Abstract
The bone morphogenetic protein (BMP)-SMAD signaling pathway is a key transcriptional regulator of hepcidin in response to tissue iron stores, serum iron, erythropoietic drive and inflammation to increase the iron supply when needed for erythropoiesis, but to prevent the toxicity of iron excess. Recently, BMP2 was reported to play a non-redundant role in hepcidin regulation in addition to BMP6. Here, we used a newly validated BMP2 ELISA assay and mice with a global or endothelial conditional knockout (CKO) of Bmp2 or Bmp6 to examine how BMP2 is regulated and functionally contributes to hepcidin regulation by its major stimuli. Erythropoietin (EPO) did not influence BMP2 expression in control mice, and still suppressed hepcidin in Bmp2 CKO mice. Lipopolysaccharide (LPS) reduced BMP2 expression in control mice, but still induced hepcidin in Bmp2 CKO mice. Chronic dietary iron loading that increased liver iron induced BMP2 expression, whereas acute oral iron gavage that increased serum iron without influencing liver iron did not impact BMP2. However, hepcidin was still induced by both iron loading methods in Bmp2 CKO mice, although the degree of hepcidin induction was blunted relative to control mice. Conversely, acute oral iron gavage failed to induce hepcidin in Bmp6−/− or CKO mice. Thus, BMP2 has at least a partially redundant role in hepcidin regulation by serum iron, tissue iron, inflammation and erythropoietic drive. In contrast, BMP6 is absolutely required for hepcidin regulation by serum iron.
Keywords: Iron, hepcidin, BMP2, BMP6, erythropoietin, inflammation
INTRODUCTION
The participation of iron in numerous physiological activities makes it indispensable for nearly all living organisms. Iron deficiency impairs cell growth and survival, is a major cause anemia, and represents a significant global health concern.1,2 Excess iron accumulation, however, generates free radicals via the Fenton reaction that can lead to cell damage,3 as seen in hereditary hemochromatosis and iron loading anemias.4,5 Therefore, iron metabolism must be carefully maintained and regulated.
Systemic iron levels are governed by the liver hormone hepcidin (HAMP), which promotes the degradation of iron exporter ferroportin in enterocytes, macrophages and hepatocytes to control iron entry into the plasma.6 Inactivating mutations in HAMP or mutations in SLC40A1 (encoding ferroportin) that interfere with hepcidin binding lead to the iron overload disorder hereditary hemochromatosis.7,8 Moreover, the mechanism of action of several other genes, including HFE, HJV (encoding hemojuvelin), TFR2 (encoding transferrin receptor 2), and BMP6 (encoding bone morphogenetic protein 6), whose mutations have been identified in humans to associate with iron overload depends on their ability to appropriately induce hepcidin expression in response to iron.9–12 Whereas iron induces hepcidin as a feedback mechanism to prevent iron overload, iron deficiency or acute blood loss suppress hepcidin expression to ensure adequate iron supply for red blood cell production. Erythrocyte precursor derived erythroferrone plays a critical role in hepcidin suppression by erythropoietic drive,13 and excess erythroferrone production and consequent hepcidin deficiency contribute to iron overload in diseases of ineffective erythropoiesis such as β-thalassemia.14 In the context of many infections, hepcidin transcription is increased by inflammatory cytokines to limit iron availability to harmful microorganisms; however, excess induction of hepcidin in the setting of chronic inflammatory diseases also contributes to iron restricted erythropoiesis and anemia.15
The BMP-SMAD signaling pathway is the central transcriptional regulator of hepcidin and is critically involved in controlling hepcidin expression in response to iron.16 Tissue iron loading increases BMP6 production in liver endothelial cells,17 which initiates a signaling cascade by binding to hepatocyte BMP type I and type II receptors and the co-receptor HJV and subsequently activates transcription factors SMAD1/5/8 and SMAD4 to translocate into the nucleus for hepcidin transcription.15 Acute increases in serum iron, presumably in the form of diferric transferrin, independently increase hepcidin expression even in the absence of tissue iron loading.18,19 Although acute serum iron loading without tissue iron loading activates the downstream SMAD1/5/8 signaling cascade, it does not impact liver BMP6 mRNA expression.18 Moreover, genetic ablation of the Hfe localizing protein beta-2-microglublin (B2m) or Tfr2, which are implicated in sensing serum diferric transferrin and functionally intersect with the SMAD1/5/8 signaling cascade to induce hepcidin production,20,21 worsens the iron overload phenotype of Bmp6−/− mice.22 These data raise the possibility that another BMP ligand may play a role in hepcidin induction by serum iron levels via HFE and TFR2.
The BMP-SMAD pathway has also been implicated in hepcidin regulation by erythropoietic drive and inflammation. Erythropoietin and erythroferrone decrease SMAD1/5/8 phosphorylation in hepatocytes, and their ability to suppress hepcidin expression is completely abrogated in hepatocyte-specific Smad1/5 CKO mice.23 However, erythropoietin retains the ability to suppress hepcidin in Bmp6−/− mice.24,25 In a similar vein, inflammation induces SMAD1/5/8 phosphorylation in hepatocytes, but retains the ability to stimulate hepcidin in Bmp6−/− mice.26 These data also suggest the possibility that erythropoietic drive and/or inflammation may also regulate the production of or response to another BMP family ligand to control hepcidin production.
Recently, we and others demonstrated that independent of BMP6, BMP2 also plays an essential role in hepcidin regulation and iron homeostasis since mice with an endothelial CKO of Bmp2 exhibit hepcidin deficiency and iron overload, similar to mice with a global or endothelial CKO of Bmp6.17,27 Although liver endothelial cell Bmp2 mRNA was shown to be regulated by chronic changes in dietary iron, albeit to a lesser extent than Bmp6, how BMP2 protein expression is regulated by tissue iron, serum iron, erythropoietic drive, and inflammation, and the functional role of BMP2 in these contexts remains unknown.
