurons in the hippocampal DG, whereas elevated endogenous α‐synuclein levels could decrease dendrite length and impair dendrite branching 31. Recently, using a 14C retrospective birth dating approach, which could determine the time point when DNA was synthesized and cells were born and then deduce the age of cells in the cortex of human brain 24, 32, it was revealed that the extent of striatal neurogenesis is significantly reduced in patients with HD 33.
Except for the studies mentioned above, stem cell‐based therapies for neurogenesis‐associated disorders have been shown in experimental animal models with neuroprotection 34, 35, 36, 37. Although many efforts have been made to elucidate the relationship between neurogenesis and human disorders, concerted efforts are needed to narrow the gap between animal models and humans. Exploring novel approaches, applying the approaches to humans with no or less toxicity and side effects, and breaking the limitations of obtaining data from surgical specimens and/or postmortem tissue samples are urgently needed. Historical breakthroughs have been made using patient‐derived or genome‐edited pluripotent stem cells and induced pluripotent stem cells (iPSCs), which provide a better understanding of the cellular physiological and pathological mechanisms and shed light on further decisions of therapeutic interventions.
Nicotinamide Phosphoribosyltransferase in Neurogenesis
The Characteristics and Functions of NAMPT
Nicotinamide phosphoribosyltransferase (NAMPT) enzymatic activity was reported in 1957 with the property of catalyzing nicotinamide mononucleotide (NMN) synthesis 38. Decades later, the NAMPT gene was recorded in GenBank by an article published in 1990 as one of a set of putative lymphocyte G0/G1 switch genes 39. Interestingly, a cytokine named pre‐B‐cell colony‐enhancing factor (PBEF) 40, which enhances the effect of stem cell factor and IL‐7 on pre‐B‐cell colony formation, was described in 1994 and was shown to be equivalent to NAMPT in 2001 and 2002 41, 42. In 2005, NAMPT was reported as a visceral fat‐derived adipokine and renamed visfatin. NAMPT is expressed in both the nucleus and cytoplasm, and its expression is regulated by circadian locomotor output cycles protein kaput (CLOCK) and aryl hydrocarbon receptor translocator‐like protein 1 (BMAL1), which is related to circadian rhythmicity and circadian oscillation of nicotinamide adenine dinucleotide (NAD) levels in vivo 43, 44. In addition to intracellular NAMPT, NAMPT can be secreted into the extracellular space in some types of cells, and both intracellular and extracellular forms of the protein have NAMPT enzymatic activity 45, 46, 47. The wide distribution of NAMPT suggests its pleiotropic functions in physiology and pathophysiology 48, 49, 50, 51.
Nicotinamide adenine dinucleotide is a ubiquitous coenzyme involved in biochemical reactions and serves as a substrate for NAD‐dependent enzymes, such as poly‐ADP‐ribose polymerases, sirtuins (SIRTs), and CD38 52, 53. There are two ways to synthesize NAD: de novo synthesis from tryptophan and the salvage pathway from nicotinamide (NAM), nicotinamide riboside, and nicotinic acid (Figure 1). NAMPT is the rate‐limiting enzyme for mammalian NAD salvage synthesis by generating NMN from NAM and 5′‐phosphoribosyl‐1‐pyrophosphate, thereby influencing NAD‐dependent enzymes and regulating cellular metabolism, mitochondrial biogenesis, and the adaptive response to inflammatory, oxidative, proteotoxic and genotoxic stresses 42, 50, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64.
Figure 1.
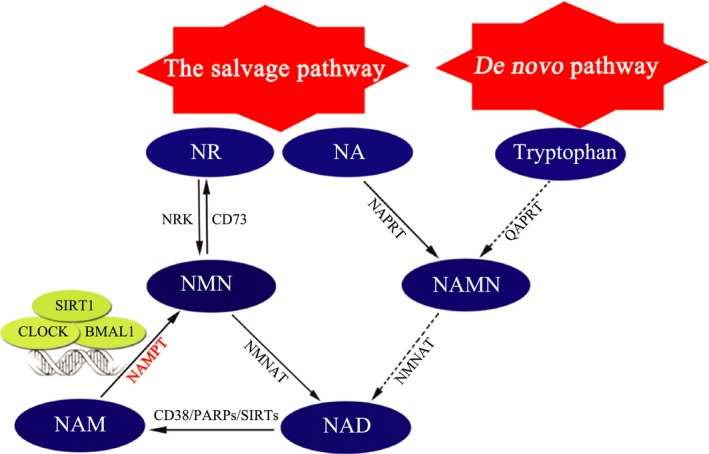
The nicotinamide adenine dinucleotide (NAD) biosynthetic pathways in mammals. There are two ways to maintain the cellular levels of NAD in mammals: the de novo pathway from tryptophan and the salvage pathway from NAM, NR, and NA with different catalyzing enzymes. NAMPT, the rate‐limiting enzyme of NAD synthesis in the salvage pathway, can be bound and upregulated by the two major circadian regulators, BMAL1 and CLOCK, acting synergistically with SIRT1 43, 52, 53, 54, 55, 57, 58, 59, 60, 61, 62, 63. BMAL1, aryl hydrocarbon receptor translocator‐like protein 1; CLOCK, circadian locomotor output cycles protein kaput; NA, nicotinic acid; NAD, nicotinamide adenine dinucleotide; NAM, nicotinamide; NAMN, nicotinic acid mononucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NAPRT, nicotinic acid phosphoribosyltransferase; NMN, nicotinamide mononucleotide; NMNAT, nicotinamide mononucleotide adenylyltransferase; NR, nicotinamide riboside; NRK, nicotinamide ribose kinase; PARPs, poly‐ADP‐ribose polymerases; QAPRT, quinolinate phosphoribosyltransferase; SIRTs, sirtuins.
