Abstract
Extracellular fluid overload (FO), which is assessed using bioimpedance technologies, is an important predictor of outcome in dialysis patients and in patients with early stages of chronic kidney disease. While traditional cardiovascular abnormalities are assumed to mediate this risk, recently also, the importance of noncardiovascular factors, such as systemic inflammation and malnutrition has been shown. While both FO and inflammation are independent risk factors for mortality, recent studies have shown that their combined presence can lead to a cumulative risk profile. From a pathophysiologic viewpoint, FO and inflammation can also be mutually reinforcing. Inflammation could contribute to FO by hypoalbuminemia, capillary leakage, and a (unnoticed) decline in lean and/or fat tissue mass resulting in incorrect estimation of dry weight. Reciprocally, FO could lead to inflammation by the translocation of endotoxins through a congested bowel wall or by a proinflammatory effect of tissue sodium. The relative importance of these putative factors is, however, not clear yet and epidemiological studies have shown no clear temporal direction regarding the relationship between FO and inflammation. FO and inflammation appear to be part of (dynamic) clusters of risk factors, including malnutrition and hyponatremia. Technology-guided fluid management of the often vulnerable dialysis patient with FO and inflammation cannot yet be based on evidence from randomized controlled trials, in which these specific patients were in general not included. In the absence of those trials, treatment should be based on identifying actionable causes of inflammation and on the judicious removal of excess volume based on frequent clinical reassessment.
Video Journal Club ‘Cappuccino with Claudio Ronco' at http://www.karger.com/?doi=485153.
Keywords: Pathophysiology, Extracellular fluid, Inflammation, Outcomes, Malnutrition, Segmental bioimpedance
Introduction
Extracellular fluid overload (FO) is a major risk factor for mortality in dialysis patients [1] but also in patients at earlier stages of chronic kidney disease (CKD) [2]. Traditionally, the relation between FO and outcome has been explained by its cardiovascular effects [3], such as left ventricular hypertrophy, systolic dysfunction, pulmonary hypertension, and increased aortic stiffness [4, 5, 6, 7], but recent evidence suggests that noncardiovascular factors are also of importance in further explaining the relation between FO and mortality [8, 9]. One important factor appears to be systemic inflammation [10, 11, 12]. In this short review, the relevance of association between noncardiovascular risk factors and FO, with special emphasis on inflammation, is discussed.
Assessment of FO
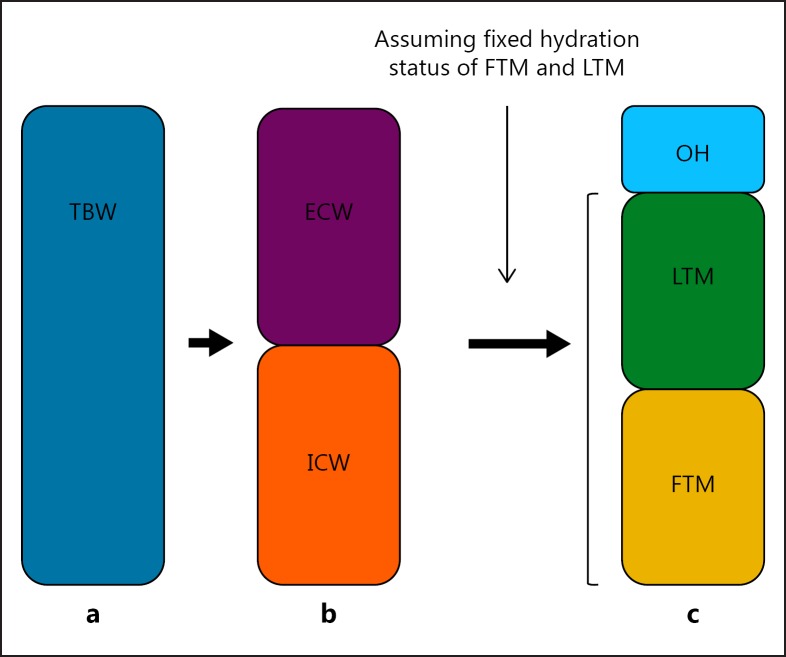