Here, we used endothelial Bmp2 CKO mice to validate a commercially available BMP2 enzyme-linked immunosorbent assay (ELISA) and investigated how BMP2 expression is controlled by different hepcidin regulators. We also explored the functional role of BMP2 in the regulation of hepcidin by tissue iron, serum iron, epoetin alfa (EPO), and lipopolysaccharide (LPS).
METHODS
Animals
Animal protocols were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Endothelial Bmp2 CKO (Bmp2fl/fl;Tek-Cre+) mice on a mixed C57BL/6J,129S4/SvJaeSor background, endothelial Bmp6 CKO (Bmp6fl/fl;Tek-Cre+) mice and global Bmp6 (Bmp6−/− mice) on a mixed C57BL/6J,129SvEvBrd background, and their littermate Cre- or Bmp6+/+ controls were generated and genotyped as previously described.17,28 All mice were maintained with ad libitum access to water and a Prolab 5P75 Isopro RMH 3000 diet containing 380 ppm iron (house diet), unless otherwise indicated. Where indicated, mice were given an intraperitoneal dosage of epoetin alfa (EPO; 200 U per mouse; Amgen) or LPS (1 mg per kg body weight; serotype 055:B5; Sigma) or an equivalent volume of PBS as controls.
To examine BMP2 regulation by dietary iron, four-week-old C57BL/6 male were placed on a low iron (2–6 ppm; Harlan #TD.80396), iron sufficient (Control; 48 ppm; Harlan #TD.80394), or a high iron (2% carbonyl iron; Harlan #TD.08496) diet for 3 weeks. To determine the functional role of BMP2 in hepcidin regulation by dietary iron, Bmp2fl/fl;Tek-Cre+ and Cre- control mice were treated with the low iron diet (2–6 ppm; Harlan #TD.80396) upon weaning for 3 weeks to prevent the iron overload that develops on a standard diet in Bmp2fl/fl;Tek-Cre+ mice. Animals were then either kept on a low iron diet or switched to the house diet (Prolab 5P75) that contains 380 ppm iron for 1 more week. To increase serum iron without affecting liver iron concentrations, endothelial CKO of Bmp2 or Bmp6, and Bmp6−/− mice were treated with the low iron diet upon weaning for 3 weeks and received a single dose of 2 mg ferrous sulfate (Sigma F8633) per kg body weight in 0.5 M ascorbic acid or equivalent volume of distilled water as control via oral gavage. After treatment, animals were kept without food but with ad libitum access to water until sacrifice 4 hours later.
RNA Isolation, Reverse Transcription, and qRT-PCR
Total liver RNA was isolated using QIAshredder and RNeasy Mini Kit (Qiagen). First-strand cDNA was synthesized from 1 μg RNA using the iScript cDNA synthesis kit (Bio-Rad) or High capacity RNA-to-cDNA Kit (Applied Biosystems). PCR reactions were performed using the PowerUp SYBR Green Master Mix on the QuantStudio3 Real-Time PCR system (Applied Biosystems) using primers in Table S1. Standard curves were run in each qRT-PCR reaction, which were validated as linear over the range of expression seen in all samples. Transcript levels were determined as previously described28 and are reported as -ΔCt = Ct Rpl19 reference – Ct target. The fold change is derived from 2-ΔΔCt.
Iron Analysis
Serum iron and unsaturated iron binding capacity were measured by colorimetric assay (Pointe Scientific) to calculate transferrin saturation according to manufacturer’s instructions. Tissue nonheme iron concentrations (in μg/g wet weight) were determined as described previously.23
BMP2 enzyme-linked immunosorbent assay (ELISA)
Serum BMP2 was measured using BMP2 ELISA Kit (R&D Systems DBP200) in 1:2 dilution according to manufacturer’s instructions. Liver samples were homogenized in ice-cold PBS containing 0.05% sodium azide, 0.5% triton x-100, 1X cOmplete Mini Protease inhibitor Cocktail (Roche) and 1X Halt Phosphatase Inhibitor Cocktail (Thermo Scientific).
Statistics
Statistical significance was determined by 2-tailed Student’s t-test or one-way analysis of variance (ANOVA) with Tukey’s post hoc test for pairwise multiple comparisons using Prism 7 (GraphPad). P < 0.05 was considered significant.
RESULTS
Validation of a BMP2 ELISA
To investigate how BMP2 protein expression is regulated by the major stimuli known to control hepcidin production, we first validated a commercially available BMP2 ELISA using endothelial Bmp2 CKO (Bmp2fl/fl;Tek-Cre+) and littermate Cre- mice. We found that the BMP2 concentration in the serum of control mice averaged approximately 1 ng/mL (Figure 1A), a concentration of BMP ligand that was previously shown to be sufficient to induce hepcidin expression in primary hepatocyte cultures.29 In Bmp2fl/fl;Tek-Cre+ mice, serum BMP2 levels were reduced by over 95% to < 50 pg/mL (Figure 1A). A significant reduction in BMP2 protein expression was also seen in Bmp2fl/fl;Tek-Cre+ compared with Cre- mouse livers (Figure 1B). Together, these data confirm the specificity of the ELISA for measuring BMP2 protein in serum and liver and suggest that in mice, circulating BMP2 arises predominantly from endothelial cells or other Tek-Cre expressing cells, which also includes T and B lymphocytes, class II MHC positive cells, natural killer cells, dendritic cells, and circulating and resident tissue macrophages.30
Figure 1: BMP2 is not regulated by EPO and is not required for hepcidin suppression by EPO.
