Abstract
Formal [5+1] cycloadditions between aryl-substituted vinylcyclopropanes and nitrenoid precursors are reported. The method, which employs Rh2(esp)2 as a catalyst, leads to the highly regioselective formation of substituted tetrahydropyridines. Preliminary mechanistic studies support a stepwise, polar mechanism enabled by the previously observed Lewis acidity of Rh-nitrenoids. Overall, this work expands the application of nitrene-transfer cycloaddition, a relatively underexplored approach to heterocycle synthesis, to the formation of six-membered rings.
Graphical Abstract

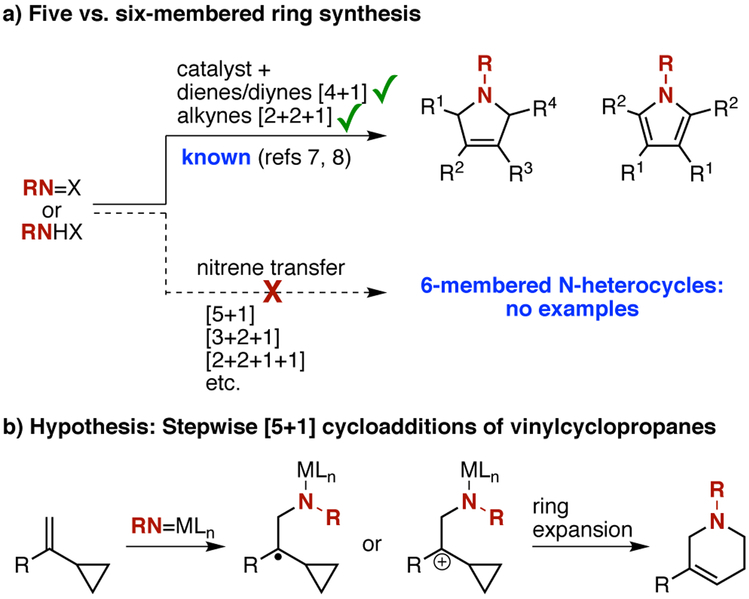
The development of nitrenes and metallonitrenes as reactive intermediates useful for addressing limitations in organic synthesis has recently been accelerated by advances in nitrene-transfer catalysis.1 Yet many potential applications remain unexplored.2 One area of potential growth is their use as one-atom components in cycloaddition reactions for the preparation of nitrogen-containing heterocycles. Even for common N-heterocycles, which are present in a majority of FDA-approved small molecule drugs,3 current synthetic methods are, in many cases, not sufficiently advanced to allow for the rapid and flexible preparation of selectively substituted variants.4 The complementarity of nitrene chemistry to other approaches might provide paths to overcoming these limitations.5 However, only a handful of reports of the use of nitrene precursors in intermolecular cycloaddition reactions have appeared in the literature.6 These include [4+1] cycloadditions with dienes,7 [2+2+1] cycloadditions with alkynes,8 and [2+2+1] cycloadditions with alkynes and nitriles (Scheme 1a).9 All are limited to the formation of five-membered rings. Here, we describe the first examples of six-membered ring synthesis via nitrene-transfer cycloaddition. The overall reaction, which employs a strategy and catalytic approach distinct from the above examples, constitutes a formal [5+1] cycloaddition between a nitrene precursor and a vinylcyclopropane. In addition, the reactions provide a single regioisomer of heterocyclic products that are not readily available through other means.
Scheme 1.
Progress and Challenges in Nitrene-Transfer Cycloaddition
Our strategy relies on the hypothesis that in certain cases, nitrene transfer to the olefin of a vinylcyclopropane (VCP) might produce a substituted cyclopropylcarbinyl cation or radical, arising either directly from the olefin or from an intermediate aziridine.10 Either cyclopropylcarbinyl species, if sufficiently long-lived, would be expected to promote cyclo-propane C–C bond cleavage, ultimately providing a ring-expanded [5+1] cycloaddition product (specifically a tetrahydropyridine) after a subsequent cyclization (Scheme 1b).11 One advantage of this strategy is the potential to exert regiocontrol over the reaction outcome by adjusting substitution on the VCP to favor selective cleavage of one of two differentiated cyclopropane C–C bonds.