When physical examination remains the mainstay of the assessment of FO in dialysis patients, in the literature exploring the relation between FO and inflammation, most recent evidence has been obtained by bioimpedance spectroscopy (BIS). BIS measures the resistance and reactance (the latter reflecting the capacitance of cell membranes) of body tissues to an alternating current at multiple frequencies from which intra- and extracellular water (ICW and ECW) can be estimated [13]. Using this method, FO was conventionally expressed by the ECW:ICW ratio or by normalized ECW [14]. Recently, a 3-compartment model was developed, differentiating between fat tissue mass (FTM), lean tissue mass (LTM) and an overhydration (OH) compartment, which is calculated based on assumptions regarding the hydration of lean- and adipose tissue [15, 16], Figure1. Different cutoff levels for this compartment have been used in the literature, varying between an OH:ECW ratio of 7–15% and absolute values of OH >+1.1 or >+2.5 L [2, 10, 14, 17]. However, BIS is not the only bioimpedance (BIA)-based technology on which the assessment of fluid status in dialysis patients is based, because segmental technologies or vector plots have also been used for this purpose [13].
Fig. 1.
Different models to assess fluid status. a Total body water (TBW) compartment. b TBW compartment divided into the intracellular water (ICW) and extracellular water (ECW) compartment. c Display of the body compartments as calculated according to the 3-compartment model assuming a fixed hydration status of lean tissue mass (LTM) and fat tissue mass (FTM).
Evidence for the Relation between FO and Inflammation
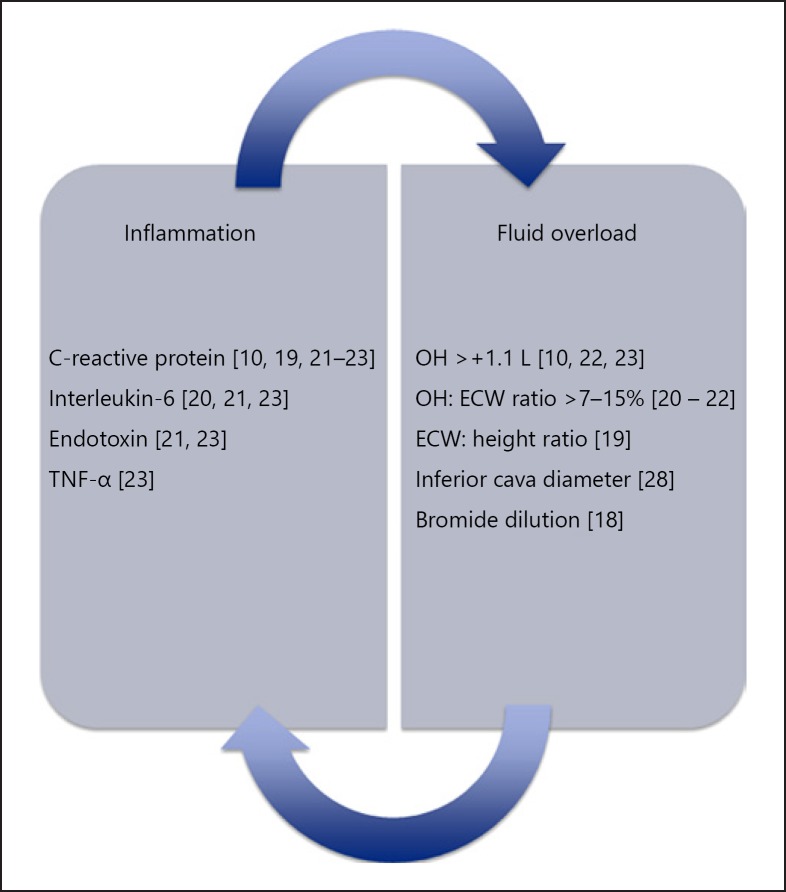
Various studies have reported a relation between FO and inflammation in both hemodialysis (HD) and peritoneal dialysis (PD) patients [10, 18, 19], Figure 2. Regarding the more recent studies, interleukin 6 (IL-6) levels were significantly related to the OH:ECW ratio as well as to N-terminal pro Brain Natriuretic Peptide in 57 PD patients [20]. The association between FO and IL-6 was confirmed in a larger study also including HD and CKD patients not on dialysis. In this same study, an association between FO and other markers of inflammation, namely, endotoxin and C-reactive protein (CRP) was also found [21]. The association between CRP as a marker of inflammation and FO was further confirmed in a study of 59 PD patients [19] and in a single-center study, using segmental BIA, in CKD patients [22]. A larger study, performed by Hung et al. [23] showed significant associations between IL-6 as well as tumor necrosis factor alpha with the OH:ECW ratio in 338 CKD patients. These results were confirmed and expanded upon by an international study in 8,883 HD patients, where we observed incrementally higher CRP levels in categories of patients with progressive levels of FO using MF-BIS [10] (Fig. 1). Most studies assessing the relation between FO and inflammation have been done using BIS in which the 3-compartment model is based on a fixed assumption regarding the hydration ratio of LTM and FTM [24]. However, theoretically, this