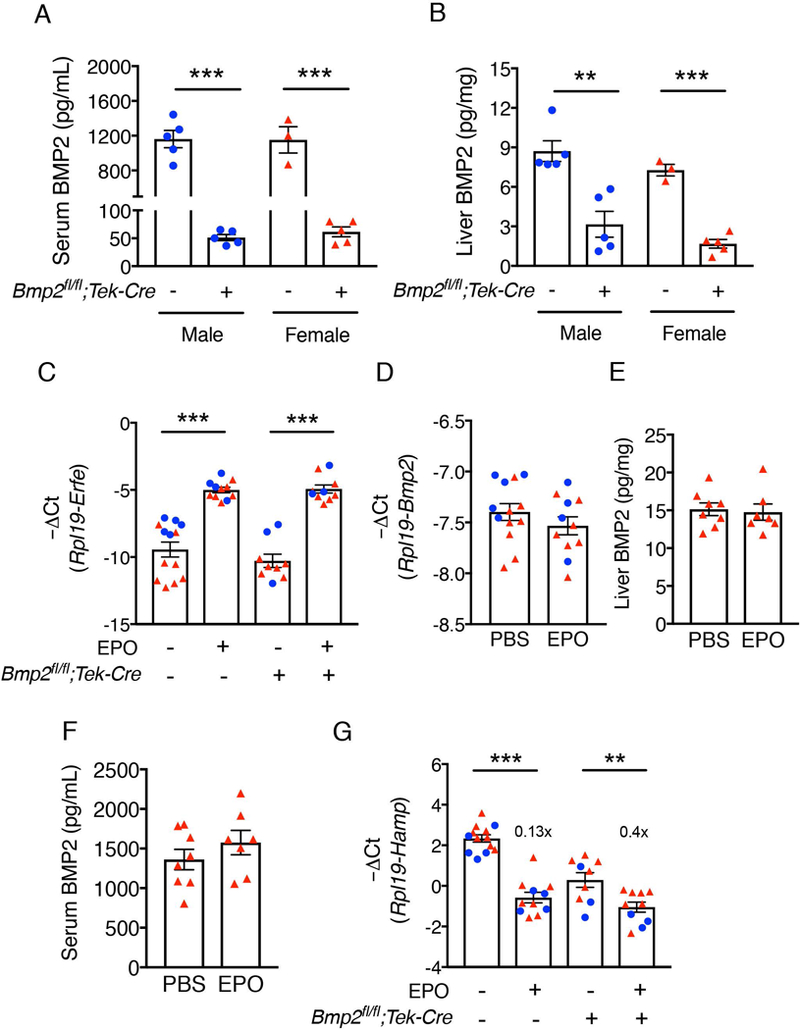
(A-B) Bmp2 endothelial CKO (Bmp2fl/fl;Tek-Cre+) and littermate Cre- control male (blue circles) and female mice (red triangles) were sacrificed at 8 weeks of age (n=3–5 per sex per group). Levels of (A) serum and (B) liver BMP2 were quantitated by ELISA. (C-G) Bmp2fl/fl;Tek-Cre+ and littermate Cre- control male (blue circles, n=3–5 per group) and female (red triangles, n=6–8 per group) mice at 6 weeks of age were injected with PBS or EPO (200 U per mouse) and tissues were collected after 15 hours to determine relative (C) bone marrow erythroferrone (Erfe), (D) liver Bmp2, and (G) liver hepcidin (Hamp) mRNA levels by qRT-PCR. Transcript levels were normalized to Rpl19. (E) Liver and (F) serum BMP2 protein levels in female Cre- animals were determined by ELISA. Values represent mean ± SEM. **P<0.01, ***P<0.001 relative to the respective Cre- controls or PBS-treated controls of the same genotype by Student’s t test. Fold-change relative to PBS treated controls of the same genotype as calculated by 2-ΔΔCt are reported in panel G.
Erythropoietin-mediated suppression of hepcidin does not require BMP2
Recently, we demonstrated that erythropoietin (EPO)- and erythroferrone-mediated suppression of hepcidin requires SMAD1/5 signaling since these stimuli failed to reduce hepcidin expression in hepatocyte Smad1/5 double-knockout mice.23 However, BMP6 ligand is not required since hepcidin was still suppressed by EPO in Bmp6−/− mice.24,25 Here, we investigated whether EPO regulates BMP2 production by the liver and whether EPO-mediated suppression of hepcidin requires BMP2. Bmp2fl/fl;Tek-Cre+ and Cre- littermate mice were injected with 200U EPO intraperitoneally and tissues were collected after 15 hours. Bone marrow Erfe mRNA (encoding erythroferrone) confirmed successful administration of EPO and stimulation of endogenous erythroferrone production for each genotype (Figure 1C), without changes in liver iron levels (Supplemental Figure S1). Liver Bmp2 mRNA and serum and liver BMP2 protein levels did not differ between EPO-treated animals and PBS-treated Cre- controls (Figure 1D-F), suggesting that EPO does not regulate BMP2 production. Moreover, EPO significantly decreased liver hepcidin (Hamp) mRNA expression in both Bmp2fl/fl;Tek-Cre+ and Cre- control mice (Figure 1G). These data suggest that EPO-mediated hepcidin suppression does not require BMP2. Although liver phosphorylated SMAD5 (p-SMAD5) and the SMAD target transcript Id1 were previously reported to be suppressed by EPO treatment in some studies,23,24 neither p-SMAD5 nor Id1 were significantly affected at the 15 hour timepoint after EPO treatment in Bmp2fl/fl;Tek-Cre+ mice (Supplemental Figure S1 B-C).