Functions and Mechanisms of NAMPT in Restoring Neurogenesis
Nicotinamide adenine dinucleotide is an essential coenzyme involved in energy production and redox metabolism, which is closely related to mitochondrial energy metabolism. Recent studies showed that the energetic demands of stem cell proliferation and lineage fate decision require distinct metabolic programs 65, and the NAD level is very likely to connect the survival and function of stem cells. The NAMPT‐driven salvage pathway plays a predominant role in maintaining the homeostasis of NAD levels. NAD depletion in brain tissue disrupts intracellular energy homeostasis and results in neural cell death 66. Additionally, cell‐autonomous NAMPT is the main source of NAD for NSPCs 67.
In the brain, NAMPT has uniquely strong expression in the hippocampus where adult neurogenesis exists 68, 69. NAMPT is mainly expressed in neurons and in NSCs in vitro and in vivo, with less expression in glial cells 67, 68, 69. Accordingly, NAMPT is particularly important for the proliferation, self‐renewal, and differentiation of NSPCs. Using adult NSPC‐specific inducible NAMPT knockout mice, Stein et al. 67 reported that the inactivation of NAMPT in the adult Nestin+ population impaired NSPC proliferation and self‐renewal. Acute deletion of NAMPT in hippocampal neurospheres significantly reduced NAD levels in NSPCs and stalled the cells in the G1 phase of the cell cycle. Chronic ablation of NAMPT in hippocampal neurospheres abrogated oligodendrogenesis, and in vivo ablation of NAMPT in the adult Nestin+ population reduced NSPC‐mediated oligodendrogenesis upon insult.
Nicotinamide phosphoribosyltransferase is a key protein in the defense mechanisms of organisms and plays a critical role in metabolic homeostasis and survival 66. The NAMPT–NAD–SIRT cascade has been demonstrated as a strong endogenous defense system 66. NAMPT may contribute to the survival of NSPCs under energy depletion, genotoxic stress, and other insults. In addition, NAMPT may be beneficial for neurogenesis in paracrine and endocrine manners. NAMPT can be secreted by several types of neural cells 45, 46, 47 and can cross the blood–brain barrier by unknown mechanisms 46. In adipocyte‐specific NAMPT knockout and knock‐in mice, adipocyte‐derived NAMPT showed remote action on brain hypothalamic NAD–SIRT1 signaling and physical activity 70. Our studies also revealed that NAMPT can regulate vascular function in paracrine and endocrine manners 71, 72.
How NAMPT regulates proliferation, differentiation, and self‐renewal of NSPCs is not fully understood. Current studies showed that the proliferative and prodifferentiation effects of NAMPT–NAD axis on NSPCs require several SIRTs 47, 67. In an in vitro model, studies using NMN and siRNA‐mediated knockdown of SIRT1‐7 revealed that SIRT1 and SIRT2 contribute to NSC proliferation, whereas SIRT1, SIRT2, and SIRT6 contribute to NSC differentiation 47. In addition, another study showed that NAMPT is vital in oligodendrocytic lineage fate decisions via a mechanism mediated by SIRT1 and SIRT2 67. They also suggested the involvement of transcriptional upregulation of cyclins E and A and their upstream regulator E2F1 during NAMPT‐mediated NSPC proliferation 67. Up to now, the mechanism by which NAMPT promotes NSPC self‐renewal remains unidentified.
Role of NAMPT in Aging‐Associated Neurogenesis
Nicotinamide adenine dinucleotide and NAMPT levels are significantly decreased in diverse organs during aging 73. Strikingly, the reduction of NSPC proliferation, self‐renewal, and insult‐induced differentiation are observed during aging, concomitant with a decreased level of NAD and NAMPT in the hippocampus 67. Long‐term NMN administration combats age‐related decline in NSPC functionality and maintains the NSPC pool 67. Furthermore, age‐related mitochondrial dysfunction can be corrected by NAD precursor supplementation 74. Modulation of the NAD level improves mitochondrial function and prevents age‐associated metabolic decline through the activation of the mitochondrial unfolded protein response and FOXO signaling, where the declining NAD level induces a pseudohypoxic state to disrupt nuclear–mitochondrial communication during aging 75, 76.
In addition, the NAMPT–NAD axis connects to SIRT signaling, constituting a strong endogenous defense system against various stresses. The activation of SIRTs, which are NAD‐dependent histone deacetylases, delays senescence and acts as a regulator of health span and life span 75, 77. Furthermore, SIRT activation has been proposed as a preventative and therapeutic measure against multiple age‐associated disorders, including metabolic syndrome 78. Furthermore, a study showed that SIRT1 delays aging by governing central circadian control to activate transcription of the two major circadian regulators, namely BMAL1 and CLOCK 77, acting synergistically to regulate the expression of the NAMPT gene.
Role of NAMPT in Stroke‐Associated Neurogenesis
The relationship between NAMPT and cerebral ischemia has been elucidated in detail 66, 79. NAMPT is a therapeutic target of ischemic stroke, including neuroprotection in acute phase, neovascularization in subacute phase, and neurorestoration in chronic phase 66.
Upon ischemic insult, NAMPT has been shown to support the neurorestoration. Recently, our group demonstrated that the NAMPT–NAD cascade promotes regenerative neurogenesis after ischemic stroke 47. Compared to wild‐type mice, NAMPT transgenic mice shows elevated brain NAD level, enhanced number of NSCs, improved neural functional recovery, increased survival rate, and accelerated body weight gain after MCAO, which are not observed in H247A mutant‐NAMPT transgenic mice due to the loss of NAD biosynthetic activity, providing evidence for proneurogenic effects of the NAMPT–NAD cascade 47. Delayed administration of NMN for 7 days with the first dose at 12 hours post‐MCAO, which cannot confer neuroprotection in acute phase, still reduces MCAO‐induced death rate during the first week, improves the neuronal recovery, and increases postischemic neurogenesis in the SVZ and DG of MCAO mice 47. NMN and NAD promote NSC proliferation and differentiation in cultured NSC neurospheres, where NAMPT inhibitor FK866 induces converse effect 47. Additionally, NAMPT may participate in poststroke synaptic plasticity 66.