As part of our initial exploration of this idea, we evaluated the possibility of achieving the desired tandem nitrene-transfer/ring expansion outcome by employing Rh(II) catalysis (Table 1). In addition to their role as catalytic intermediates in C–H amination12 and aziridination13 reactions, there is evidence that Rh-nitrenoids can act as mild Lewis acids, promoting dipolar ring opening of aziridines.13 In our case, we examined whether this dual nature of the nitrenoid could enable successful nitrene-transfer [5+1] cycloaddition of VCP 1a. Our initial experiments focused in the use of iminoiodinanes as nitrene precursors and Rh2(esp)2 as the catalyst.14 Previous studies have shown a high dependence of reaction yield for intermolecular Rh2(esp)2-catalyzed nitrene transfer reactions on iminoiodinane structure.15 Consistent with this, we observed only trace amounts of the desired [5+1] product 2a using PhINTs (A, entry 1) as the nitrene precursor, but obtained 40% isolated yield of 2,5-diphenyl substituted tetrahydropyridine 3a using PhINTces16 (B, entry 2). In situ formation of the iminoiodinane from TcesNH2 was not compatible with the reaction conditions (entry 3). Further reaction optimization enabled the use of benzyl tosyloxycarbamate C, providing a product 4a that bears a Cbz protecting group (entry 4). The use of the Cbz group offers synthetic advantages over Tces, which requires the use of a large excess of Zn/Cu under acidic conditions for deprotection,16 rather than the catalytic hydrogenolysis conditions required for Cbz removal. Using the related Boc-protected nitrene precursor D, no formation of product 5a was observed (entry 5). Screening of alternative Rh(II) catalysts revealed the particular suitability of Rh2(esp)2 for the desired reaction. Of the other catalysts evaluated, only the use of Rh2(OPiv)4 provided any amount of 4a at room temperature, albeit in substantially reduced yield. Given that Rh2(esp)2 was designed to have enhanced stability over other tetracarboxylate Rh(II) catalysts for the specific purpose of enabling greater turnover in nitrene-transfer reactions,14 this might indicate that the efficiency of the nitrene transfer step is critical to achieving a productive [5+1] cycloaddition. Using the optimal protocol (entry 11), which employs a slight excess of C and lower concentration compared to the initial conditions (0.05 M), a synthetically useful yield could be obtained.
Table 1.
Optimization of a Rh(II)-catalyzed [5+1] reactiona
 | ||||
|---|---|---|---|---|
| entry | nitrene precursor (equiv) |
catalyst (mol %) | R | yield (%) |
| 1b | A (1.1) | Rh2(esp)2 (2) | Ts | <5c |
| 2b | B (1.1) | Rh2(esp)2 (2) | Tces | 40 |
| 3b | B (1.5)d | Rh2(esp)2 (2) | Tces | 0 |
| 4 | C (1.1) | Rh2(esp)2 (2) | Cbz | 36 |
| 5 | D (1.1) | Rh2(esp)2 (2) | Boc | NR |
| 6 | C (1.1) | Rh2(OAc)4 (2) | Cbz | NR |
| 7 | C (1.1) | Rh2(OPiv)4 (2) | Cbz | 10c |
| 8 | C (1.1) | Rh2(tpa)4 (2) | Cbz | 0 |
| 9 | C (1.5) | Rh2(esp)2 (2) | Cbz | 53 |
| 10 | C (2.0) | Rh2(esp)2 (2) | Cbz | 35 |
| 11e | C (1.5) | Rh2(esp)2 (2) | Cbz | 60 |
| 12 | C (1.1) | None | Cbz | NR |
All reactions were conducted on a 0.2 mmol scale of 1a, with isolated yields reported except where noted. NR = no reaction observed.
No NaOAc used, reaction time 10 min.
NMR yield using methyl pivalate as an internal standard.
Formed in situ using 1.5 equiv each of TcesNH2 and PhI(OAc)2.
Reaction concentration 0.05 M in 1a and reaction time 30 h.
