Abstract
Neuroinflammation is one of the mechanisms leading to neurodegenerative brain damage induced by chronic alcohol (ethanol) exposure. Microglia play a major role in the development of innate immune responses to environmental injuries including ethanol. Adenosine 5″-triphosphate (ATP)-activated purinergic P2X receptor (P2XR) subtypes, P2X4Rs and P2X7Rs, are endogenously expressed in microglia and can modulate their activity. These 2 P2XR subtypes differ pharmacologically and functionally: 1) P2X4Rs are activated at lower (≤0.1 mM) whereas P2X7Rs – at higher (≥1.0 mM) ATP concentrations; 2) P2X4R activation contributes to the release of brain derived neurotrophic factor and its role in tactile allodynia and neuropathic pain is demonstrated; 3) Due to its role in the secretion of pro-inflammatory IL-1β, P2X7Rs have been implicated in the development of neurodegenerative pathologies, pain and morphine tolerance. To date, the roles of individual P2XR subtypes in ethanol effects on microglia and the functional consequences are not completely understood. Based on the existing knowledge on the pharmacological and functional differences between P2X4Rs and P2X7Rs, the present work tested the hypothesis that P2X4Rs and P2X7Rs play differential roles in ethanol action in microglia. Effects of ethanol on P2X4R and P2X7R activity, expression and functional consequences were determined using murine BV2 microglial cells. Ethanol (≥100 mM) inhibited P2X4Rs but was inactive on P2X7 channel activity. Ethanol (25, 100 mM) inhibited P2X4R-mediated microglia migration whereas it potentiated pore formation in P2X7Rs. Furthermore, ethanol (25, 100 mM) potentiated P2X7R-mediated IL-1β secretion from BV2 microglia. Ethanol also induced protein expression for both P2XR subtypes. Overall, the findings identify differential roles for P2X4Rs and P2X7Rs in regards to ethanol effects on microglia which may be linked to different stages of ethanol exposure.
Keywords: Purinergic P2X4 and P2X7 receptors, Ethanol, BV2 microglia, P2X antagonists, IL-1β secretion, Patch-clamp electrophysiology
1. Introduction
Chronic alcohol abuse and alcoholism are significant socioeconomic problems worldwide that often result in cognitive impairment and permanent brain damage (Vetreno et al., 2011). Despite substantial efforts to treat alcohol use disorder, no effective pharmacotherapies have been developed so far. Excessive high relapse rates further aggravate the problem thus signifying the need for novel strategies to ameliorate the consequences of chronic ethanol (alcohol) consumption. To overcome these issues, it is important to understand the mechanisms and targets of ethanol action in the brain.
Recent advances suggest that one of the mechanisms leading to neurodegenerative brain damage induced by chronic alcohol use is associated with persistent neuroinflammation (Crews and Vetreno, 2014; Kelley and Dantzer, 2011). Microglia, immune cells of the nervous system, play a major role in the development of neuro-inflammatory responses (Alfonso-Loeches et al., 2010). Microglia comprise 10% of the total cell population in the CNS and are of myeloid origin (Tay et al., 2017). Microglia serve as immune guards for surveillance of neuronal injury and play an important role in glia-neuron communication (Fernandes et al., 2014; Sierra et al., 2014). In this regard, microglia express various immunomodulatory proteins and upon activation are able to produce variety of cytokines, chemokines, reactive oxygen species, neurotrophic factors, proteases that contribute to neuroinflammation (Graeber, 2010).
Building evidence suggests that ethanol exposure can cause activation of microglia in the CNS. Studies on different animal models of ethanol exposure demonstrated variety of responses of microglia, ranging from partial to full activation as well as microglial apoptosis (Crews et al., 2015; Marshall et al., 2013; McClain et al., 2011). In addition, increases in pro-inflammatory mediators in both animal models of chronic ethanol exposure as well as in post-mortem alcoholic human brain have been linked to changes in glial cells and in particular to microglia (Crews and Nixon, 2009; He and Crews, 2008).