Except for the benefits from NAMPT‐induced neurogenesis in stroke, NAMPT also confers neuroprotection to reduce OGD‐induced injury in neurons 45, 46, 69, 80, 81, 82, improves ischemic brain damage, and increases myelinated fibers in the striatum and corpus callosum in the experimental MCAO model 69, 83, 84, 85, 86, 87. Moreover, NAMPT indirectly provides a positive microenvironment for neurogenesis by enhancing neovascularization in endothelial cells, vascular smooth cells, and endothelial progenitor cells 88, 89, 90. Our studies have demonstrated that the NAMPT–NAD cascade improves the mobilization and functions of endothelial progenitor cells and promotes the growth of vascular smooth muscle cells 71, 91. Moreover, an increase in newly formed blood vessels was found in the brain's penumbra zone after MCAO in NAMPT transgenic mice, and increased cerebral blood flow at 7 and 14 days was detected in ischemic brain area 89.
With regard to the above‐mentioned positive effects of NAMPT in restoring neurogenesis, targeting NAMPT can be applied as a powerful strategy to treat neurogenesis‐associated disorders. Although novel NAMPT inhibitors have been discovered recently 92, 93, 94, there is no report about NAMPT activators, except for a recent study demonstrating that P7C3 chemicals activate the NAMPT–NAD pathway 95. Hence, exploring novel NAMPT activators as candidates to treat neurological diseases is quite essential.
P7C3 Neuroprotective Chemicals as Potential NAMPT Activators
The deficiency of effective proneurogenic and neuroprotective drugs to treat neurogenesis‐associated disorders, including AD, PD, HD, TBI, and amyotrophic lateral sclerosis as well as age‐related cognitive decline, needs to be resolved. Surprisingly, a recent study reported that the administration of active P7C3 chemicals could result in a rebound in the intracellular levels of NAD and protect cultured cells from doxorubicin‐mediated toxicity caused by NAD depletion 95. The evidence showed that P7C3 could bind NAMPT and function by enhancing the activity of the rate‐limiting enzyme in the salvage pathway of NAD synthesis 95, suggesting the potential role of the P7C3 family as NAMPT activators.
The Characteristics of Compound P7C3 and Its Derivatives
McKnight's group initially discovered neuroprotective chemicals using an unbiased in vivo screen 96. The authors screened 1000 druglike compounds and found eight compounds with enhanced neuron formation in the SVZ of the DG 96. Among the compounds, P7C3, an aminopropyl carbazole chemical, has the highest potential of favorable pharmacological properties, with better absorption, distribution, metabolism, and excretion characteristics, and has an appropriate half‐life, rate of clearance, and bioavailability 96. Moreover, P7C3 is orally bioavailable and readily able to cross the blood–brain barrier and is nontoxic at doses several folds higher than the efficacious dose 96.
To further evaluate the properties of P7C3, several more derivatives of P7C3 were designed in an in vivo structure activity relationship study to assess its proneurogenic activity 96. The single derivative of P7C3 (P7C3‐A20), replacing the hydroxyl group at the chiral center of the linker with fluorine, exhibits better proneurogenic activity compared to the parent compound 96. In the follow‐up study, (‐)‐P7C3‐S243 was designed with improved druglike properties compared to previously reported compounds, replacing the aniline moiety of P7C3‐A20 with an alternative heterocycle 97 (Figure 2). All of the analogs display no observed toxicity, no binding of the hERG channel whose dysfunction causes cardiac toxicity, good blood/brain distribution, oral availability, and excellent metabolic stability in mice, rats, and cell culture 97, 98. P7C3 and its more active analogs, namely P7C3‐A20 and (‐)‐P7C3‐S243, have been employed in almost all functional studies to date (Table 1). Based on the aminopropyl carbazole scaffold, Yoon et al. 99 also synthesized a series of aminopropyl carbazole derivatives (compounds 1–26) and screened them in cultured NSCs to assess their biological activity. Among the compounds, compound 9 is safe, is nontoxic to NSCs, and increases the viability of NSCs better than P7C3‐A20 99.
Figure 2.