With optimized conditions in hand, we surveyed the scope and limitations of this new reaction with regard to electronic modification of each aryl substituent (Figure 1, examples 4a to 4aa). Overall, the expected [5+1] cycloaddition product could be isolated in each case, with effects on reaction yield observed as a function of the nature and position of the substitution. While these effects were generally moderate, certain trends could be observed. For example, the inclusion of electron-donating groups in the para position of the styrenyl aryl group led to reduced yields (e.g. 4a vs 4b and 4s vs 4u), and a similar effect was observed for electron-withdrawing groups in the para position of the cyclopropyl aryl group (e.g. 4i and 4l vs 4a). Reasonable success was observed with fluorinated and trifluoromethylated aryl groups in the R2position, increasing the value of the method for the potential preparation of medicinal agents.17 Other halogenated products (e.g. 4f, 4o, 4x, 4aa) present handles for further diversification via cross coupling. The 2,5-diaryl tetrahydropyridine products have rarely appeared in the literature.18 In addition, more extensively substituted heterocyclic products can also be obtained, as in the tricyclic 4ac. In many cases, the reaction does not reach 100% conversion and a small amount of starting material remains. For selected examples where a substantial amount of the VCP was recovered, yields based on recovered starting material are reported (e.g. 4a, 4d, 4e, and others). In other cases (e.g. 4b), the starting material is completely consumed. In all cases, VCP that is unaccounted for appears to be nonselectively converted to an inseparable mixture of other, unidentifiable products formed in trace amounts. Finally, at the largest scale evaluated (1 mmol of 1a), no substantial effect on reaction yield was observed.19
Figure 1.
Scope and limitations of the [5+1] cycloaddition. Yields shown are of isolated products. All reactions employed 0.2 mmol of vinylcyclopropane as the limiting reagent.
A synthetically advantageous feature of this reaction is, in each case, the observation of only a single regioisomer of the product. This is reflected in both the high degree of selectivity for cleavage of the cyclopropane C–C bond adjacent to the aryl group, leading to a preference for the 2,5-disubstituted product, as well as the position of the olefin. This regioselectivity, which has been confirmed by X-ray crystallography,19 would be consistent with a heterolytic ring opening of the cyclopropane to a stabilized benzylic cation, as part of a stepwise mechanism promoted by the Lewis acidity of the Rh-nitrenoid. Further highlighting a potential role for resonance stabilization of cationic intermediates, alkyl substitution at either the R1 or R2 positions of the VCP abrogated the formation of the desired cycloaddition product. (4ad and 4ae).
To obtain additional preliminary evidence of the reaction mechanism, 24-hour 1H-NMR time course experiments were performed. These data indicate that that the VCP is ~90% consumed after 10 hours, but at the same time point only ~50% of the eventual product formation has occurred. Several long-lived intermediates can be observed but not conclusively identified. Therefore, the data are consistent with the hypothesis of a stepwise [5+1] cycloaddition initiated by nitrene transfer to the olefin of the VCP. An alternative pathway involving initial cleavage of the cyclopropane ring can be tentatively ruled out given that we observe no reaction of cyclopropylbenzene under the reaction conditions. It has previously been shown that VCP 5 undergoes oxyamination to provide 6 under Rh2(esp)2-catalyzed conditions using PhINTces generated in situ. Mechanistic studies of this reaction suggested the involvement of a benzylic carbocation intermediate (Scheme 2a).13 These observations and precedent prompt us to propose the stepwise cationic pathway outlined in Scheme 2b. Aziridination of the substrate20 could lead, after dipolar ring-opening, to formation of an equilibrium among 7 and geometric isomers 8 and 9, with the latter ultimately undergoing ring closure to the observed [5+1] product. This mechanism is also consistent with the regioselectivity of the reaction and the observed trends in yield. For example, an electron-deficient R2 group less able to stabilize a carbocation would disfavor the formation of key intermediate 9, whereas an electron-rich VCP olefin (dependent on the R1 substituent) might be expected to react in an unproductive sense with intermediates 7 or 8 at a higher rate than a comparatively electron-deficient olefin.21 This possibility of oligomerization or other unwanted reactivity of an intermediate carbocation is consistent with the aforementioned observation of complete consumption of the unaccounted-for starting material to a complex mixture of products.