LPS decreases both BMP2 and BMP6 expression, but LPS-induced hepcidin production remains intact in Bmp2fl/fl;Tek-Cre+ mice
Next, we examined whether inflammation induced by LPS regulates hepcidin production by controlling BMP2 production. Bmp2fl/fl;Tek-Cre+ and littermate Cre- mice were given a single intraperitoneal dose of 1 mg/kg LPS and sacrificed after 6 hours. Liver Il6 mRNA, the main inflammatory cytokine implicated in liver hepcidin regulation,15 was induced by LPS compared to PBS controls confirming an inflammatory response in both genotypes (Figure 2A). Liver Bmp2 mRNA, liver BMP2 protein, and serum BMP2 protein were all significantly decreased by LPS (Figure 2B-D). Liver Bmp6 mRNA levels were also reduced by LPS in both genotypes (Figure 2E). Despite the suppression of BMP2 and BMP6 ligand production, LPS still significantly induced Hamp mRNA in Bmp2fl/fl;Tek-Cre+ mice relative to PBS treatment with comparable or even higher potency (11.6-fold) than in Cre- control mice (4.7-fold, Figure 3F), similar to prior report for Bmp6−/− mice.26 Although our study was not powered to compare genotype effects in addition to treatment effects, the maximal Hamp mRNA levels achieved appeared to be lower in the Cre+ mice compared to Cre- controls in the setting of lower baseline Hamp mRNA, similar to the prior report for Bmp6−/− mice. 26
Figure 2: BMP2 is suppressed by LPS and is not required for hepcidin induction by LPS.
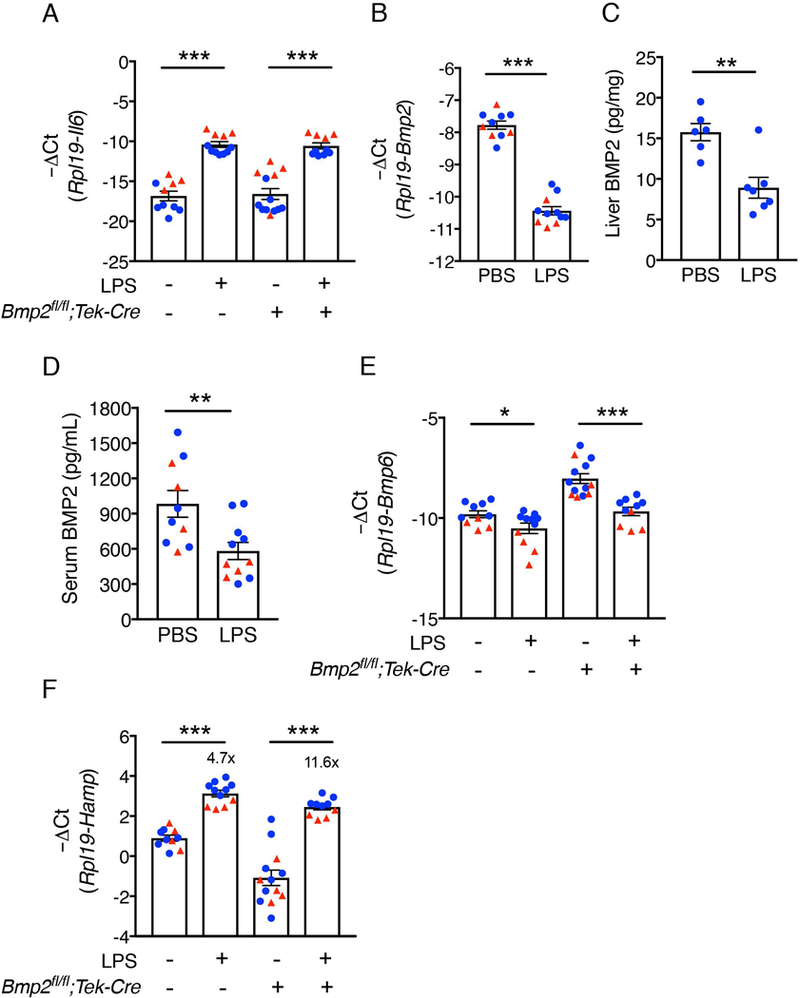
Bmp2fl/fl;Tek-Cre+ and littermate Cre- control male (blue circles, n=6–8 per group) and female (red triangles, n=4–5 per group) mice at 6 weeks of age were injected with PBS or LPS (1 mg per kg body weight) and sacrificed after 6 hours. Livers were collected to determine (A) Il6, (B) Bmp2, (E) Bmp6 and (F) Hamp mRNA levels. Transcripts measured by qRT-PCR were normalized to Rpl19. (C) Liver and (D) serum BMP2 protein levels in Cre- animals were determined by ELISA. Values represent mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 relative to the PBS-treated controls of the same genotype by Student’s t test. Fold-change relative to PBS treated controls of the same genotype as calculated by 2-ΔΔCt are reported in panel F.
Figure 3: BMP2 is regulated concordantly with hepcidin by chronic changes in dietary iron, but BMP2 is partially redundant for hepcidin induction by chronic dietary iron loading.

(A-E) 4-week-old C57BL/6 male mice were treated with a low (2–6 ppm), sufficient (48 ppm, Control) or high iron (2% carbonyl iron) diet for 3 weeks (n=8 per group). (F-I) 3-week-old male (blue circles) and female (red triangles) Bmp2fl/fl;Tek-Cre+ and littermate Cre- controls were weaned to a low iron diet for 3 weeks and then either switched to the house diet (380 ppm iron) or kept on a low iron diet for 1 more week (n=6 per group, 2–4 of each sex). (A) Serum iron and (B,F) liver iron levels were quantified by colorimetric assay. (C) Liver Bmp2, (G) Bmp6, and (H) Hamp mRNA levels were measured by qRT-PCR and normalized to Rpl19. (D) Liver and (E) serum BMP2 protein levels were quantified by ELISA. (I) Liver Hamp normalized to Rpl19 mRNA relative to liver iron content was calculated for mice switched to the house diet. Values represent mean ± SEM. *P<0.05, **P<0.01, *** P<0.001 relative to mice treated with a control diet by one-way ANOVA with Tukey’s post-hoc test (Panels A-E), a low iron diet of the same genotype by Student’s t test (Panels F-H) or Cre- mice switched to a house diet by Student’s t test (Panel I). Fold-change relative to low iron diet controls of the same genotype as calculated by 2-ΔΔCt are reported in panel H.