ATP activated purinergic P2X receptors (P2XRs) are expressed in immune cells including microglia and play a vital role in modulation of microglia activity (Boumechache et al., 2009; Monif et al., 2010). P2X4Rs and P2X7Rs are the major P2XR subtypes expressed in microglia. One important difference between the two P2XR subtypes is related to their sensitivities to ATP. At P2X4Rs 100 μM ATP exerts maximum inducible currents whereas P2X7Rs are activated at dramatically high, pathological ATP concentrations (1.0 mM and above). Other differences include ability of P2X7Rs to form a pore or interact with other pore-forming proteins in plasma membranes and the unusual long C-terminus of P2X7Rs that has sites for regulation by other molecules. Overall, P2X7Rs possess features that make these receptors unique among all the members of P2XR superfamily (Bartlett et al., 2014).
Both P2XR subtypes can modulate microglia function. Activation of P2X4Rs is sufficient to trigger brain derived neurotrophic factor release and subsequent tactile allodynia (Ulmann et al., 2008). P2X4-null mice retain normal sensory function but lose mechanical hypersensitivity after peripheral nerve injury (Tsuda et al., 2013; Ulmann et al., 2008). In addition, it has been shown that pro-inflammatory agents such as lypopolysaccharide (LPS) are able to dramatically increase the number of P2X4Rs on microglial cell membranes (Boumechache et al., 2009; Raouf et al., 2007). P2X4Rs are also involved in chemotaxis, long-term potentiation (LTP) in spinal dorsal horn, and opioid signaling (Gong et al., 2009; Horvath et al., 2010; Ohsawa et al., 2007). For the involvement in pathology, the role of P2X4Rs in neuropathic pain has been demonstrated (Burnstock, 2016; Masuda et al., 2014; Tsuda et al., 2013). In addition, P2X4R-signaling plays a role in ability to migration of microglia in response to ethanol or morphine treatment (Gofman et al., 2014; Horvath et al., 2010).
P2X7R functioning was found to be sufficient to trigger microglia activation and proliferation (Monif et al., 2009). P2X7Rs have been shown to modulate the release of IL-1β, a recognized mediator of neurodegeneration and pain sensation (Clark et al., 2010; Honore et al., 2009; Takenouchi et al., 2008). P2X7Rs are also mediators of TNFα and CC-chemokine ligand 3 secretion (Kataoka et al., 2009; Suzuki et al., 2004), production of superoxide (Parvathenani et al., 2003) and nitric oxide in microglial cells (Gendron et al., 2003) and matrix metalloproteinase 9 (Choi et al., 2010; Shin et al., 2010). As such, P2X7Rs have been implicated in CNS disease states such as neurodegenerative pathologies, pain, morphine tolerance (Sperlagh and Illes, 2014; Takenouchi et al., 2010; Zhou et al., 2010). Elimination of P2X7R activity via blockade or knockout decreases nociception in neuropathic and inflammatory pain models (Chessell et al., 2005; Honore et al., 2009).
For the past decade our group has been extensively studying P2X4Rs as targets of ethanol action and in regards to their involvement in alcohol-induced behaviors (reviewed in (Franklin et al., 2014)). For example, using recombinant expression systems and electrophysiological approaches we have identified that among several P2XR subtypes, including P2X1, P2X2 and P2X3Rs, P2X4Rs are the most ethanol sensitive receptors directly inhibited by ethanol (Davies et al., 2005; Ostrovskaya et al., 2011; Popova et al., 2010). Moreover, genetic studies have linked the differential p2rx4 gene expression to drinking phenotypes (reviewed in (Franklin et al., 2014)). In addition, p2rx4 knockdown caused increased drinking behaviors in the early phases in mice suggesting a role of P2X4Rs in ethanol consumption. Lastly, a recent report suggests that P2X4Rs play a role in the functional response of microglial to ethanol (Gofman et al., 2014). With regards to ethanol effects on P2X7Rs, our recent work demonstrated an increased expression of P2X7Rs in ethanol-sensitive brain regions that paralleled neuroinflammatory responses in C57BL/6J mice exposed to chronic intragastric ethanol combined with high fat diet (Asatryan et al., 2015).
Despite these advances, the role of each individual P2XR subtype in ethanol modulation of microglia and the link to neuroinflammation and behavioral effects is yet to be determined. Based on the existing knowledge on pharmacological and functional differences between P2X4R and P2X7Rs, we hypothesized that these two P2XRs play differential roles in ethanol action in microglia. The present study used murine BV2 microglia which endogenously express P2X4Rs and P2X7Rs to test acute effects of ethanol on P2XR activation, P2XR expression and P2XR-mediated functional consequences.