Scheme 2.
Stepwise hypothesis for the Rh-catalyzed cycloaddition based on precedent and preliminary observations.
We have found that the use of 3 equivalents of NaOAc as an additive is essential to achieving the best yields for these reactions. The use of non-nucleophilic bases (e.g. DIPEA) and other carboxylates (e.g. NaOBz, NaOPiv, NaOCOCF3) results in significantly reduced yields in all cases.19 While these results could point to a number of potential roles for acetate in the reaction mechanism beyond serving as a base to deprotonate the nitrene precursor, one possibility is that it reacts as a nucleophile with carbocations 7, 8, or 9 to form one or more of the long-lived intermediates that are observed by NMR. A similar role for tosylate liberated from C might be envisioned; nosylate and mesylate analogs of C perform substantially less well in the reaction.19 Our attempts to synthesize the potential aziridine and acetate intermediates derived from 1a have so far been unsuccessful using a variety of reaction conditions; decomposition of the starting material is observed rather than a productive reaction. This might indicate that if these intermediates are formed, they are prone to further reactivity such as that which we observe in the [5+1] reaction.
Finally, we envision that the tetrahydropyridine products of this reaction can serve as key intermediates in the synthesis of regio- and stereoselectively substituted piperidines, which are the most common N-heterocycle in FDA-approved drugs.3 Preliminary investigations that illustrate this capability are shown in Scheme 3. Hydrogenation of 4a to the fully saturated piperidine, with concurrent removal of the Cbz protecting group, proceeds in high yield to reveal the free amine 10. Oxidation or other non-hydrogenative functionalization of the olefin provides an avenue to access increasingly complex piperidines. In this regard, epoxidation of 4a proceeds with remarkably high diastereoselectivity, setting two new stereocenters and providing 2,4,5-trisubstituted piperidine derivative 11.
Scheme 3.
Derivatization of product 4a.
In summary, the first examples of the use of nitrene-transfer cycloaddition for the preparation of six-membered nitrogen-containing heterocycles have been presented. The method, which uses Rh2(esp)2 to catalyze nitrene transfer to a vinylcyclopropane in an overall [5+1] cycloaddition, exhibits high regioselectivity, enhancing its synthetic utility and allowing for further derivatization to complex piperidines. Furthermore, the expansion of the nitrene-transfer cycloaddition approach illustrates new possibilities for this underexplored reaction paradigm. Overall, this strategy offers an alternative synthetic entry to a class of compounds that are highly valued in medicinal chemistry, and we anticipate that further development of this approach could lead to broad application in the preparation of bioactive compounds.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Diane Dickie (Department of Chemistry, University of Virginia) for assistance with X-ray crystallography. Partial financial support of this work from the National Institutes of Health (R01 GM124092) the University of Virginia is gratefully acknowledged.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures as well as spectroscopic and analytical data for all new compounds (PDF)
Crystallographic data (CIF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Darses B; Rodrigues R; Neuville L; Mazurais M; Dauban P Transition Metal-Catalyzed Iodine(III)-Mediated Nitrene Transfer Reactions: Efficient Tools for Challenging Syntheses. Chem. Commun. 2016, 53, 493. [DOI] [PubMed] [Google Scholar]
- (2).Dequirez G; Pons V; Dauban P Nitrene Chemistry in Organic Synthesis: Still in Its Infancy? Angew. Chem. Int. Ed. 2012, 51, 7384. [DOI] [PubMed] [Google Scholar]
- (3).Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles Among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257. [DOI] [PubMed] [Google Scholar]