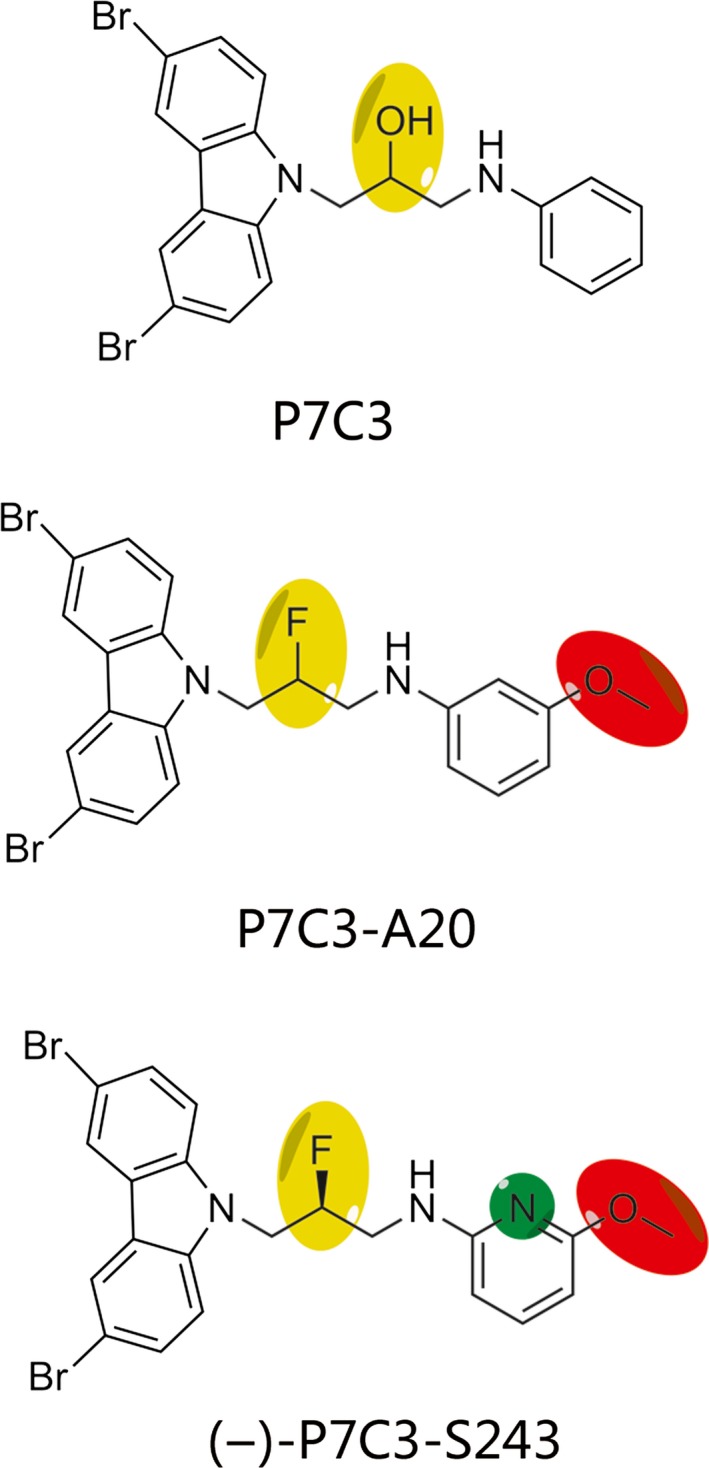
The structures of P7C3 and two active analogs, P7C3‐A20 and (‐)‐P7C3‐S243. Parts with yellow, green, and red colors represent modified sites compared to P7C3 96, 97.
Table 1.
Overview of the proneurogenic and neuroprotective effects of the P7C3 family
| Year | Studied chemical | Experimental model | Proneurogenic and neuroprotective effect |
|---|---|---|---|
| 2010 96 | P7C3 and its analogs |
NPAS3−/− mouse model of neurological malfunctions U2OS cell model of calcium‐induced mitochondrial dissolution Aged rat |
Enhance neuron formation in the SGZ of the DG Rescue NPAS3−/−‐induced loss of hippocampal neurogenesis Ameliorate cognitive decline in aged rat |
| 2012 102 |
P7C3 P7C3‐A20 |
MPTP‐mediated mouse model of PD MPP+‐mediated worm model of PD |
Protect mature neurons in the mouse brain region outside of hippocampus Protect substantia nigra dopaminergic neurons from cell death |
| 2012 109 |
P7C3 P7C3‐A20 |
G93A‐SOD1 mutant mouse model of ALS | Protect ventral horn spinal cord motor neurons from cell death |
| 2013 99 | Compound 9 | Cultured rat cortical NSCs | Induce neurogenesis to enhance neuronal differentiation and inhibit astrocytogenesis |
| 2014 108 |
P7C3 P7C3‐A20 |
Depression‐prone ghrelin receptor‐null mouse model |
Increase proliferating DG cell survival and mature neuron formation Augment hippocampal neurogenesis following focal ablation of hippocampal stem cells |
| 2014 104 | P7C3‐A20 | Moderate fluid percussion brain injury rat model of TBI | Increase proliferating neurons and improve cognitive function after TBI |
| 2014 97 | (‐)‐P7C3‐S243 |
Mouse model of hippocampal neurogenesis MPTP‐mediated mouse model of PD |
Protect developing neurons in hippocampal neurogenesis Protect mature neurons within the substantia nigra in PD |
| 2014 105 | (‐)‐P7C3‐S243 | Blast‐mediated rodent model of TBI | Block brain axonal degeneration and preserve learning, memory, and motor coordination |
| 2015 106 | P7C3 | Rat model of nerve crush injury | Enhance neuron survival, axonal regeneration and prevent microglia proliferation |
| 2015 107 | P7C3 | Ts65Dn mouse model of DS | Increase hippocampal neurogenesis in Ts65Dn mice |
| 2015 103 | Compound 1 | Cultured rat cortical NSCs | Enhance neurogenesis by increasing final cell division |
AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; DG, dentate gyrus; DS, Down syndrome; MPP, 1‐methyl‐4‐phenylpyridinium; MPTP, 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine; NAD, nicotinamide adenine dinucleotide; NPAS3, PAS domain protein 3; NSCs, neural stem cells; PD, Parkinson's disease; SGZ, subgranular zone; TBI, traumatic brain injury.