ratio might be altered by a fluid shift from the intra- to the extracellular fluid compartments due to a loss of cellular membrane integrity (“sick cell syndrome”) or by excretion of inorganic phosphate and potassium from diseased cells [25, 26, 27]. However, in a previous study, we also found a relation between FO, assessed by the bromide dilution method, and inflammation, assessed by CRP levels in PD patients, suggesting that the relation between FO and inflammation is not an artificial construct but a real biological phenomenon [18]. In addition, Goncalves et al. [28] observed higher endotoxin, but not CRP levels, in fluid overloaded patients according to the inferior cava vein diameter.
Fig. 2.
Known associations between fluid overload (FO) and inflammation. Known associations between FO and inflammation in patients with end stage renal disease, peritoneal dialysis and on hemodialysis.
The relation between FO and inflammation has not been confirmed in all studies. For instance, Vega et al. [44] observed lower pre-albumin levels in FO dialysis patients (defined as the OH/ECW level >10%), whereas CRP levels were not significantly different. Moreover, in a longitudinal study of 44 patients, we observed a strong negative correlation between CRP levels and phase angle but not with normalized ECW assessed by BIS [29]. Antlanger et al. [30] did not observe a relation between the OH:ECW ratio and CRP levels in 126 dialysis patients. However, in the largest studies on this topic, this relation appears to be robust [10, 21].
Combined Effects of FO and Inflammation on Outcome
While FO in itself is an independent risk factor for mortality, after correction for other risk markers [12], the combined presence of FO and inflammation is also associated with an additive risk of mortality. For instance, the presence of mild FO (defined as OH >+1.1 to +2.5 L before dialysis) was associated with an hazard ratio (HR) of 1.67 (95% CI 1.19–2.33) for mortality in the absence of inflammation (defined as CRP levels >6 mg/L) but increased to an HR of 3.99 (95% CI 2.94–5.43) in the presence of inflammation. The increased mortality risk appears to persist, albeit to a lesser degree, even after the resolution of both risk factors during a subsequent 3 months follow-up period (HR 3.28 with resolution of both risk factors, as compared to a HR of 9.44 with persistent presence of inflammation and FO) [10]. Interestingly, these results are contradicted by another study in which the ECW:ICW ratio, assessed by segmental BIA, nullified the effects of CRP on outcome [22]. However, this ratio is a combined marker of FO and/or malnutrition, given the fact that a loss of LTM may be reflected by a reduction of ICW. In another study in the same cohort, we found that inflammation, defined by CRP levels >6 mg/L, remained an independent predictor of survival after correction for FO, hyponatremia, and the presence of a LTM below the 10th percentile [12].