2. Materials and methods
2.1. Materials
ATP disodium salt, 2′(3′)-O-(4-Benzoylbenzoyl) adenosine-5′-triphosphate triethylammonium salt (BzATP), LPS, ivermectin (IVM), ethanol (190 proof, USP), ethidium bromide dye (EB), dimethylsulfoxide (DMSO) were purchased from Sigma Co. (St. Louis, MO, USA). Mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) was obtained from Vector Labs (Burlingame, CA). Five-(3-Bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (5-BDBD), N-cyano-N”-[(1S)-1-phenylethyl]-N′-5-quinolinyl-guanidine (A804598) were from Tocris Biosciences (Bio-Techne, Minneapolis, MN). Antibodies to P2X4 and P2X7 proteins were purchased from Alomone Labs (Jerusalem, Israel), antibody to beta actin — from Sigma Co. All other chemicals were of reagent grade.
2.2. BV2 microglia culture and treatments
The current experiments used immortalized murine BV2 microglial cell line, a kind gift from Dr. B. Khakh (UCLA). As in all cell models, BV2 microglial cells have some limitations including a partial activated state, lack of diverse morphological states and less sensitivity to stimulatory challenges. Even with these limitations, this cell line is widely used for investigations of microglial activation (Henn et al., 2009). The cells were grown at a humidified atmosphere of 5% CO2 in DMEM:F12 (1:1) medium containing Glutamax (Thermo Fisher Scientific, Canoga Park, CA) and supplemented with 5—10% FBS and penicillin/streptomycin. Cells were cultured twice a week, trypsinized and plated in 35 mm culture plates for electrophysiological recordings or multi-well plates before appropriate treatments.
2.3. Patch-clamp electrophysiology
Whole-cell current recordings were performed according to the procedures published before (Ostrovskaya et al., 2011). Briefly, BV2 cells were voltage clamped at −70 mV at room temperature, and agonist-induced currents were acquired using Axopatch 200B amplifier, Digidata 1320 interface and pClamp 9.0 software (Molecular Devices, Union City, CA). Data was digitized at 5 kHz and filtered at 1 kHz. Patch electrodes (2—6 MΩ) were filled with (in mM): KCl 140; MgCl2 2; EGTA 2.5; TEA-Cl 2; K2ATP 4; HEPES 10; pH 7.25 with KOH and osmolarity of 310 mmol/kg. Composition of the external solution was (in mM): NaCl 135, KCl 5.4, CaCl2 1.8, MgCl2 1, HEPES 10, and glucose 10 with pH of 7.4 adjusted with NaOH. Osmolarity was adjusted to 315 mmol/kg with sucrose. Agonist and drug containing solutions were prepared freshly on the day of an experiment. Agonists were applied for the shortest time-period sufficient to reach the peak in most experiments. In experiments with ethanol, cells were pre-treated with ethanol for 30 s - 1 min before agonist applications. IVM was pre-applied to the cells for 2 min before the addition of agonists. Time between applications was 15—120 s depending on receptor recovery from desensitization. Agonist/drug applications were performed through a 3-barell flowpipe using a Warner SB-77B Fast Perfusion apparatus and VC-6 Valve Controller (Hamden, CT).
2.4. Microglia migration assay
Cells were plated in 12-well plates (5 × 105/well) and treated next day with ATP at 0; 0.1; or 1.0 mM in the absence and presence of ethanol at 0, 25 and 100 mM for a period of 30 min. In separate series of tests, cells were pre-treated for 15 min with DMSO or antagonists (5-BDBD, A804598) or 2 min with IVM prior to the ATP addition. After 30 min incubation with ATP medium was replaced with OPTI-MEM and cells continued to incubate for 3 more h. Cells then were trypsinized, collected in a 1 ml growth medium containing 1% FBS and counted using disposable hemocytometers (INCYTO C-Chip, Fisher Sci). Thirty to 50 μl of the cell suspension was added into Transwell tissue culture inserts (8 μm pore size, Corning, NY) in duplicates. The cells were allowed to settle for 15 min before the addition of 200 μl of medium containing 1% FBS and 800 μl of medium containing 20% FBS to the lower well to stimulate migration. After overnight incubation, the non-migrated cells and the medium were removed from the inserts using cotton swabs, inserts washed once with PBS and fixed using 70% ethanol for 10 min. At each additional step the upper chambers of the inserts were dried with a cotton swab. The inserts then were washed twice in distilled water, membranes were excised using a scalpel, placed on a glass slide facing up and immediately cover slipped using a mounting solution with DAPI.
