Abstract
Qualitative studies suggest that social relationships play an important role in HIV pre-exposure prophylaxis (PrEP) use, but there have been few quantitative assessments of the role of social relationships in PrEP uptake or adherence. We examined the association between disclosure of study participation or LGBT identity and PrEP use in the 1603 HIV-negative participants enrolled in the iPrEx OLE study. We also evaluated the association between LGBT social group involvement and PrEP use. Study participation disclosure to parents and LGBT identity disclosure to anyone in a participant’s social network were associated with greater PrEP uptake. Study participation disclosure to partners was associated with higher probability of having protective PrEP drug concentrations compared (risk difference 0.15 95%CI [0.01, 0.30]). For each additional type of LGBT organization a participant was involved in, the probability of PrEP uptake and having protective drug concentrations increased by 0.04 (95%CI [0.03, 0.06]) and 0.04 (95%CI [0.02, 0.07]) respectively. Overall, social context was associated with PrEP use in iPrEx OLE, and should be taken into consideration when designing future PrEP implementation programs.
Keywords: pre-exposure prophylaxis, social relationships, disclosure, social integration, adherence
Introduction
Pre-exposure prophylaxis (PrEP) is effective for preventing HIV infection when taken consistently, but its public health impact depends on its uptake and use among those at greatest risk of HIV infection. Understanding the important barriers and facilitators of PrEP use is essential for designing effective PrEP programs.1,2
In considering the important factors that influence an individual’s decision to use PrEP, we adopt the conceptual model developed by Sorensen et al. in which health behaviors are affected by the interplay of individual demographic characteristics and social context.3–9 Though prior studies have mostly focused on the individual-level demographic characteristics associated with PrEP use, there has been less attention devoted to exploring the role of social relationships in PrEP uptake or adherence.10–15 Qualitative studies suggest that social support from close relationships might help PrEP users remember to take their dose and may also mitigate some of the stigma associated with study-participation or LGBT identity.16–19 Social integration –or engagement in social groups or organizations—may provide social pressure, motivation, and a sense of community that is thought to promote healthy behaviors in general.20 Thus far, however, there have been no quantitative studies measuring the impact of social relationships on PrEP uptake or adherence.
Here, we aim to examine the role that social relationships played in PrEP uptake and adherence in the iPrEx open-label extension (iPrEx OLE) study.15 In particular, we assess whether disclosure of study participation or LGBT identity to various members of a participant’s social network predicted PrEP uptake or adherence. We also evaluate the association between social integration and PrEP use during iPrEx OLE.
Methods
Study Population
iPrEx OLE enrolled 1603 HIV-negative men who have sex with men and transgender women in 11 study sites from 6 countries (Peru, Brazil, the United States, Ecuador, Thailand, and South Africa). All participants had previously been enrolled in one of three PrEP randomized controlled trials (iPrEx21, ATN08222, US Safety Study23). Enrollment occurred between June 2011 and July 2012, and participants were followed for up to 72 weeks. Participants could elect to begin taking PrEP at any time within the first 48 weeks of follow-up.15 When the study was conducted, PrEP was not yet readily available outside of the study setting, and those who decided not to take PrEP as part of iPrEx OLE were unlikely to have received it elsewhere.
Disclosure of study participation and LGBT identity
All participants were asked whether they disclosed their study participation or LGBT identity to parents, partners, friends, relatives, or other acquaintances on an interviewer-administered questionnaire. Those participants who had previously enrolled in the iPrEx trial (N=1450) answered the questionnaire at the unblinding visit of the randomized study, and those participants who had previously enrolled in ATN 082 (N=46) or US Safety Study (N=104) answered the questionnaire at the enrollment visit of iPrEx OLE.
For each type of disclosure (study participation and LGBT identity), participants were asked if they had told “all”, “most”, “one or a few”, or “none” of each relationship type. Because participants did not report the number of individuals that fall into each relationship category, we coded disclosure as a binary indicator variable for each relationship type where disclosure is considered to have occurred if the participant reported having told at least one person in a given category. For the secondary dose-response analyses, we created an average disclosure score by relationship type by taking the mean of all items in each relationship category.
Social integration
Participants were also asked about their involvement in 6 different categories of LGBT social groups or websites during a computer assisted structured interview (CASI) questionnaire at the enrollment visit of iPrEx OLE. The total number of categories reported was used to rate each participant on a scale of 0 to 6 to quantify the extent of social integration.
The text of the questionnaires is provided in Appendix 1.