Pathophysiological Explanations for the Relation between FO and Inflammation
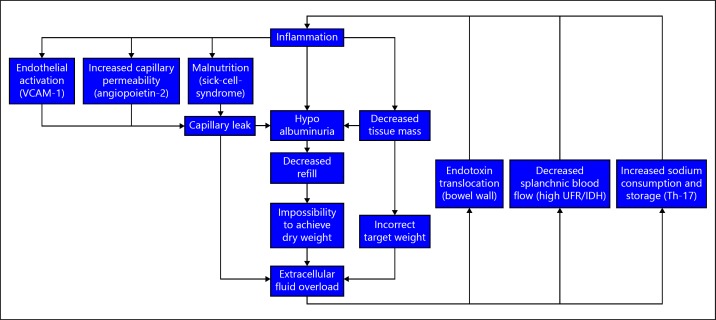
There are different potential explanations for the relation between FO and inflammation, Figure 3. Inflammation can theoretically lead to FO because of an unnoticed reduction in FTM or LTM not followed by a reduction in prescribed dry weight in HD patients. Another important potential culprit is hypoalbuminemia, which can be strongly related to inflammation [31]. The relation between hypoalbuminemia and FO in dialysis patients has been established previously [32], and was confirmed in our recent study by progressively lower serum albumin levels in groups with incremental levels of FO [10]. Hypoalbuminemia can lead to a translocation of intravascular volume to the interstitial compartment and thus hamper fluid removal during dialysis [33]. On the other hand, FO per se can also lower serum albumin levels by dilution, as evidenced by the increase in serum albumin levels after increased ultrafiltration [34]. Inflammation might also induce interstitial fluid accumulation by an increased capillary permeability. In the single study, which evaluated capillary leakage by the Iodine-125 albumin methodology, capillary permeability was more strongly related to markers of platelet activation as compared to inflammatory markers [35]. However, we recently observed, both in a CKD-5 non-dialysis, as well as in prevalent HD patients population, a significant relation between vascular cell adhesion protein-1 levels, a marker of endothelial activation, and FO assessed by BIS (submitted data). Moreover, in CKD non-dialysis patients, angiopoietin 2, a factor that stimulates capillary permeability, was significantly related to FO, and had an additive effect on mortality risk [17].
Fig. 3.
Potential pathophysiological explanations of the fluid overload and inflammation axis. VCAM, vascular cell adhesion protein; UFR, ultrafiltration rate; IDH, intradialytic hypotension; Th-17, T-helper 17 cells.
On the other hand, FO might also induce inflammation by passage of endotoxin fragments through a congested bowel wall, or by inducing splanchnic ischemia [36, 37, 38, 39, 40, 41]. Until now, the relative importance of FO in the pathogenesis of the systemic endotoxemia is not fully clear. In a pilot study in 8 PD patients and 9 healthy controls where splanchnic perfusion was assessed by MRI, endotoxin levels were higher in PD patients, but no relation with volume status or splanchnic blood flow was observed, although patients in this study did not appear to be severely fluid overloaded [42]. In another study, the same group observed a relation between endotoxin levels and ultrafiltration volume in HD patients, but not with N-terminal pro Brain Natriuretic Peptide levels [41]. Another factor by which FO can induce inflammation is by the proinflammatory effects of tissue sodium by inducing T-helper 17 cells [37]. In a CKD rat model, it was also shown that a high salt diet induced systemic inflammation [43].
At present, the relative importance of the proposed mechanisms is not yet clear. Also from longitudinal epidemiological data, we could not distill a clear order of priority between both possibilities, as FO both preceded and followed a period with elevated CRP levels in a comparable percentage of patients [10].