Serum and liver BMP2 protein are regulated by chronic changes in dietary iron.
We previously demonstrated that chronic changes in dietary iron significantly regulate liver Bmp2 mRNA expression, although to a lesser extent than Bmp6.17 To examine whether chronic changes dietary iron also regulate BMP2 protein expression, serum and liver BMP2 protein levels were quantitated by the validated BMP2 ELISA from Figure 1 in wildtype mice treated with a low iron (2–6 ppm), an iron sufficient diet (Control, 48 ppm) or a high iron diet (2% carbonyl) for 3 weeks. Serum and liver iron concentrations were measured to confirm dietary iron effects (Figure 3A-B). Liver Bmp2 mRNA was significantly reduced by a low iron diet and increased by a high iron diet (Figure 3C) similar to our prior study.17 Liver BMP2 protein levels were also significantly reduced by the low iron diet, although no significant increase in liver BMP2 protein expression was seen in mice treated with the high iron diet (Figure 3D). Serum BMP2 levels were significantly increased by a high iron diet with a trend towards reduced levels in mice treated with a low iron diet (Figure 3E). In the overall population, there was a significant correlation between liver iron content and serum BMP2 levels (R=0.899, P<0.001). A survey of other tissues showed that although a low iron diet reduced Bmp2 mRNA in the bone marrow and pancreas, and a high iron diet increased Bmp2 mRNA in the spleen, the liver was the only tissue tested where Bmp2 mRNA levels were both reduced by a low iron diet and increased by a high iron diet (Supplemental Figure S2).
BMP2 is not required for hepcidin induction by chronic dietary iron loading.
To examine if BMP2 is functionally required for hepcidin induction by chronic dietary iron loading, Bmp2fl/fl;Tek-Cre+ and littermate Cre- mice were fed a low iron diet (2–6 ppm) for 3 weeks upon weaning to prevent the iron overload that develops on a standard rodent diet in Bmp2fl/fl;Tek-Cre+ mice. Animals were then either kept on the low iron diet for 1 more week or switched to the house diet that contains 380 ppm iron. Mice switched to the house diet had significantly increased liver iron levels compared to mice kept on the low iron diet (Figure 3F) with associated increases in liver Bmp6 mRNA (Figure 3G). In the Cre- control mice, Hamp mRNA was greatly induced by dietary iron by 240-fold, consistent with prior observations (Figure 3H).23,28 In Bmp2fl/fl;Tek-Cre+ mice, Hamp mRNA was still significantly induced by 226-fold. 26 However, the maximal hepcidin levels achieved were lower in Cre+ mice compared to Cre- mice, particularly when considered relative to the higher degree of liver iron loading in the Cre+ mice (Figure 3I). Together, these data suggest that although BMP2 facilitates the maximal induction of hepcidin by chronic dietary iron, there is a residual ability of iron to induce hepcidin in the absence of BMP2. Similar findings have previously been reported for BMP6.17
Hepcidin induction by acute serum iron loading requires BMP6, but not BMP2
Chronic changes in dietary iron increases both serum and liver iron levels, which are known to independently stimulate hepcidin transcription.18,19 We next sought to investigate whether an isolated increase in serum iron and transferrin saturation regulates serum or liver BMP2 expression and whether BMP2 or BMP6 is required for hepcidin induction in this context. Bmp2fl/fl;Tek-Cre+, littermate Cre- controls, or Bmp6fl/fl;Tek-Cre+ mice were given a single dose of ferrous sulfate via oral gavage, which increased serum iron and transferrin saturation without affecting liver iron stores (Figure 4A-B). As previously reported, iron gavage did not increase liver Bmp6 mRNA expression (Figure 4C). Similarly, iron gavage did not significantly impact serum or liver Bmp2 mRNA or protein expression (Figure 4D-F). Notably, whereas Hamp mRNA expression was still induced by iron gavage 5-fold in Bmp2fl/fl;Tek-Cre+ mice, albeit to a lesser extent than Cre- mice (37-fold), iron gavage completely failed to upregulate Hamp in Bmp6;Tek-Cre+ mice (Figure 4G). Iron gavage also completely failed to upregulate Hamp in Bmp6−/− mice (Supplemental Figure S3). These data suggest that neither BMP2 nor BMP6 is regulated by serum iron; however, BMP6 is required for hepcidin induction by serum iron. In contrast, although the presence of BMP2 facilitates maximal hepcidin induction, serum iron has a residual ability to induce hepcidin in the absence of BMP2.
Figure 4: Neither BMP2 nor BMP6 are regulated by acute serum iron loading, but BMP2 has a partially redundant role, whereas BMP6 is required for hepcidin induction by acute serum iron loading.