Proneurogenic and Neuroprotective Effects of the P7C3 Family
Prolonged P7C3 administration in adult mice enhances hippocampal neuron formation with no abundance of hippocampal astrocytes or oligodendrocytes, protecting newborn neurons from cell death during a month‐long differentiation instead of increasing cell proliferation 96. Mice with the loss of the gene encoding the neuronal PAS domain protein 3 (NPAS3) have impaired adult neurogenesis with 3‐fold increased cleaved caspase‐3‐positive (apoptotic) cells in the SGZ, display severe attenuation in dendritic branching and spine density in the DG granular neurons, and have aberrant hyperexcitability of synaptic transmission in the outer molecular layer of the DG and in the CA1 region of the hippocampus compared to wild‐type littermates 96, 100, 101. Prolonged administration of P7C3 normalizes the elevated levels of hippocampal apoptosis and corrects the deficits in the DG of NPAS3−/− mice but has no effects in the CA1 region of the hippocampus 96. The administration of P7C3 or its active analogs could protect against mitochondrial dissolution induced by calcium ionophore A23187 and preserve mitochondrial membrane integrity in cultured U2OS cells 96. Furthermore, P7C3 enhances hippocampal neurogenesis, ameliorates cognitive decline, prevents weight loss in terminally aged rats 96, and shows protection of mature neurons in brain region outside of the hippocampus 102. Yoon et al. 99 demonstrated that compound 9 could induce neurogenesis and inhibit astrocytogenesis in cultured rat cortical NSCs. Compound 1 was identified to enhance neurogenesis by increasing final cell division to generate neurons during NSC differentiation 103. Due to the proneurogenic and neuroprotective activity of P7C3 compounds, substantial studies are needed to focus on exploring its role in neurological disease.
In the mouse model of TBI, both proneurogenic and neuroprotective effects account for the anti‐TBI effects of P7C3 compounds. Administration P7C3‐A20 initially at 30 min postsurgery for 7 days decreases contusion volume, blocks mature cortical neuron death, increases the generation of new hippocampal neurons in the DG, and improves functional neurological outcome at 1 and 4 weeks after TBI 104. Similarly, long‐term administration of (‐)‐P7C3‐S243 blocks widespread axonal degeneration and preserves learning, memory, and motor coordination in TBI mice, given the hypotheses that the protective effects may be regulated by activating NAMPT 105. Moreover, the administration of P7C3 doubles motor and sensory neuron survival after sciatic nerve crush injury, promotes axon regeneration, enhances behavioral and muscle functional recovery, and reverses pathological mobilization of spinal microglia following neonatal nerve injury, suggesting the potential application of the P7C3 family in nerve injury‐related diseases 106.
Down syndrome (DS) is a genetic disease with intellectual disability caused by the triplication of human chromosome 21. Eisch et al. 107 first discovered that patients with DS are marked by diminished adult hippocampal neurogenesis. Surprisingly, chronic P7C3 treatment has no effect on hippocampal neurogenesis in wild‐type mice but is sufficient to restore the neurogenic deficits in Ts65Dn mice, an animal model with similar neuropathological features in patients with DS 107. In addition, Walker et al. 108 found that P7C3 and its analogs exert an antidepressant‐like effect in depression‐prone ghrelin receptor‐null mice exposed to chronic social defeat stress or caloric restriction by increasing hippocampal neurogenesis in which the ablation of hippocampal stem cells with radiation eliminates the antidepressant effect.
Moreover, P7C3 compounds show neuroprotective efficacy to prevent the death of existing mature neurons in neurodegenerative disease; however, no further studies were carried out on neurogenesis in the corresponding disease models. In the mouse model of PD, P7C3 blocks the cell death of dopaminergic neurons in the substantia nigra of adult mice, P7C3‐A20 shows greater potency and efficacy, and an antihistaminergic drug Dimebon confers no protection 102. Of note, (‐)‐P7C3‐S243 is more efficacious than P7C3 and P7C3‐A20 in the animal model of PD with almost complete protection 97. In the mouse model of amyotrophic lateral sclerosis, P7C3 delays disease progression when administration is initiated substantially earlier than the expected time of symptom onset 109. P7C3‐A20 protects ventral horn spinal cord motor neurons from cell death, correlates with the preservation of motor function, and is efficacious when administered at disease onset, where Dimebon is also not active 109.
However, the effects of the P7C3 family on stroke remain unclear. Further studies need to be performed to explore the role of P7C3 compounds in functional recovery poststroke. In addition, there is no study reporting changes in the cellular or plasma NAMPT levels following P7C3 compound administration in any disease model, which may align with their effects on neuroprotection and neurorestoration. More efforts should be made to provide more evidence for P7C3 compounds as NAMPT activators, which will bring hope to patients suffering from neurological diseases.
Recent tremendous progress in techniques and methods has allowed the modeling of human diseases, including how to mimic hippocampal circuitry and neurogenesis in the adult brain. Successful generation of iPSCs from adult human dermal fibroblasts brings hope to novel experimental models 110. Lancaster et al. 111 initially introduced a human pluripotent stem cell (hPSC)‐derived three‐dimensional (3D) organoid culture system to develop various discrete brain regions, termed cerebral organoids. Interestingly, cerebral organoids could recapitulate features of human cortical development, that is, characteristic progenitor zone organization with abundant outer radial glial stem cells. In addition, the authors also successfully modeled microcephaly using RNA interference and patient‐specific iPSCs 111, suggesting a novel approach to study the human neurodevelopment process and model developmental diseases through the in vitro culture of cerebral organoids from hPSCs. Thus, we suggest that P7C3 compounds can be tested in the model of human patient‐derived organoids to verify its reported neuroprotective and proneurogenic efficacy.
Conclusions
Adult neurogenesis continues to be a hot topic in the neuroscience field. Due to the intricate structure of the human brain, it is difficult to obtain a detailed process of adult neurogenesis and mimic the process of neurogenesis‐associated human disorders. Future studies will aim to unveil the mysterious masks of those diseases and link new detection techniques to neurogenesis in normal or diseased human conditions. In addition, the proposed novel approach, namely 3D culture of organoids, can be used for drug testing to screen more effective and specific chemicals to restore adult neurogenesis. Current studies have provided much evidence supporting NAMPT as a potential therapeutic target to restore adult neurogenesis. However, more efforts are still needed to fully clarify the mechanisms by which NAMPT regulates the neurogenesis, and its role in neurodegenerative diseases, such as AD and PD. On the other hand, mining NAMPT activators as a powerful therapy is extremely urgent. Meanwhile, the proneurogenic and neuroprotective effects of P7C3 compounds should be further demonstrated in humanized disease models before clinical trials. Finally, the mechanisms of action underlying P7C3 compounds in neurological diseases need to be further explored.