- (4).Blakemore DC; Castro L; Churcher I; Rees DC; Thomas AW; Wilson DM; Wood A Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 1. [DOI] [PubMed] [Google Scholar]
- (5).For selected recent reports of novel cycloaddition reactions to form N-heterocycles, see:Lin T-Y; Wu H-H; Feng J-J; Zhang J Chirality Transfer in Rhodium(I)-Catalyzed [3+2] Cycloaddition of Vinyl Aziridines and Oxime Ethers: Atom-Economical Synthesis of Chiral Imidazolidines. Org. Lett. 2018, 20, 3587.Liu W ; Chen C; Zhou P; Tan H Preparation of 1,2-Oxazetidines from Styrenes and Arylamines via a Peroxide-Mediated [2+1+1] Cycloaddition Reactions. Org. Lett. 2017, 19, 5830–5832.Hao W; Wu X Sun JZ; Siu JC; MacMillan SN; Lin S Radical Redox-Relay Catalysis: Formal [3+2] Cycloaddition of N-Acylaziridines and Alkenes. J. Am. Chem. Soc. 2017, 139, 12141.(d) Chu JK; Dalton DM; Rovis T Zn-Catalyzed Enantio- and Diastereoselective Formal [4+2] Cycloaddition Involving Two Electron-Deficient Partners: Asymmetric Synthesis of Piperidines from 1-Azadienes and Nitro-Alkenes. J. Am. Chem. Soc. 2015, 127, 4445.Kramer S; Fu GC Use of a New Spirophosphine to Achieve Catalytic Enantioselective [4+1] Annulations of Amines with Allenes to Generate Dihydropyrroles. J. Am. Chem. Soc. 2015, 137, 3803.Feng J-J; Lin T-Y ; Wu H-H; Zhang J Transfer of Chirality on the Rhodium-Catalyzed Intramolecular Formal Hetero-[5+2] Cycloaddition of Vinyl Aziridines and Alkynes: Stereoselective Synthesis of Fused Azepine Derivatives. J. Am. Chem. Soc. 2015, 137, 3787.
- (6).For early investigations of intramolecular variants, see:Naruta Y; Nagai N; Arita Y; Maruyama K Copper-Catalyzed Double Cyclization Reaction of Azidoquinones: One-Step Synthesis of Dihydropyrroloindoloquinones and Related Quinolinoquinones. J. Org. Chem. 1987, 52, 3956.Pearson WH; Poon Y-F Assembly of the Gephyrotoxin Ring System via a [4+1] Approach to 3-Pyrrolines. Tetrahedron Lett. 1989, 30, 6661.Pearson WH; Bergmeier SC; Degan S; Lin K-C; Poon Y-F; Schkeryantz JM; Williams JP Synthesis of Pyrrolizidines and Indolizidines by the Intramolecular Cycloaddition of Azides with Electron-Rich 1,3-Dienes. A Synthetic Equivalent of a Nitrene-Diene Cycloaddition. J. Org. Chem. 1990, 55, 5719.Pearson WH; Hines JV Total Syntheses of (+)-Australine and (−)-7-Epialexine. J. Org. Chem. 2000, 65, 5785.
- (7).Wu Q; Hu J,; Ren X; Zhou J An Efficient, Overall [4+1] Cycloaddition of 1,3-Dienes and Nitrene Precursors. Chem. Eur. J. 2011, 17, 11553. [DOI] [PubMed] [Google Scholar]
- (8) (a).Gilbert ZW; Hue RJ; Tonks IA Catalytic Formal [2+2+1] Synthesis of Pyrroles from Alkynes and Diazenes via TiII/TiIV Redox Catalysis. Nat. Chem. 2016, 8, 63. [DOI] [PubMed] [Google Scholar]; (b) Chiu H-S; Tonks IA Trimethylsilyl-Protected Alkynes as Selective Cross-Coupling Partners in Titanium-Catalyzed [2+2+1] Pyrrole Synthesis. Angew. Chem. Int. Ed. 2018, 57, 6090. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Davis-Gilbert ZW; Wen X; Goodpaster JD; Tonks IA Mechanism of Ti-Catalyzed Oxidative Nitrene Transfer in [2+2+1] Pyrrole Synthesis from Alkynes and Azobenzene. J. Am. Chem. Soc. 2018, 140, 7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Saito A; Kambara Y; Yagyu T; Noguchi K; Yoshimura A; Zhdankin VV Metal-Free [2+2+1] Annulation of Alkynes, Nitriles, and Nitrogen Atoms from Iminoiodanes for Synthesis of Highly Substituted Imidazoles. Adv. Synth. Catal. 2015, 357, 667. [Google Scholar]
- (10).For related examples of cycloadditions initiated by an aziridination/ring opening sequence, see:Stoll AH; Blakey SB Rhodium Catalyzed Allene Amination: Diastereoselective Synthesis of Aminocyclopropanes via a 2-Amidoallylcation Intermediate. J. Am. Chem. Soc. 2010, 132, 2108.Stoll AH; Blakey SB Rhodium Catalyzed Allene Amidation: A Facile Entry into 2-Amidoallycations for Unusual [3+3] Annulation Reactions. Chem. Sci. 2011, 2, 112.Gerstner NC; Adams CS; Tretbar M; Schomaker JM Stereocontrolled Syntheses of Seven-Membered Carbocycles by Tandem Allene Aziridination/[4+3] Reaction. Angew. Chem. Int. Ed. 2016, 128, 13434.