PrEP uptake and adherence
The primary outcomes were decision to take PrEP by week 48 and longitudinal adherence to PrEP during follow-up.
Adherence was quantified by measuring concentrations of tenofovir diphosphate (TVF-DP) in dried blood spots (DBS) collected in a nested case-cohort sample of the overall study population that had elected to take PrEP (N=349).24 The drug concentration cohort included all participants who became HIV infected during study follow-up and a site-stratified random sample of participants who elected to take PrEP during iPrEx OLE.15 In this sample, drug concentrations were measured at all study visits after initiating PrEP (weeks 4, 8, 12, and every 12 weeks thereafter), and were categorized according to dosing to reflect the estimated number of pills taken per week. For these analyses, we use an indicator of having TFV-DP concentrations of at least 700 fmol per punch as our primary outcome. This concentration is commensurate with taking at least 4 pills/week, and is sufficient for maximum PrEP efficacy.25
Baseline Confounders
We expected that the effects of social relationships on PrEP uptake and adherence were confounded by other important baseline characteristics. Age and education likely affected the extent of one’s social integration and were also associated with both PrEP initiation and adherence in iPrEx OLE.15 Additionally, the iPrEx OLE cohort comprised participants across diverse regions of the world, and differences in cultural norms between regions may have affected disclosure and social integration. For example, in the Andes, children often live with their parents or other family members well into adulthood, whereas in the United States this is generally less common. There was wide variation in PrEP uptake and adherence across the study sites of iPrEx OLE, and thus these regional differences may have confounded any association between our measures of social relationships and PrEP use. Finally, the experiences of trans women compared to men who have sex with men are distinct and may affect both social support and social integration. Prior studies have found that trans women in iPrEx and iPrEx OLE were also less likely to use PrEP consistently.26
Statistical Methods
We assessed the association between PrEP uptake and disclosure separately by study participation and LGBT identity and by each relationship type through logistic regressions. In each regression, we controlled for geographic region, age, level of education, and gender identity, and we used Bonferroni adjusted confidence intervals to account for multiple comparisons. Using STATA’s “margins” command, we extracted the marginal probabilities of electing to take PrEP from the fitted logistic regression, and estimated the absolute risk differences associated with each disclosure category and for each level of social integration.27–29
The analyses exploring the associations between disclosure and social integration and PrEP adherence over time were conducted in the sample of the study population for whom DBS data were available (N=349). We used generalized estimating equations with sampling weights to reflect the study design, and we calculated robust standard errors to account for repeated measures on each individual. We calculated the marginal probability of having drug concentrations commensurate with taking at least 4 pills per week for each disclosure category and each level of social integration.
Because of the geographically and culturally heterogeneous nature of the iPrEx OLE cohort, we hypothesized that the effects of social support and social integration on PrEP initiation or use may vary according to geographic region. We similarly hypothesized that the effects of social support and social integration may differ between men who have sex with men and transgender women. We tested for effect heterogeneity across geographic region and gender identity in all models.
As a secondary post-hoc analysis, for any relationship category that was found to be associated with either uptake or adherence, we assessed whether there was a dose-response between extent of disclosure within the relationship category and PrEP uptake and adherence. All analyses were conducted using STATA 13.1.
Results
Table I shows the distribution of disclosure and social integration by region, level of education, age, and gender.
Table 1.