FO and Inflammation: Part of a Broader Spectrum?
Inflammation and FO may be present in isolation as well as in combination but may also be part of a wider spectrum of both cardiovascular as well as noncardiovascular risk factors [44]. The relation between markers of FO and malnutrition has also been described in the literature [23, 45, 46]. However, sometimes the interpretation may be hampered by the fact that the ECW:total body water was used as a marker of FO [46], whereas this is likely a combined risk marker expressing either FO, malnutrition, or both [22]. FO has also been associated with the malnutrition-inflammation-atherosclerosis syndrome [19, 23].
Strictly speaking, whether FO forms part of a syndrome (defined as a group of symptoms which consistently occur together) or whether it is part of, often temporally dynamic, phenotypical clusters in which risk factors are associated with a cumulative risk of adverse outcome, as suggested by a recent study from our group, yet remains a question [10].
These results were expanded in a study where we defined clusters based on the presence of FO (OH> +1.1 L pre-dialysis) assessed by BIS, inflammation (CRP levels >6 mg/L), and malnutrition (Lean Tissue Index below the 10th percentile of a healthy gender matched control population) and showed that the largest risk for mortality was observed in clusters where all 3 risk factors were present (OR 5.89, 95% CI 2.28–8.01) as compared to patients with only inflammation (OR 2.06, 95% CI 1.41–2.99) or FO (OR 1.79, 95% CI 1.26–2.52) as a single risk factor (submitted data). Surprisingly, malnutrition, in the absence of other risk factors was not significantly related to outcome.
Implications for Management
While the epidemiologic relation between FO, inflammation and outcome has been firmly established and their frequent assessment may add to a powerful risk profile of the patient, there are very limited data regarding the effects of therapeutic interventions in the inflamed and fluid overloaded patient. In chronic heart failure patients, a reduction in inflammatory markers was observed after decongestion [47], but we are not aware of comparable data in patients with renal failure. Two randomized controlled trials have addressed the effect of a BIS-guided fluid strategy compared to conventional fluid management in HD patients. Both studies found a significant reduction in blood pressure and pulse wave velocity. Mean pre-dialysis FO levels were +1.48 and +2.0 L before intervention, and relatively young HD patients were included with a mean age of 51 and 52 years in these studies. In the study by Hur et al. [48], no significant decrease in CRP level was observed; in the study by Onofriescu et al. [49], no data on CRP-levels were available. Importantly, in the latter study, a significant reduction in mortality was observed in the intervention group after a 2.5 year follow-up period. Despite the fact that the results of these trials look very promising, it should be noted that patients in these studies were relatively young and the results may not be transposable to an elderly population with extensive comorbidity and systemic inflammation. Therefore, we do not know if attaining euvolemia, for example, by BIS-guided strategies, is feasible or desirable under these circumstances. It cannot be excluded that rapid and overzealous ultrafiltration may put the patient at risk for hypotension and organ ischemia, especially when the refill of plasma volume from the interstitial compartments is hampered by hypoalbuminemia [50]. We suggest that under these circumstances, a pragmatic approach be followed based on frequent clinical reevaluation, with judicious ultrafiltration and increased dialysis frequency where needed, in combination with nutritional support and identification of treatable causes of inflammation.