Conflict of Interest
The authors declare no conflict of interests.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81373414 and 81130061 to C.‐Y.M).
The first two authors contributed equally to this work.
References
- 1. Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 1965;124:319–335. [DOI] [PubMed] [Google Scholar]
- 2. Eriksson PS, Perfilieva E, Bjork‐Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998;4:1313–1317. [DOI] [PubMed] [Google Scholar]
- 3. Jessberger S, Gage FH. Adult neurogenesis: Bridging the gap between mice and humans. Trends Cell Biol 2014;24:558–563. [DOI] [PubMed] [Google Scholar]
- 4. Alvarez‐Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004;41:683–686. [DOI] [PubMed] [Google Scholar]
- 5. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008;132:645–660. [DOI] [PubMed] [Google Scholar]
- 6. Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013;153:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergmann O, Liebl J, Bernard S, et al. The age of olfactory bulb neurons in humans. Neuron 2012;74:634–639. [DOI] [PubMed] [Google Scholar]
- 8. Bergmann O, Spalding KL, Frisen J. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol 2015;7:a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age‐related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging 2010;31:151–161. [DOI] [PubMed] [Google Scholar]
- 10. Jin K, Sun Y, Xie L, et al. Neurogenesis and aging: FGF‐2 and HB‐EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2003;2:175–183. [DOI] [PubMed] [Google Scholar]
- 11. Lugert S, Basak O, Knuckles P, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 2010;6:445–456. [DOI] [PubMed] [Google Scholar]
- 12. Decker L, Picard‐Riera N, Lachapelle F, Baron‐Van Evercooren A. Growth factor treatment promotes mobilization of young but not aged adult subventricular zone precursors in response to demyelination. J Neurosci Res 2002;69:763–771. [DOI] [PubMed] [Google Scholar]
- 13. Moraga A, Pradillo JM, Garcia‐Culebras A, et al. Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia. J Neuroinflammation 2015;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA 2001;98:4710–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin K, Wang X, Xie L, et al. Evidence for stroke‐induced neurogenesis in the human brain. Proc Natl Acad Sci USA 2006;103:13198–13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim DH, Lee HE, Kwon KJ, et al. Early immature neuronal death initiates cerebral ischemia‐induced neurogenesis in the dentate gyrus. Neuroscience 2015;284:42–54. [DOI] [PubMed] [Google Scholar]
- 17. Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma 2002;19:693–703. [DOI] [PubMed] [Google Scholar]
- 18. Rice A. Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp Neurol 2003;183:406–417. [DOI] [PubMed] [Google Scholar]
- 19. Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res 2001;63:313–319. [DOI] [PubMed] [Google Scholar]
- 20. Agosta F, Weiler M, Filippi M. Propagation of pathology through brain networks in neurodegenerative diseases: From molecules to clinical phenotypes. CNS Neurosci Ther 2015;21:754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jabir NR, Firoz CK, Baeesa SS, et al. Synopsis on the linkage of Alzheimer's and Parkinson's disease with chronic diseases. CNS Neurosci Ther 2015;21:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Camilleri A, Vassallo N. The centrality of mitochondria in the pathogenesis and treatment of Parkinson's disease. CNS Neurosci Ther 2014;20:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular‐associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis 2006;24:1–14. [DOI] [PubMed] [Google Scholar]
- 24. Curtis MA, Penney EB, Pearson AG, et al. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci USA 2003;100:9023–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science 2007;315:336–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peng GP, Feng Z, He FP, et al. Correlation of hippocampal volume and cognitive performances in patients with either mild cognitive impairment or Alzheimer's disease. CNS Neurosci Ther 2015;21:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 2004;7:726–735. [DOI] [PubMed] [Google Scholar]
- 28. O'Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine‐induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci USA 2009;106:8754–8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mezey E, Dehejia A, Harta G, Papp MI, Polymeropoulos MH, Brownstein MJ. Alpha synuclein in neurodegenerative disorders: Murderer or accomplice? Nat Med 1998;4:755–757. [DOI] [PubMed] [Google Scholar]
- 30. Goedert M. Alpha‐synuclein and neurodegenerative diseases. Nat Rev Neurosci 2001;2:492–501. [DOI] [PubMed] [Google Scholar]
- 31. Winner B, Regensburger M, Schreglmann S, et al. Role of alpha‐synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci 2012;32:16906–16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell 2005;122:133–143. [DOI] [PubMed] [Google Scholar]
- 33. Ernst A, Alkass K, Bernard S, et al. Neurogenesis in the striatum of the adult human brain. Cell 2014;156:1072–1083. [DOI] [PubMed] [Google Scholar]
- 34. Tang YH, Ma YY, Zhang ZJ, Wang YT, Yang GY. Opportunities and challenges: Stem cell‐based therapy for the treatment of ischemic stroke. CNS Neurosci Ther 2015;21:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen J, Leak RK, Yang GY. Perspective for stroke and brain injury research: Mechanisms and potential therapeutic targets. CNS Neurosci Ther 2015;21:301–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Y, Guan YM, Huang HL, Wang QS. Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacol Sin 2014;35:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tripathy D, Verma P, Nthenge‐Ngumbau DN, Banerjee M, Mohanakumar KP. Regenerative therapy in experimental Parkinsonism: Mixed population of differentiated mouse embryonic stem cells, rather than magnetically sorted and enriched dopaminergic cells provide neuroprotection. CNS Neurosci Ther 2014;20:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Preiss J, Handler P. Enzymatic synthesis of nicotinamide mononucleotide. J Biol Chem 1957;225:759–770. [PubMed] [Google Scholar]
- 39. Siderovski DP, Blum S, Forsdyke RE, Forsdyke DR. A set of human putative lymphocyte G0/G1 switch genes includes genes homologous to rodent cytokine and zinc finger protein‐encoding genes. DNA Cell Biol 1990;9:579–587. [DOI] [PubMed] [Google Scholar]