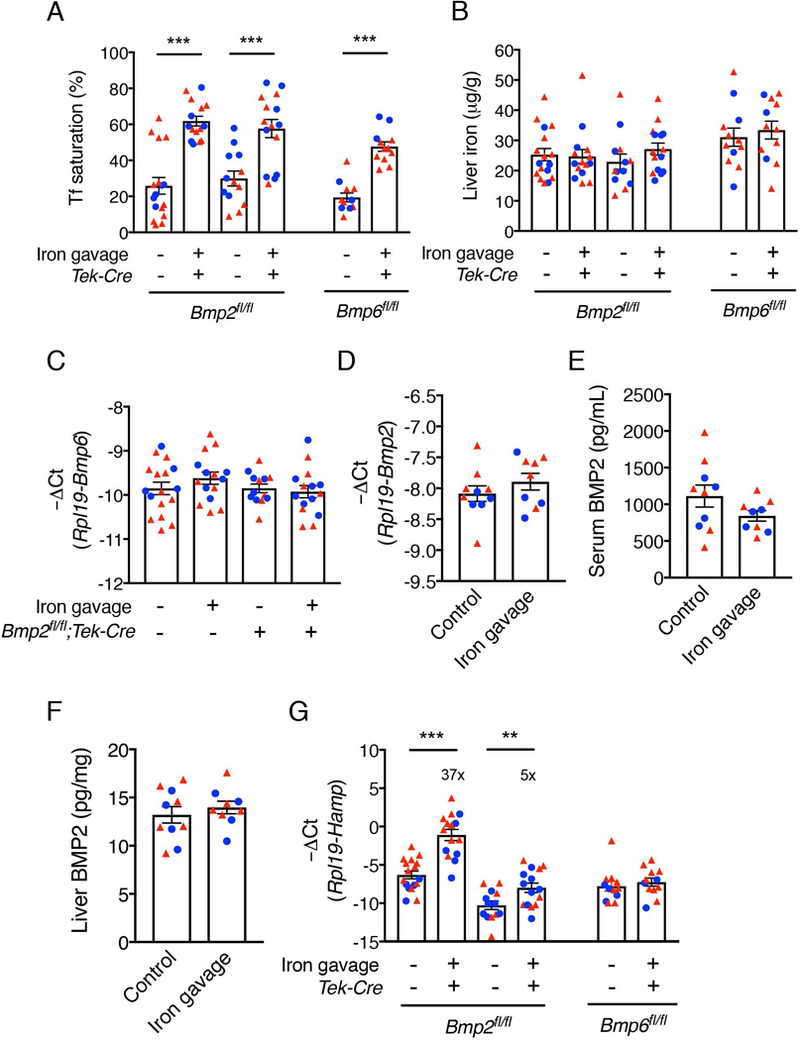
3-week-old male (blue circles) and female (red triangles) Bmp2fl/fl;Tek-Cre+ and littermate Cre- controls (n=13–17 per group, 5–10 of each sex) and Bmp6fl/fl;Tek-Cre+ mice (n=12 per group, 3–4 males and 8–9 females) were weaned to a low iron (2–6 ppm) diet for 3 weeks before treated with 2 mg/kg ferrous sulfate in 0.5 M ascorbic acid or equivalent volume of distilled water by oral gavage. Mice were sacrificed 6 hours after gavage. (A) Serum transferrin saturation and (B) liver iron levels were determined by colorimetric assays. (C) Liver Bmp6, (D) Bmp2 and (G) Hamp mRNA levels were quantified and normalized to Rpl19 by qRT-PCR. (E) Serum and (F) liver BMP2 protein concentrations were measured by ELISA. Values represent mean ± SEM. **P<0.01, ***P<0.001 relative to mice treated with distilled water of the same genotype by Student’s t test. Fold-change relative to vehicle treated controls of the same genotype as calculated by 2-ΔΔCt are reported in panel G.
DISCUSSION
The BMP-SMAD signaling pathway is a major transcriptional regulator of hepcidin expression.31 This pathway not only regulates hepcidin transcription in response to serum and tissue iron levels,18 but also intersects with other major hepcidin regulatory stimuli including erythropoietic drive23 and inflammation.32,33 BMP2 was recently identified as a key ligand in hepcidin and iron homeostasis regulation17,27 in addition to BMP6.34,35 Here we define how BMP2 expression is controlled by major hepcidin regulatory stimuli and the functional contribution of BMP2 relative to BMP6 in hepcidin regulation by these stimuli.
Using a newly validated BMP2 ELISA, our results demonstrated that BMP2 protein levels were regulated proportionally to chronic changes in body iron loading to some extent both in the circulation and liver. Interestingly, the induction of BMP2 by a high iron diet was only detected in circulation, not in the liver, whereas the reduction of BMP2 by a low iron diet was more readily detected in the liver than circulation. Total liver Bmp2 mRNA was reduced by a low iron diet and induced by a high iron diet, suggesting that iron does modulate liver BMP2 production. A similar regulation of Bmp2 mRNA by iron was previously reported in liver endothelial cells,17 which are the predominant cells in the liver responsible for BMP2 production.17,27 One explanation for why we did not detect an increase in liver BMP2 protein by a high iron diet despite an increase in liver Bmp2 mRNA and circulating BMP2 protein could be that BMP2 is more rapidly secreted and/or utilized by the liver under conditions of iron loading. Another possibility is that circulating BMP2 may arise from an extra-hepatic source in response to iron loading. Interestingly, the concentration of circulating BMP2 in wildtype mice, averaging ~1 ng/mL, is in the biological range of Bmp ligand activity,29 which could be consistent with endocrine effects. The loss of circulating BMP2 levels in Bmp2fl/fl;Tek-Cre+ mice suggests that the primary sources to consider are other Tek-Cre expressing cells, including other endothelial cell populations or some hematopoietic cells.30 However, a survey of other tissues found that although a low iron diet reduced Bmp2 in the bone marrow and pancreas and a high iron diet induced Bmp2 in the spleen, the liver was the only organ tested where Bmp2 mRNA was both reduced by a low iron diet and increased by a high iron diet. Thus, the liver is most likely the predominant source of circulating BMP2 in response to changes in dietary iron, although we cannot definitively rule out a contribution from another source. Future experiments will be needed to test whether iron loading impacts liver BMP2 secretion.