- (11).Recently, a low-yielding formation of 2a from 1a (stereochemistry of the latter was undefined) was observed as an outcome of a mechanistic probe of a radical-based photoredox aziridination. See:Yu W-L; Chen J-Q; We Y-L; Wang Z-Y; Xu P-F Alkene Functionalization for the Stereospecific Synthesis of Substituted Aziridines by Visible-Light Photoredox Catalysis. Chem. Commun. 2018, 54, 1948.
- (12).Park Yoonsu, Kim You young, Chang S Transition Metal-Catalyzed C–H Amination: Scope, Mechanism and Applications. Chem. Rev. 2017, 117, 9247. [DOI] [PubMed] [Google Scholar]
- (13) (a).Dequirez G; Ciesielski J; Retailleau P; Dauban P Catalytic Intermolecular Alkene Oxyamination with Nitrenes. Chem. Eur. J. 2015, 20, 8929. [DOI] [PubMed] [Google Scholar]; (b) Ciesielski J Dequirez G; Retailleau P; Gandon V; Dauban P Rhodium-Catalyzed Alkene Difunctionalization with Nitrenes. Chem. Eur. J. 2016, 22, 9338. [DOI] [PubMed] [Google Scholar]
- (14).Espino CG; Fiori KW; Kim M; Du Bois J Expanding the Scope of C–H Amination through Catalyst Design. J. Am. Chem. Soc. 2004, 126, 15378. [DOI] [PubMed] [Google Scholar]
- (15).Fiori KW; Du Bois J Catalytic Intermolecular Amination of C–H Bonds: Method Development and Mechanistic Insights. J. Am. Chem. Soc. 2007, 129, 562. [DOI] [PubMed] [Google Scholar]
- (16).Guthikonda K; Du Bois J A Unique and Highly Efficient Method for Catalytic Olefin Aziridination. J. Am. Chem. Soc. 2002, 124, 13672. [DOI] [PubMed] [Google Scholar]
- (17).Gillis EP; Eastman KJ; Hill MD; Donnelly DJ; Meanwell NA Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315. [DOI] [PubMed] [Google Scholar]
- (18).A SciFinder substructure search revealed that only 2 unique 2,5-diaryl tetrahydropyridines have previously appeared in the chemical or patent literature.
- (19).See Supporting Information for details.
- (20).An alternative explanation is that intermediate 7 is formed directly upon nitrene transfer to the olefin. The intermediacy of the aziridine cannot be conclusively established at this time.
- (21).Alternatively, formation of a benzylic radical as a consequence of the mechanism of Rh-catalyzed aziridination could result in the observed cycloaddition via a radical clock process. It has been calculated that a preferred mechanistic pathway for Rh-catalyzed aziridination using the unprotected aminating agent DPH proceeds through a singlet diradical:Jat JL; Paudyal MP; Gao H; Xu Q-L; Yousufuddin M; Devarajan D; Ess DH; Kürti L; Falck JR Direct Stereospecific Synthesis of Unprotected N-H and N-Me Aziridines from Olefins. Science 2014, 343, 61–65.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.