- 40. Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre‐B‐cell colony‐enhancing factor. Mol Cell Biol 1994;14:1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin PR, Shea RJ, Mulks MH. Identification of a plasmid‐encoded gene from Haemophilus ducreyi which confers NAD independence. J Bacteriol 2001;183:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rongvaux A, Shea RJ, Mulks MH, et al. Pre‐B‐cell colony‐enhancing factor, whose expression is up‐regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol 2002;32:3225–3234. [DOI] [PubMed] [Google Scholar]
- 43. Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT‐mediated NAD+ biosynthesis. Science 2009;324:651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone‐Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK‐SIRT1. Science 2009;324:654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao Y, Liu XZ, Tian WW, Guan YF, Wang P, Miao CY. Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury. CNS Neurosci Ther 2014;20:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jing Z, Xing J, Chen X, et al. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. J Cereb Blood Flow Metab 2014;34:1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao Y, Guan YF, Zhou XM, et al. Regenerative neurogenesis after ischemic stroke promoted by nicotinamide phosphoribosyltransferase‐nicotinamide adenine dinucleotide cascade. Stroke 2015;46:1966–1974. [DOI] [PubMed] [Google Scholar]
- 48. Kitani T, Okuno S, Fujisawa H. Growth phase‐dependent changes in the subcellular localization of pre‐B‐cell colony‐enhancing factor11The nucleotide sequence of cDNA for rat PBEF has been submitted to the DDBJ/EMBL/GenBank under the accession number AB081730. FEBS Lett 2003;544:74–78. [DOI] [PubMed] [Google Scholar]
- 49. Revollo JR, Korner A, Mills KF, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 2007;6:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang H, Yang T, Baur JA, et al. Nutrient‐sensitive mitochondrial NAD+ levels dictate cell survival. Cell 2007;130:1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pittelli M, Formentini L, Faraco G, et al. Inhibition of nicotinamide phosphoribosyltransferase: Cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J Biol Chem 2010;285:34106–34114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 2015;11:535–546. [DOI] [PubMed] [Google Scholar]
- 53. Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol 2014;24:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 2004;279:50754–50763. [DOI] [PubMed] [Google Scholar]
- 55. Bowlby SC, Thomas MJ, D'Agostino RB, Kridel SJ. Nicotinamide phosphoribosyl transferase (Nampt) is required for de novo lipogenesis in tumor cells. PLoS One 2012;7:e40195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rodgers JT, Lerin C, Gerhart‐Hines Z, Puigserver P. Metabolic adaptations through the PGC‐1 alpha and SIRT1 pathways. FEBS Lett 2008;582:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bruzzone S, Fruscione F, Morando S, et al. Catastrophic NAD+ depletion in activated T lymphocytes through Nampt inhibition reduces demyelination and disability in EAE. PLoS One 2009;4:e7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Koltai E, Szabo Z, Atalay M, et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev 2010;131:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Gool F, Galli M, Gueydan C, et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin‐dependent manner. Nat Med 2009;15:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP‐ribose) polymerase‐1‐dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem 2005;280:43121–43130. [DOI] [PubMed] [Google Scholar]
- 61. van der Horst A, Tertoolen LG, de Vries‐Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J Biol Chem 2004;279:28873–28879. [DOI] [PubMed] [Google Scholar]
- 62. Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol 2006;4:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Luo X, Kraus WL. On PAR with PARP: Cellular stress signaling through poly(ADP‐ribose) and PARP‐1. Genes Dev 2012;26:417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Song J, Ke SF, Zhou CC, et al. Nicotinamide phosphoribosyltransferase is required for the calorie restriction‐mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J Gerontol A Biol Sci Med Sci 2014;69:44–57. [DOI] [PubMed] [Google Scholar]
- 65. Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 2012;11:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang P, Miao CY. NAMPT as a therapeutic target against stroke. Trends Pharmacol Sci 2015;36:891–905. [DOI] [PubMed] [Google Scholar]
- 67. Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J 2014;33:1321–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang W, Xie Y, Wang T, et al. Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab 2010;30:1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang P, Xu TY, Guan YF, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1‐dependent adenosine monophosphate‐activated kinase pathway. Ann Neurol 2011;69:360–374. [DOI] [PubMed] [Google Scholar]
- 70. Yoon MJ, Yoshida M, Johnson S, et al. SIRT1‐Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab 2015;21:706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue‐derived visfatin is a vascular smooth muscle cell growth factor: Role of nicotinamide mononucleotide. Cardiovasc Res 2009;81:370–380. [DOI] [PubMed] [Google Scholar]
- 72. Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol 2012;165:643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet‐ and age‐induced diabetes in mice. Cell Metab 2011;14:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Karamanlidis G, Lee CF, Garcia‐Menendez L, et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 2013;18:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gomes AP, Price NL, Ling AJ, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear‐mitochondrial communication during aging. Cell 2013;155:1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mouchiroud L, Houtkooper RH, Moullan N, et al. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013;154:430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 2013;153:1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Satoh A, Stein L, Imai S. The role of mammalian sirtuins in the regulation of metabolism, aging, and longevity. Handb Exp Pharmacol 2011;206:125–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang P, Vanhoutte PM, Miao CY. Visfatin and cardio‐cerebro‐vascular disease. J Cardiovasc Pharmacol 2012;59:1–9. [DOI] [PubMed] [Google Scholar]