Social support and social integration by region, education, age, and gender
| Disclosure | Social Integration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Disclosed Study Participation To: N (%) | Disclosed LGBT Identity To: N (%) | Number of Groups Participated In (Range 0–6) | ||||||||
| Parents | Partners | Friends or Relatives | Acquaintances | Parents | Partners | Friends or Relatives | Acquaintances | Mean | |||
| Region | United States | 287 | 89 (31.0) |
203 (70.7) |
239 (83.3) |
121 (42.2) |
207 (72.1) |
262 (91.3) |
270 (94.1) |
230 (80.1) |
2.6 |
| Brazil | 208 | 108 (51.9) |
175 (84.1) |
195 (93.8) |
126 (60.6) |
143 (68.8) |
181 (87.0) |
201 (96.6) |
164 (78.8) |
1.7 | |
| Andes | 999 | 571 (57.2) |
719 (72.0) |
876 (87.7) |
499 (49.9) |
496 (49.6) |
738 (73.9) |
847 (84.8) |
611 (61.2) |
0.8 | |
| Cape Town | 48 | 25 (52.1) |
34 (70.8) |
44 (91.7) |
22 (45.8) |
34 (70.8) |
42 (87.5) |
43 (89.6) |
37 (77.1) |
1.9 | |
| Thailand | 61 | 21 (34.4) |
36 (59.0) |
55 (90.2) |
20 (32.8) |
21 (34.4) |
31 (50.8) |
33 (54.1) |
18 (29.5) |
1.1 | |
| Education | Less Than Secondary School | 327 | 208 (63.6) |
239 (73.1) |
297 (90.8) |
188 (57.5) |
198 (60.6) |
254 (77.7) |
286 (87.5) |
221 (67.6) |
0.9 |
| Completed Secondary School | 547 | 289 (52.8) |
382 (69.8) |
467 (85.4) |
263 (48.1) |
288 (52.7) |
401 (73.3) |
455 (83.2) |
343 (62.7) |
0.9 | |
| More than Secondary School | 716 | 307 (42.9) |
537 (75.0) |
632 (88.3) |
329 (45.9) |
407 (56.8) |
588 (82.1) |
641 (89.5) |
488 (68.2) |
1.8 | |
|
Age |
18–24 | 418 | 257 (61.5) |
295 (70.6) |
378 (90.4) |
173 (41.4) |
240 (57.4) |
319 (76.3) |
359 (85.9) |
266 (63.6) |
1 |
| 25–29 | 403 | 228 (56.6) |
281 (69.7) |
346 (85.9) |
193 (47.9) |
211 (52.4) |
296 (73.4) |
334 (82.9) |
239 (59.3) |
1.1 | |
| 30–40 | 435 | 215 (49.4) |
326 (74.9) |
388 (89.2) |
243 (55.9) |
248 (57.0) |
340 (78.2) |
384 (88.3) |
303 (69.7) |
1.3 | |
| >=40 | 347 | 114 (32.9) |
265 (76.4) |
297 (85.6) |
179 (51.6) |
202 (58.2) |
299 (86.2) |
317 (91.4) |
252 (72.6) |
1.8 | |
| Gender | MSM | 1578 | 702 (44.5) |
1036 (65.7) |
1246 (79.0) |
682 (43.2) |
778 (49.3) |
1119 (70.9) |
1248 (79.1) |
931 (59.0) |
1.6 |
| TGW | 192 | 112 (58.3) |
131 (68.2) |
163 (84.9) |
106 (55.2) |
123 (64.1) |
135 (70.3) |
146 (76.0) |
129 (67.2) |
0.9 | |
Of the 1603 HIV-negative participants who enrolled in iPrEx OLE, 1225 elected to take PrEP during study follow-up. After adjusting for region, education, age, and gender, those who reported telling their parents about their study participation were more likely to elect to take PrEP compared to those who did not disclose their study participation to their parents (0.83 vs. 0.76 respectively; risk difference 0.07 95%CI [0.01, 0.12]). Participants who disclosed their LGBT identity to parents, partners, friends or relatives, or other acquaintances were also more likely to elect to take PrEP during iPrEx OLE compared to those who did not (Table 2).
Table 2.
Disclosure and PrEP uptake and adherence
| PrEP Uptake | PrEP Adherence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Any Disclosure# | Extent of Disclosure% | Any Disclosure# | Extent of Disclosure% | ||||||
| Risk Difference1 | 95% Cl | Risk Difference2 | 95% Cl | Risk Difference3 | 95% Cl | Risk Difference4 | 95% Cl | ||
| 0.07 | [0.01, 0.12] | 0.08 | [0.01, 0.14] | 0.01 | [−0.12, 0.14] | ||||
| Partners | 0.06 | [−0.01, 0.13] | 0.15 | [0.01, 0.30] | 0.1 | [0.02, 0.18] | |||
| Friends or Relatives | 0.07 | [−0.02, 0.16] | 0.13 | [−0.08, 0.34] | |||||
| Others | 0.04 | [−0.01, 0.10] | 0.08 | [−0.04, 0.22] | |||||
| LGBT Identity | Parents | 0.09 | [0.04, 0.15] | 0.09 | [0.03, 0.15] | −0.01 | [−0.14, 0.13] | ||
| Partners | 0.08 | [0.00, 0.16] | 0.05 | [0.02, 0.08] | 0.07 | [−0.12, 0.27] | |||
| Friends or Relatives | 0.09 | [0.00, 0.18] | 0.05 | [0.03, 0.08] | 0.05 | [−0.16, 0.26] | |||
| Others | 0.10 | [0.04, 0.16] | 0.04 | [0.02, 0.07] | 0.05 | [−0.10, 0.20] | |||
Dichotomous indicator variable where disclosure is considered to have occurred if the participant reported disclosure to at least one member of the relationship category.