Conclusion
FO is an important risk factor for mortality in CKD non-dialysis, as well as in PD and HD patients. Recent studies have suggested that apart from its cardiovascular effects, FO also has important noncardiovascular associations, notably systemic inflammation. There are various, mutually reinforcing mechanisms by which FO and inflammation may be connected but epidemiological studies have so far not identified a clear temporal pattern between both risk factors. FO and inflammation are both powerful independent risk factors for outcome, but their combination yields an additive risk. Most likely, both factors are part of an often dynamic cluster of risk factors including malnutrition. While frequent measurement of FO aids in the construction of a detailed, important risk profile, the therapeutic implications in fluid overloaded patients with inflammation should be the focus of future studies, as trials in the field of guided fluid management have not specifically addressed this vulnerable subgroup.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–1579. doi: 10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai YC, Chiu YW, Tsai JC, Kuo HT, Hung CC, Hwang SJ, et al. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol. 2015;10:39–46. doi: 10.2215/CJN.03610414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz A, Yilmaz B, Kucukseymen S, Ozpelit E, Pekel N. Association of overhydration and cardiac dysfunction in patients have chronic kidney disease but not yet dialysis. Nephrol Ther. 2016;12:94–97. doi: 10.1016/j.nephro.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Kocyigit I, Sipahioglu MH, Orscelik O, Unal A, Celik A, Abbas SR, et al. The association between arterial stiffness and fluid status in peritoneal dialysis patients. Perit Dial Int. 2014;34:781–790. doi: 10.3747/pdi.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yilmaz S, Yildirim Y, Taylan M, Demir M, Yilmaz Z, Kara AV, et al. The relationship of fluid overload as assessed by bioelectrical impedance analysis with pulmonary arterial hypertension in hemodialysis patients. Med Sci Monit. 2016;22:488–494. doi: 10.12659/MSM.896305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan K, Hassan D, Shturman A, Rubinchik I, Fadi H, Shadi H, et al. The impact of sub-clinical over-hydration on left ventricular mass in peritoneal dialysis patients. Int J Clin Exp Med. 2015;8:5890–5896. [PMC free article] [PubMed] [Google Scholar]
- 8.Onofriescu M, Siriopol D, Voroneanu L, Hogas S, Nistor I, Apetrii M, et al. Overhydration, cardiac function and survival in hemodialysis patients. PLoS One. 2015;10:e0135691. doi: 10.1371/journal.pone.0135691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jotterand Drepper V, Kihm LP, Kalble F, Diekmann C, Seckinger J, Sommerer C, et al. Overhydration is a strong predictor of mortality in peritoneal dialysis patients - independently of cardiac failure. PLoS One. 2016;11:e0158741. doi: 10.1371/journal.pone.0158741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekker MJ, Marcelli D, Canaud BJ, Carioni P, Wang Y, Grassmann A, et al. Impact of fluid status and inflammation and their interaction on survival: a study in an international hemodialysis patient cohort. Kidney Int. 2017;91:1214–1223. doi: 10.1016/j.kint.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Reyes-Bahamonde J, Raimann JG, Thijssen S, Levin NW, Kotanko P. Fluid overload and inflammation - a vicious cycle. Semin Dial. 2013;26:31–35. doi: 10.1111/sdi.12024. [DOI] [PubMed] [Google Scholar]
- 12.Dekker MJ, Marcelli D, Canaud B, Konings CJ, Leunissen KM, Levin NW, et al. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: results from the international MONDO initiative. Eur J Clin Nutr. 2016;70:779–784. doi: 10.1038/ejcn.2016.49. [DOI] [PubMed] [Google Scholar]
- 13.Zhu F, Rosales L, Kotanko P. Techniques for assessing fluids status in patients with kidney disease. Curr Opin Nephrol Hypertens. 2016;25:473–479. doi: 10.1097/MNH.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 14.Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 2014;86:489–496. doi: 10.1038/ki.2014.207. [DOI] [PubMed] [Google Scholar]