2.5. Ethidium bromide (EB) uptake assay
Cells were plated in 96-well black plates with clear bottom at 5 × 104/well density the day before treatments. For treatments, the growth medium was removed and replaced with OPTI-MEM containing mixture of 50 μg/ml EB dye and ATP at 0; 0.1; or 1.0 mM ATP in the absence or presence of 0, 25,100 mM ethanol. Control wells were incubated with EB solution without agonists and/or ethanol. In experiments including P2X4R or P2X7R antagonists, those were added to the cells 15 min prior to the addition of the mixture of EB and/or ATP. To test the effects of IVM, cells were pre-treated with the drug for 2 min with IVM prior to the addition of the mixture of EB and/or ATP and/or the drug. EB solution with DMSO was added to the control wells for antagonist or IVM experiments. Assays were performed in triplicates. After 15 min of incubation at 37 °C, the cells were washed twice with OPTI-MEM and the EB fluorescence (excitation/emission filter cutoffs — 540 nm/605 nm) visualized using a BD Pathway 435 High-Content Bioimager (BD Biosciences, San Jose, CA). Transmitted light microscopic images were also acquired to further use in data analysis.
2.6. Treatments for IL-1β and TNFα determinations
Cells were plated in 6-well plates a day before the treatments at 1 × 106/well. Cells were treated with 1.0 μg/ml LPS for 2.5—3 h. After initial LPS treatments, ATP was added at 0; 0.1, or 1.0 mM in the absence and presence of 25 or 100 mM ethanol and continued to incubate for 30 min. At the end of the treatments, the supernatants were collected, briefly spun to remove any large cellular debris and stored at −80 °C for IL-1β and TNFα ELISA assays.
2.7. IL-1β and TNF-α measurements
IL-1β and TNF-α measurements in cell supernatants were performed using ELISA kits respectively from Affimetrix and R&D Systems (both available now at ThermoScientific, Waltham, MA) following instructions from manufacturers.
2.8. BV2 cell treatments and Western immunoblotting (WB) for P2X4R and P2X7R expression
For all treatments, cells were plated in 12-well plates at a density of 5 × 105/well in the complete medium the day before the treatments. On the day of the treatment the culture medium was replaced by phenol-red free DMEM supplemented with 0.5% BSA and penicillin/streptomycin. Ethanol was added at different concentrations (0, 25, 50 and 100 mM) and incubated for 24 h in a closed chamber. A 35 mm dish with DPBS supplemented with 100 mM ethanol was placed open in the chamber to reduce evaporation of ethanol from the wells. At the end of the treatments, the cells were washed with PBS and collected in RIPA lysis buffer (10 mM Tris, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, pH 8.0) and kept at −20 °C for further WB analysis. For WB, 10 μg of protein was separated using 10 or 12% SDS-PAGE, protein transferred to PVDF membranes and blotted against P2X4, P2X7 and beta actin. The following antibodies and dilutions were used: rabbit polyclonal P2X4 - 1:2000, rabbit polyclonal P2X7 – 1:1000, mouse monoclonal beta actin — 1:10,000. P2X7 and P2X4 antibodies were incubated overnight at 4 °C, beta actin — 10—15 min at room temperature. The protein bands were visualized following appropriate HRP-conjugated secondary anti-rabbit or anti-mouse antibody incubations for 1 h at room temperature and incubation with enhanced chemiluminescence substrate (Clarity Western, Bio-Rad, Hercules, CA).
2.9. Data analyses
Patch-clamp data were analyzed with pClamp 9.0 (Molecular Devices). Drug effects are presented as normalized percentage of peak current responses obtained with agonist alone. Two agonist responses measured before the drug applications were averaged and taken as the control current. Each experiment was carried out with 3 or more cells from at least two different batches. Densitometry data for Western blots were analyzed using Image J software (http://imagej.nih.gov/ij). P2X4 and P2X7 band densities were normalized over the corresponding beta actin bands. Changes are presented as % difference from no ethanol control taken as “1”. To obtain migrated cell number, DAPI fluorescence images were obtained using Nikon Diaphot 300 fluorescence microscope (Nikon Instruments, Inc., Melville, NY) at 20× magnification and images analyzed using Image J software. Averages of the cell numbers from 10 to 15 different fields were normalized over the cell densities obtained before seeding into the Transwell inserts. Changes are expressed as % difference from “no ATP” control taken as “1”. EB uptake was analyzed using Image J software by calculating the total fluorescence signal on each image normalized on the number of cells in the field. The threshold for the fluorescence images was set to “black&white”. The number of cells in each image was obtained via particle analysis in Image J. The changes were compared to the “no ATP” controls set at “1 ”. The antagonist data were fitted to nonlinear one-site inhibition curve. All analyzed data are presented as mean ± SEM using GraphPAD Prism software (San Diego, CA). Statistical analysis was performed using Student’s t-test or 1-way or 2-way ANOVA followed by multiple comparison Tukey test where appropriate with the significance defined as p < 0.05.