Average extent of disclosure within each relationship category. Scores range from 0 “I told none” to 3 “I told all”.
Absolute difference in probability of taking PrEP between those who disclosed to at least one member of the relationship category compared to those who did not disclose to any members of the relationship category.
Absolute difference in probability of taking PrEP for each unit increase in mean extent of disclosure in the relationship category.
Absolute difference in probability of having protective drug concentrations between those who disclosed to at least one member of the relationship category compared to those who did not disclose to any members of the relationship category.
Absolute change in probability of having protective drug concentrations for each unit increase in mean extent of disclosure in the relationship category.
After controlling for region, education, age, and gender, participants who told their partners about their study participation were more likely to have protective levels of drug concentrations than those who did not tell their partners about their study participation (0.40 vs. 0.25 respectively; risk difference 0.15 95%CI [0.01, 0.30]). However, neither disclosure of study participation to parents, other relatives, or friends, nor disclosure of LGBT identity to any group was associated with having protective drug concentrations (Table II).
Among those relationship categories in which disclosure was associated with PrEP uptake or adherence, we found evidence of a dose-response relationship where more disclosure was more strongly associated with uptake and adherence (Table II).
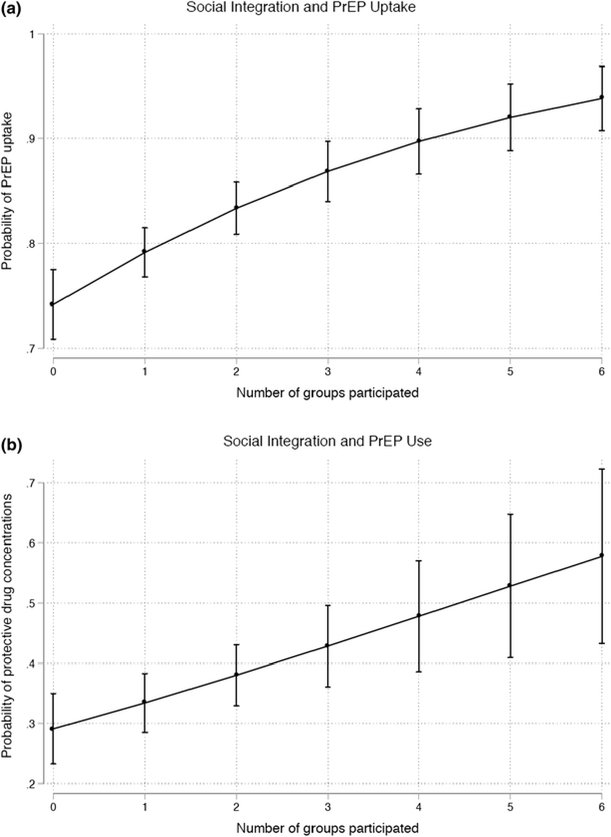
Figure 1 shows the association between increased participation in LGBT social groups and probability of PrEP uptake (Figure 1a) and PrEP adherence (Figure 1b). Overall, the probability of electing to take PrEP and having protective drug concentrations increased by 0.04 (95%CI [0.03, 0.06]) and 0.04 (95%CI [0.02, 0.07]) respectively for each additional reported type of social group participation.
Figure 1.
Social integration and PrEP uptake and adherence
There was no evidence of significant effect heterogeneity in the association between disclosure or social integration on PrEP uptake or use across geographic region or gender identity for any of the analyses (results not shown).
Discussion
Overall, we find that uptake and adherence to PrEP in iPrEx OLE was associated with disclosure of prior study participation and LGBT identity and involvement in LGBT organizations. The role of disclosure in PrEP uptake and use varied depending on the type of relationship in which the disclosure occurred. Disclosure of study participation to parents and disclosure of LGBT identity to anybody in the participant’s social network were associated with greater PrEP uptake in iPrEx OLE. Since average PrEP uptake in iPrEx OLE was already high (76%),15 the magnitudes of the associations between disclosure and PrEP uptake were consistent yet relatively minor. This result echoes prior findings in qualitative studies in which potential PrEP users reported that fear of unintentionally disclosing LGBT identity or being mistaken for being HIV-positive was a significant barrier for using PrEP.19,30,31 Here, a possible interpretation is that those who had already disclosed their study participation or LGBT identity to members of their social network may have been less concerned about others knowing about their PrEP use, and were therefore more likely to initiate PrEP.