- 15.Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27:75–80. doi: 10.1159/000167013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broers NJ, Martens RJ, Cornelis T, Diederen NM, Wabel P, van der Sande FM, et al. Body composition in dialysis patients: a functional assessment of bioimpedance using different prediction models. J Ren Nutr. 2015;25:121–128. doi: 10.1053/j.jrn.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YC, Chiu YW, Kuo HT, Lee JJ, Lee SC, Chen TH, et al. The interaction between fluid status and angiopoietin-2 in adverse renal outcomes of chronic kidney disease. PLoS One. 2017;12:e0173906. doi: 10.1371/journal.pone.0173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konings CJ, Kooman JP, Schonck M, Struijk DG, Gladziwa U, Hoorntje SJ, et al. Fluid status in CAPD patients is related to peritoneal transport and residual renal function: evidence from a longitudinal study. Nephrol Dial Transplant. 2003;18:797–803. doi: 10.1093/ndt/gfg147. [DOI] [PubMed] [Google Scholar]
- 19.Demirci MS, Demirci C, Ozdogan O, Kircelli F, Akcicek F, Basci A, et al. Relations between malnutrition-inflammation-atherosclerosis and volume status. The usefulness of bioimpedance analysis in peritoneal dialysis patients. Nephrol Dial Transplant. 2011;26:1708–1716. doi: 10.1093/ndt/gfq588. [DOI] [PubMed] [Google Scholar]
- 20.Sikorska D, Pawlaczyk K, Roszak M, Czepulis N, Oko A, Karczewski M, et al. Preliminary observations on the association between serum IL-6 and hydration status and cardiovascular risk in patients treated with peritoneal dialysis. Cytokine. 2016;85:171–176. doi: 10.1016/j.cyto.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Hassan MO, Duarte R, Dix-Peek T, Vachiat A, Naidoo S, Dickens C, et al. Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin Nephrol. 2016;86:131–135. doi: 10.5414/CNP86S127. [DOI] [PubMed] [Google Scholar]
- 22.Kim EJ, Choi MJ, Lee JH, Oh JE, Seo JW, Lee YK, et al. Extracellular fluid/intracellular fluid volume ratio as a novel risk indicator for all-cause mortality and cardiovascular disease in hemodialysis patients. PLoS One. 2017;12:e0170272. doi: 10.1371/journal.pone.0170272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014;85:703–709. doi: 10.1038/ki.2013.336. [DOI] [PubMed] [Google Scholar]
- 24.Chamney PW, Wabel P, Moissl UM, Muller MJ, Bosy-Westphal A, Korth O, et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007;85:80–89. doi: 10.1093/ajcn/85.1.80. [DOI] [PubMed] [Google Scholar]
- 25.Flear CT, Florence I, Williams JA. Water, sodium, potassium, and chloride content of skeletal muscle in fit and ill subjucts. J Clin Pathol. 1968;21:555–563. doi: 10.1136/jcp.21.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill GV, Osypiw JC, Shearer E, English PJ, Watson ID. Critical illness with hyponatraemia and impaired cell membrane integrity - the ‘sick cell syndrome' revisited. Clin Biochem. 2005;38:1045–1048. doi: 10.1016/j.clinbiochem.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Cherney DZ, Zevallos G, Oreopoulos D, Halperin ML. A physiological analysis of hyponatremia: implications for patients on peritoneal dialysis. Perit Dial Int. 2001;21:7–13. [PubMed] [Google Scholar]
- 28.Goncalves S, Pecoits-Filho R, Perreto S, Barberato SH, Stinghen AE, Lima EG, et al. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21:2788–2794. doi: 10.1093/ndt/gfl273. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs LH, van de Kerkhof JJ, Mingels AM, Passos VL, Kleijnen VW, Mazairac AH, et al. Inflammation, overhydration and cardiac biomarkers in haemodialysis patients: a longitudinal study. Nephrol Dial Transplant. 2010;25:243–248. doi: 10.1093/ndt/gfp417. [DOI] [PubMed] [Google Scholar]