Results from the Bmp2fl/fl;Tek-Cre+ mice demonstrated that BMP2 is required for optimal hepcidin induction in response to chronic dietary iron loading. Although the fold induction of hepcidin was only marginally lower in Bmp2fl/fl;Tek-Cre+ compared with Cre- mice, this may be considered inappropriately low relative to the higher degree of iron loading in the Bmp2fl/fl;Tek-Cre+ mice. However, the significant residual hepcidin induction in the Bmp2fl/fl;Tek-Cre+ mice suggests that BMP2 has a partially redundant role. Presumably, BMP6 accounts for the residual hepcidin induction by chronic iron loading in the Bmp2 CKO mice given its demonstrated functional role in hepcidin induction by iron.34 Similar findings have previously been reported in Bmp6−/− mice.17 Thus, BMP2 and BMP6 have partially redundant roles in response to chronic iron loading. Each ligand individually appears to retain some residual ability to stimulate hepcidin in this context, but both are required for a maximal effect.
Previous studies have demonstrated that in addition to chronic changes in iron stores, acute changes in serum iron levels (presumably by holo-transferrin) independently regulate hepcidin expression.18,19 Although chronic dietary iron loading and deficiency modulated BMP2 expression, BMP2 levels were not changed by an isolated increase in serum iron and transferrin saturation in response to oral iron gavage. Although a prior report suggested that liver BMP2 may be modulated by apo-transferrin administration, we were not able to validate the specificity of BMP2 antibody used in that study using our Bmp2fl/fl;Tek-Cre+ mice (data not shown).36 A similar lack of modulation by serum iron was also seen for BMP6, at least at the mRNA level, as previously reported.18 Thus, whereas both BMP6 and BMP2 expression are modulated by iron stores and thus contribute to hepcidin regulation in this context, neither BMP2 nor BMP6 levels are modulated by serum iron or transferrin saturation in response to oral iron gavage. One caveat to this conclusion is that assays are not readily available to determine if liver and/or circulating BMP6 protein levels are modified by changes in serum iron levels, so it is still feasible that serum iron could regulate BMP6 protein production, stability and/or secretion.
Interestingly, although its expression is not modulated by serum iron, BMP6 is absolutely required for hepcidin induction by serum iron since iron gavage failed to induce hepcidin in Bmp6−/− and Bmp6fl/fl;Tek-Cre+ mice. In contrast, although BMP2 facilitates maximal hepcidin induction by serum iron, BMP2 appears to be partially dispensable, since hepcidin was still induced to some extent by iron gavage in Bmp2fl/fl;Tek-Cre+ mice. A caveat to these findings is that there is a low level of residual liver Bmp2 mRNA, liver BMP2 protein, and serum BMP2 protein expression in Bmp2fl/fl;Tek-Cre+ mice, which could account for residual hepcidin induction in these mice, but a similar limitation (mild residual liver Bmp6 mRNA expression) is also present in the Bmp6fl/fl;Tek-Cre+ mice28 where iron gavage failed to induce hepcidin. These results do not support the model proposed in a prior publication22 suggesting that BMP2 preferentially functions in serum diferric transferrin sensing and BMP6 preferentially functions in intracellular tissue iron sensing. Future studies will be needed to understand what accounts for the differences in response to iron gavage in the Bmp6fl/fl;Tek-Cre+ compared with the Bmp2fl/fl;Tek-Cre+ mice.
Whereas BMP2 has a critical role in hepcidin regulation by iron, BMP2 does not appear to have a critical role hepcidin regulation by erythropoietic drive. Indeed, BMP2 levels were not regulated by EPO and loss of BMP2 did not prevent hepcidin suppression by EPO. However, a previous study showed that EPO and erythroferrone suppressed SMAD5 phosphorylation and neither EPO nor erythroferrone suppressed hepcidin in hepatocyte Smad1/5 CKO mice.23 These data suggest that although EPO and its mediator erythroferrone target the SMAD signaling cascade and require SMAD1/5 to regulate hepcidin in response to erythropoietic drive, this does not occur as a consequence of modulating BMP2 production. Moreover, EPO’s hepcidin suppressive effect appears to be independent of BMP2. The failure of EPO to significantly reduce liver phosphorylated SMAD5 and Id1 expression in Bmp2fl/fl;Tek-Cre+ mice could suggest an alternative mechanism for EPO-mediated hepcidin suppression in these mice. Notably, EPO-mediated suppression of SMAD5 phosphorylation and Id1 mRNA was relatively modest and not always reproducible in wildtype mice, which also raises the possibility of an alternative pathway. 13,23,24 However, a caveat to these conclusions is that BMP effects on SMAD5 phosphorylation and Id1 expression are less robust and occur with different kinetics compared with BMP effects on hepcidin, and therefore these readouts may not be sensitive enough to detect changes in SMAD pathway activity in this system. Interestingly, a recent report published during the preparation of this manuscript demonstrated that erythroferrone selectively inhibits hepcidin induction by BMP6, BMP5, and BMP7 ligands, but not BMP2, in cell culture. 37 These data are consistent with a functional role for BMP-SMAD signaling in hepcidin regulation by erythroferrone, but a dispensable role for BMP2. However, 2 previous studies reported that hepcidin is still suppressed by EPO in Bmp6 knockout mice,24,25 suggesting that BMP6 is not required for hepcidin suppression by erythropoietic drive in vivo. Future studies will be needed to understand the precise role of the BMP-SMAD pathway and potential alternative pathways in hepcidin suppression by erythroferrone and erythropoietic drive in vivo.
Inflammatory cytokines stimulate hepcidin production predominantly via interleukin 6 (IL6) and a STAT3 binding element on the hepcidin promoter.15 Prior studies have suggested that the inflammatory pathway requires BMP-SMAD signaling because IL6 loses the ability to stimulate hepcidin in Alk3 or Smad4 hepatocyte CKO mice.32,33 Moreover, in vitro studies suggested an interaction at the level of the hepcidin promoter via the STAT3 binding element and a proximal BMP responsive SMAD binding element.38 Here, we demonstrated that LPS retains its potency to induce hepcidin in the absence of BMP2 and similar findings have been reported for BMP6.26 However, loss of either ligand does reduce basal hepcidin expression17,28,34–35 and thereby tends to lower the maximal hepcidin levels reached in response to inflammation. Thus, BMP ligands influence hepcidin levels in the context of inflammation by altering the basal setpoint. Notably, endogenous BMP2 and BMP6 expression are reduced by LPS in control mice, which was also previously shown for the BMP co-receptor HJV,39 suggesting that dampening iron-responsive hepcidin mediators may be desirable under conditions of inflammation.