On the other hand, only disclosure of study participation to partners was associated with increased PrEP adherence in iPrEx OLE. Though we cannot directly assess the reason for why disclosure of study participation to partners was so strongly associated with higher adherence in iPrEx OLE, we suspect partners played an integral role in reminding participants to take their doses as had been reported in the HPTN 067/ADAPT qualitative studies.16 The magnitude of this association is striking and is underscored by the additional evidence of a dose-response relationship between amount of disclosure and adherence. This highlights a potential avenue for developing interventions to promote adherence by involving partners in PrEP programs.
Social integration and more frequent interaction with LGBT organizations appeared to provide resources that fostered both PrEP uptake and adherence in iPrEx OLE. In addition to the sense of community and motivation afforded by greater social integration, we expect that LGBT organizations played an important role in dissemination of information regarding PrEP’s efficacy. Thus, those participants who were more socially integrated at the time of iPrEx OLE enrollment may have been more inclined to elect to take PrEP, and similarly may have been more motivated to continue taking PrEP. This is consistent with qualitative analyses of other open-label PrEP users and the Mutuality Framework, in which peer-based support improves trust and knowledge of PrEP, and can also provide adherence reminders and pressure to maintain PrEP use.16
Finally, we found no evidence that associations between disclosure or social integration and PrEP uptake or use varied according to geographic region or gender identity. This is notable, given the diverse cultural contexts and experiences represented by the participants in iPrEx OLE, and suggests that social relationships may be important for PrEP implementation across a wide range of settings and populations.
Limitations of this work include the fact that the measurements did not comprehensively assess all aspects of social support or social integration; rather these measures examined components of each of these constructs at a single time point. Additionally, our questionnaire did not measure the number of people disclosed to, and therefore could not precisely capture degree of disclosure. As such, the findings presented here cannot fully explore the extent to which social support or social integration may affect PrEP uptake and use, but rather are a preliminary step in understanding and quantifying the role of social relationships in PrEP implementation.
In interpreting these results for future PrEP implementation programs, it is important to highlight the fact that iPrEX OLE enrolled participants prior to PrEP’s FDA approval or widespread availability. Awareness of PrEP’s efficacy has undoubtedly increased among potential users—particularly in the United States and other countries where PrEP has received regulatory approval, and these changes may alter the specific roles social relationships play in PrEP use. For example, as knowledge about PrEP’s efficacy becomes more widespread, it’s possible that the role of LGBT organizations in encouraging PrEP uptake may diminish. Similarly, as PrEP use becomes more common, we anticipate that many of the concerns surrounding disclosure of PrEP use may diminish in certain populations. However, PrEP is still not available in most of the world, and issues surrounding awareness and stigma are likely to persist for years to come.
Overall, it is clear that social relationships are important for PrEP uptake and use, and their role is likely to persist and evolve as knowledge and uptake increases. Future PrEP implementation programs should be designed with social context in mind, and should consider incorporating interventions to improve social support or social integration with traditional PrEP programs. In particular, PrEP implementation programs should explore programs that involve informing partners about PrEP in order to foster support in dose-taking reminders. In addition, given the role LGBT organizations played in PrEP uptake and adherence in iPrEx OLE, PrEP implementation programs should collaborate with these organizations to ensure that they have the necessary information and resources available to promote the spread of accurate and useful information about PrEP use.
Supplementary Material
Funding sources.
The iPrEx Open Label Extension was sponsored by the NIH (NIAID UO1 AI064002, RO1 AI062333, and RO1 AI118575) with study medication donated by Gilead Sciences.