- 30.Antlanger M, Hecking M, Haidinger M, Werzowa J, Kovarik JJ, Paul G, et al. Fluid overload in hemodialysis patients: a cross-sectional study to determine its association with cardiac biomarkers and nutritional status. BMC Nephrol. 2013;14:266. doi: 10.1186/1471-2369-14-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaysen GA, Greene T, Daugirdas JT, Kimmel PL, Schulman GW, Toto RD, et al. Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am J Kidney Dis. 2003;42:1200–1211. doi: 10.1053/j.ajkd.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Jones CH, Akbani H, Croft DC, Worth DP. The relationship between serum albumin and hydration status in hemodialysis patients. J Ren Nutr. 2002;12:209–212. doi: 10.1053/jren.2002.35295. [DOI] [PubMed] [Google Scholar]
- 33.John B, Tan BK, Dainty S, Spanel P, Smith D, Davies SJ. Plasma volume, albumin, and fluid status in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2010;5:1463–1470. doi: 10.2215/CJN.09411209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones CH, Wells L, Stoves J, Farquhar F, Woodrow G. Can a reduction in extracellular fluid volume result in increased serum albumin in peritoneal dialysis patients? Am J Kidney Dis. 2002;39:872–875. doi: 10.1053/ajkd.2002.32010. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Tan BK, Dainty S, Mattey DL, Davies SJ. Hypoalbuminaemia, systemic albumin leak and endothelial dysfunction in peritoneal dialysis patients. Nephrol Dial Transplant. 2012;27:4437–4445. doi: 10.1093/ndt/gfs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kooman J, Dekker M, Usvyat LA, Kotanko P, van der Sande F, Schalkwijk CG, et al. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Renal Physiol. 2017;313:F938–F950. doi: 10.1152/ajprenal.00256.2017. [DOI] [PubMed] [Google Scholar]
- 37.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jantsch J, Schatz V, Friedrich D, Schroder A, Kopp C, Siegert I, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 2015;21:493–501. doi: 10.1016/j.cmet.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau WL, Kalantar-Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron. 2015;130:92–98. doi: 10.1159/000381990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 41.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:133–141. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grant C, Harrison L, Hoad C, Marciani L, Cox E, Buchanan C, et al. Endotoxemia in peritoneal dialysis patients: a Pilot Study to Examine the Role of intestinal perfusion and congestion. Perit Dial Int. 2017;37:111–115. doi: 10.3747/pdi.2016.00079. [DOI] [PubMed] [Google Scholar]
- 43.Hung SC, Lai YS, Kuo KL, Tarng DC. Volume overload and adverse outcomes in chronic kidney disease: clinical observational and animal studies. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001918. pii: e001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vega A, Quiroga B, Abad S, Ruiz C, Lopez-Gomez JM. Study on overhydration in dialysis patients and its association with inflammation. Nefrologia. 2014;34:579–583. doi: 10.3265/Nefrologia.pre2014.Jun.12422. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda M, Honda H, Takahashi K, Shishido K, Shibata T. N-terminal pro-B-type natriuretic peptide as a biomarker for loss of muscle mass in prevalent hemodialysis patients. PLoS One. 2016;11:e0166804. doi: 10.1371/journal.pone.0166804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Q, Yi C, Li J, Wu X, Yang X, Yu X. Prevalence and risk factors of fluid overload in Southern Chinese continuous ambulatory peritoneal dialysis patients. PLoS One. 2013;8:e53294. doi: 10.1371/journal.pone.0053294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato Y, Takatsu Y, Kataoka K, Yamada T, Taniguchi R, Sasayama S, et al. Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol. 1999;22:811–813. doi: 10.1002/clc.4960221211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013;61:957–965. doi: 10.1053/j.ajkd.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 49.Onofriescu M, Hogas S, Voroneanu L, Apetrii M, Nistor I, Kanbay M, et al. Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2014;64:111–118. doi: 10.1053/j.ajkd.2014.01.420. [DOI] [PubMed] [Google Scholar]
- 50.Huang SH, Filler G, Lindsay R, McIntyre CW. Euvolemia in hemodialysis patients: a potentially dangerous goal? Semin Dial. 2015;28:1–5. doi: 10.1111/sdi.12317. [DOI] [PubMed] [Google Scholar]