In summary, our results have validated a commercial BMP2 ELISA assay and demonstrated that BMP2 is regulated concordantly with hepcidin by tissue iron, but not serum iron, erythropoietic drive, or inflammation. Thus, regulation of BMP2 expression, in addition BMP6, is one mechanism by which tissue iron levels impact hepcidin production. However, BMP2 is at least partially dispensable for hepcidin regulation by all of its major stimuli, whereas BMP6 is absolutely required for hepcidin induction by serum iron. These findings demonstrate important distinctions between the functional roles of BMP2 and BMP6 and provide new insights into how the liver senses iron levels and integrates this with other signals to regulate hepcidin production and iron homeostasis.
Supplementary Material
GRANT SUPPORT AND ACKNOWLEDGEMENTS
This work was supported by NIH grant RO1-DK087727 to JLB. CW is supported in part by a Cooley’s Anemia Foundation Research Fellowship.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
JLB has ownership interest in Ferrumax Pharmaceuticals, and has received consulting fees from Keryx Biopharmaceuticals and Disc Medicine. All other authors have nothing to declare.
REFERENCE
- 1.Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull. 2003;24(4 Suppl):S99–103. [DOI] [PubMed] [Google Scholar]
- 2.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454. [DOI] [PubMed] [Google Scholar]
- 3.Chevion M A site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metals. Free Radic Biol Med. 1988;5(1):27–37. [DOI] [PubMed] [Google Scholar]
- 4.Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21 Suppl 1:S6–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crielaard BJ, Lammers T, Rivella S. Targeting iron metabolism in drug discovery and delivery. Nat Rev Drug Discov. 2017;16(6):400–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. [DOI] [PubMed] [Google Scholar]
- 7.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98(15):8780–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Njajou OT, Vaessen N, Joosse M, et al. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat Genet. 2001;28(3):213–214. [DOI] [PubMed] [Google Scholar]
- 9.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. [DOI] [PubMed] [Google Scholar]
- 10.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36(1):77–82. [DOI] [PubMed] [Google Scholar]
- 11.Camaschella C, Roetto A, Cali A, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25(1):14–15. [DOI] [PubMed] [Google Scholar]
- 12.Daher R, Kannengiesser C, Houamel D, et al. Heterozygous Mutations in BMP6 Pro-peptide Lead to Inappropriate Hepcidin Synthesis and Moderate Iron Overload in Humans. Gastroenterology. 2016;150(3):672–683 e674. [DOI] [PubMed] [Google Scholar]
- 13.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kautz L, Jung G, Du X, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of beta-thalassemia. Blood. 2015;126(17):2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016;23(3):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Core AB, Canali S, Babitt JL. Hemojuvelin and bone morphogenetic protein (BMP) signaling in iron homeostasis. Front Pharmacol. 2014;5:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canali S, Wang CY, Zumbrennen-Bullough KB, Bayer A, Babitt JL. Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6. Am J Hematol. 2017;92(11):1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corradini E, Meynard D, Wu Q, et al. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54(1):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos E, Kautz L, Rodriguez R, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53(4):1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9(3):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corradini E, Rozier M, Meynard D, et al. Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology. 2011;141(5):1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latour C, Besson-Fournier C, Meynard D, et al. Differing impact of the deletion of hemochromatosis-associated molecules HFE and transferrin receptor-2 on the iron phenotype of mice lacking bone morphogenetic protein 6 or hemojuvelin. Hepatology. 2016;63(1):126–137. [DOI] [PubMed] [Google Scholar]
- 23.Wang CY, Core AB, Canali S, et al. Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice. Blood. 2017;130(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nai A, Rubio A, Campanella A, et al. Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood. 2016;127(19):2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frydlova J, Prikryl P, Truksa J, et al. Effect of Erythropoietin, Iron Deficiency and Iron Overload on Liver Matriptase-2 (TMPRSS6) Protein Content in Mice and Rats. PLoS One. 2016;11(2):e0148540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latour C, Besson-Fournier C, Gourbeyre O, Meynard D, Roth MP, Coppin H. Deletion of BMP6 worsens the phenotype of HJV-deficient mice and attenuates hepcidin levels reached after LPS challenge. Blood. 2017;130(21):2339–2343. [DOI] [PubMed] [Google Scholar]
- 27.Koch PS, Olsavszky V, Ulbrich F, et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood. 2017;129(4):415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canali S, Zumbrennen-Bullough KB, Core AB, et al. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood. 2017;129(4):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corradini E, Garuti C, Montosi G, et al. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. 2009;137(4):1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Constien R, Forde A, Liliensiek B, et al. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30(1):36–44. [DOI] [PubMed] [Google Scholar]
- 31.Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117(7):1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. [DOI] [PubMed] [Google Scholar]
- 33.Mayeur C, Lohmeyer LK, Leyton P, et al. The type I BMP receptor Alk3 is required for the induction of hepatic hepcidin gene expression by interleukin-6. Blood. 2014;123(14):2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andriopoulos B Jr., Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–481. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Choesang T, Li H, et al. Increased hepcidin in transferrin-treated thalassemic mice correlates with increased liver BMP2 expression and decreased hepatocyte ERK activation. Haematologica. 2016;101(3):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arezes J, Foy N, McHugh K, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132(14):1473–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med (Berl). 2009;87(5):471–480. [DOI] [PubMed] [Google Scholar]
- 39.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115(8):2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