Footnotes
Potential Conflicts of Interest: R.M.G has received fees from and a research grant from ViiV, a manufacturer of an investigational compound being investigated for use as PrEP. D.V.G is a member of an advisory board for Gilead Sciences. K.R.A. has a Gilead Sciences education grant through the University of Michigan. All other authors: no reported conflicts.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Hankins C, Macklin R & Warren M Translating PrEP effectiveness into public health impact: key considerations for decision-makers on cost-effectiveness, price, regulatory issues, distributive justice and advocacy for access. J. Int. AIDS Soc 18, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaton LA & Kalichman S Translating Pre-Exposure Prophylaxis Evidence into Practice and Public Health Impact in Biomedical Advances in HIV Prevention: Social and Behavioral Perspectives (Springer Science & Business Media, 2013). [Google Scholar]
- 3.Sorensen G et al. Model for incorporating social context in health behavior interventions: applications for cancer prevention for working-class, multiethnic populations. Prev. Med 37, 188–197 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Berkman LF & Syme SL Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am. J. Epidemiol 109, 186–204 (1979). [DOI] [PubMed] [Google Scholar]
- 5.Cohen S Social Relationships and Health. Am. Psychol 59, 676–684 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Uchino BN Social Support and Health: A Review of Physiological Processes Potentially Underlying Links to Disease Outcomes. J. Behav. Med 29, 377–387 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb BH & Bergen AE Social support concepts and measures. J. Psychosom. Res 69, 511–520 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Calvo R, Avendano M, Sivaramakrishnan K & Berkman LF Social support, volunteering and health around the world: cross-national evidence from 139 countries. Soc. Sci. Med. 1982 74, 696–706 (2012). [DOI] [PubMed] [Google Scholar]
- 9.DiMatteo MR Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc 23, 207–218 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Ware NC et al. What’s Love Got to Do With It? Explaining Adherence to Oral Antiretroviral Pre-exposure Prophylaxis (PrEP) for HIV Serodiscordant Couples. J. Acquir. Immune Defic. Syndr 1999 59, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu A et al. Patterns and correlates of PrEP drug detection among MSM and transgender women in the Global iPrEx Study. J. Acquir. Immune Defic. Syndr. 1999 67, 528–537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnell D et al. HIV Protective Efficacy and Correlates of Tenofovir Blood Concentrations in a Clinical Trial of PrEP for HIV Prevention. J. Acquir. Immune Defic. Syndr. 1999 66, 340–348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haberer JE et al. Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa. PLOS Med. 10, e1001511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeten JM, Haberer JE, Liu AY & Sista N Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J. Acquir. Immune Defic. Syndr. 1999 63 Suppl 2, S122–129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant RM et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect. Dis 14, 820–829 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amico KR et al. Experiences with HPTN 067/ADAPT Study-Provided Open-Label PrEP Among Women in Cape Town: Facilitators and Barriers Within a Mutuality Framework. AIDS Behav. 21, 1361–1375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore HJ et al. Participant Experiences and Facilitators and Barriers to Pill Use Among Men Who Have Sex with Men in the iPrEx Pre-Exposure Prophylaxis Trial in San Francisco. AIDS Patient Care STDs 27, 560–566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold T et al. Social, structural, behavioral and clinical factors influencing retention in Pre-Exposure Prophylaxis (PrEP) care in Mississippi. PLOS ONE 12, e0172354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goicochea P Barriers and facilitators to PrEP adherence in iPrEx: a prevention trial that started in 3 Andean cities. (2009).
- 20.Berkman LF, Glass T, Brissette I & Seeman TE From social integration to health: Durkheim in the new millennium☆☆This paper is adapted from Berkman, L.F., & lass, T. Social integration, social networks, social support and health. In L. F. Berkman & I. Kawachi, Social Epidemiology. New York: Oxford University Press; and Brissette, I., Cohen S., Seeman, T. Measuring social integration and social networks. In S. Cohen, L. Underwood & B. Gottlieb, Social Support Measurements and Intervention. New York: Oxford University Press. Soc. Sci. Med 51, 843–857 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Grant RM et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med 363, 2587–2599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosek SG et al. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J. Acquir. Immune Defic. Syndr. 1999 62, 447–456 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grohskopf LA et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J. Acquir. Immune Defic. Syndr. 1999 64, 79–86 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Castillo-Mancilla J et al. Emtricitabine-Triphosphate in Dried Blood Spots as a Marker of Recent Dosing. Antimicrob. Agents Chemother. 60, 6692–6697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson PL et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Sci. Transl. Med 4, 151ra125–151ra125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutsch MB et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV (2015). doi: 10.1016/S2352-3018(15)00206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Localio AR, Margolis DJ & Berlin JA Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J. Clin. Epidemiol 60, 874–882 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Williams R Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 12, 308–331 (2012). [Google Scholar]
- 29.STATA Statistical Software: Release 13. (StataCorp LP; ). [Google Scholar]
- 30.Tangmunkongvorakul A et al. Facilitators and barriers to medication adherence in an HIV prevention study among men who have sex with men in the iPrEx study in Chiang Mai, Thailand. AIDS Care 25, 961–967 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Galea JT et al. Acceptability of Pre-Exposure Prophylaxis (PrEP) as an HIV prevention strategy: Barriers and facilitators to PrEP uptake among at-risk Peruvian populations. Int. J. STD AIDS 22, 256–262 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.