- 80. Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP‐ribose) polymerase‐1‐mediated neuronal death. J Neurosci 2010;30:2967–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bi J, Li H, Ye SQ, Ding S. Pre‐B‐cell colony‐enhancing factor exerts a neuronal protection through its enzymatic activity and the reduction of mitochondrial dysfunction in in vitro ischemic models. J Neurochem 2012;120:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang S, Xing Z, Vosler PS, et al. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: Role of enhanced DNA repair. Stroke 2008;39:2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F. Nampt/PBEF/visfatin exerts neuroprotective effects against ischemia/reperfusion injury via modulation of Bax/Bcl‐2 ratio and prevention of caspase‐3 activation. J Mol Neurosci 2015;56:237–243. [DOI] [PubMed] [Google Scholar]
- 84. Erfani S, Khaksari M, Oryan S, et al. Visfatin reduces hippocampal CA1 cells death and improves learning and memory deficits after transient global ischemia/reperfusion. Neuropeptides 2015;49:63–68. [DOI] [PubMed] [Google Scholar]
- 85. Erfani S, Aboutaleb N, Oryan S, et al. Visfatin inhibits apoptosis and necrosis of hippocampus CA3 cells following transient global ischemia/reperfusion in rats. Int J Pep Res Ther 2015;21:223–228. [Google Scholar]
- 86. Zheng C, Han J, Xia W, Shi S, Liu J, Ying W. NAD(+) administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett 2012;512:67–71. [DOI] [PubMed] [Google Scholar]
- 87. Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 2012;8:77–87. [DOI] [PubMed] [Google Scholar]
- 88. Wang P, Li WL, Liu JM, Miao CY. NAMPT and NAMPT‐controlled NAD metabolism in vascular repair. J Cardiovasc Pharmacol 2015. DOI: 10.1097/FJC.0000000000000332 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 89. Wang P, Guan YF, Li WL, Lu GC, Liu JM, Miao CY. Nicotinamide phosphoribosyltransferase facilitates post‐stroke angiogenesis. CNS Neurosci Ther 2015;21:475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lovren F, Pan Y, Shukla PC, et al. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: Translational implications for atherosclerosis. Am J Physiol Endocrinol Metab 2009;296:E1440–E1449. [DOI] [PubMed] [Google Scholar]
- 91. Wang P, Du H, Zhou CC, et al. Intracellular NAMPT‐NAD+‐SIRT1 cascade improves post‐ischaemic vascular repair by modulating Notch signalling in endothelial progenitors. Cardiovasc Res 2014;104:477–488. [DOI] [PubMed] [Google Scholar]
- 92. Xu TY, Zhang SL, Dong GQ, et al. Discovery and characterization of novel small‐molecule inhibitors targeting nicotinamide phosphoribosyltransferase. Sci Rep 2015;5:10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang X, Xu TY, Liu XZ, et al. Discovery of novel inhibitors and fluorescent probe targeting NAMPT. Sci Rep 2015;5:12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sampath D, Zabka TS, Misner DL, O'Brien T, Dragovich PS. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol Ther 2015;151:16–31. [DOI] [PubMed] [Google Scholar]
- 95. Wang G, Han T, Nijhawan D, et al. P7C3 neuroprotective chemicals function by activating the rate‐limiting enzyme in NAD salvage. Cell 2014;158:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pieper AA, Xie S, Capota E, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell 2010;142:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Naidoo J, De Jesus‐Cortes H, Huntington P, et al. Discovery of a neuroprotective chemical, (S)‐N‐(3‐(3,6‐dibromo‐9H‐carbazol‐9‐yl)‐2‐fluoropropyl)‐6‐methoxypyridin‐2‐amine [(‐)‐P7C3‐S243], with improved druglike properties. J Med Chem 2014;57:3746–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. MacMillan KS, Naidoo J, Liang J, et al. Development of proneurogenic, neuroprotective small molecules. J Am Chem Soc 2011;133:1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yoon HJ, Kong SY, Park MH, et al. Aminopropyl carbazole analogues as potent enhancers of neurogenesis. Bioorg Med Chem 2013;21:7165–7174. [DOI] [PubMed] [Google Scholar]
- 100. Erbel‐Sieler C, Dudley C, Zhou Y, et al. Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc Natl Acad Sci USA 2004;101:13648–13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pieper AA, Wu X, Han TW, et al. The neuronal PAS domain protein 3 transcription factor controls FGF‐mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci USA 2005;102:14052–14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. De Jesus‐Cortes H, Xu P, Drawbridge J, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci USA 2012;109:17010–17015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shin JY, Kong SY, Yoon HJ, Ann J, Lee J, Kim HJ. An aminopropyl carbazole derivative induces neurogenesis by increasing final cell division in neural stem cells. Biomol Ther (Seoul) 2015;23:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Blaya MO, Bramlett HM, Naidoo J, Pieper AA, Dietrich WD. Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J Neurotrauma 2014;31:476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yin TC, Britt JK, De Jesus‐Cortes H, et al. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep 2014;8:1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kemp SW, Szynkaruk M, Stanoulis KN, et al. Pharmacologic rescue of motor and sensory function by the neuroprotective compound P7C3 following neonatal nerve injury. Neuroscience 2015;284:202–216. [DOI] [PubMed] [Google Scholar]
- 107. Latchney SE, Jaramillo TC, Rivera PD, Eisch AJ, Powell CM. Chronic P7C3 treatment restores hippocampal neurogenesis. Neurosci Lett 2015;591:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Walker AK, Rivera PD, Wang Q, et al. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatry 2015;20:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tesla R, Wolf HP, Xu P, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 2012;109:17016–17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- 111. Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]