3. Results
3.1. P2X4Rs and P2X7Rs in BV2 microglia have different pharmacological properties
To distinguish between P2X4Rs and P2X7Rs endogenously expressed in BV2 cells, we tested currents evoked by two different concentrations of ATP, 0.1 and 1.0 mM, using patch-clamp electrophysiology (Fig. 1A). ATP (0.1 mM for 0.5 s) evoked a fast-desensitizing current typical of P2X4R response. Higher concentration of ATP (1.0 mM) applied for 0.5 s caused larger and slowly deactivating currents. Alternatively, ATP (1.0 mM) applied for a longer time interval (10 s) activated large current that had a biphasic kinetics. The first phase of fast activation was followed by a larger sustained current upon the presence of ATP. It is likely that both P2X4 and P2X7 channels contributed to the response evoked by short-term application of high ATP concentration (1.0 mM ATP for 0.5 s). On the other hand, the high capacity sustained current as visualized during longer-term ATP application (1.0 mM for 10 s) suggested activation of a pore associated with P2X7Rs. To further confirm the presence and contribution of P2X7Rs to the observed currents induced by 1.0 mM ATP, we also tested BzATP. BzATP is a known synthetic ATP agonist with activity in several P2XRs, however it demonstrates higher potency compared to ATP in activating P2X7Rs (Donnelly-Roberts et al., 2009). Application of 0.1 mM BzATP evoked inward currents which were smaller than those induced by 1.0 mM ATP but comparable to the responses to 0.1 mM ATP (Fig. 1A). To further characterize the two receptors, we tested the effect of IVM on ATP-induced currents, which is known to allosterically modulate P2X4Rs (Khakh et al., 1999). As expected, IVM (3.0 μM) potentiated currents evoked by 0.1 mM ATP (Fig. 1B). IVM also potentiated currents activated by 0.1 mM BzATP (Fig. 1B). The fold change in the current amplitude and the increase in current deactivation time determined as t1/2 for IVM effects were comparable for both ATP and BzATP (Fig. 1C).
Fig. 1. Pharmacological characterization of ATP-induced currents in BV2 microglia.
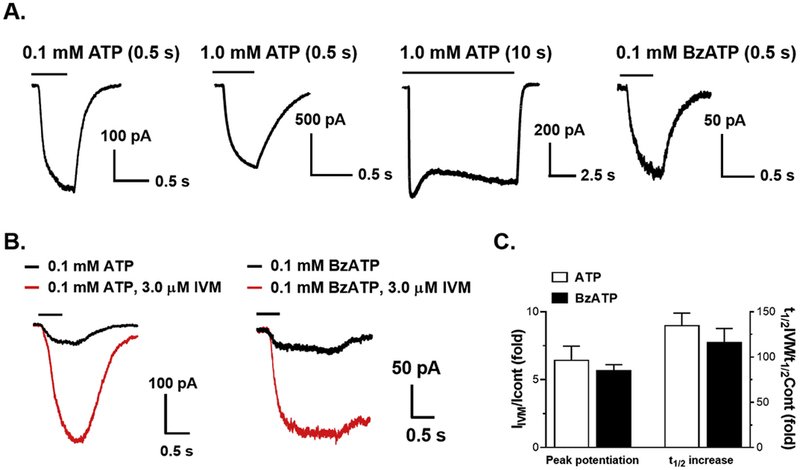
A. Representative current traces evoked by 0.1,1.0 mM ATP and 0.1 mM BzATP. B, C. Effect of 3.0 μM IVM on 0.1 mM ATP and 0.1 mM BzATP-induced currents. B illustrates representative traces. C. Bar graph demonstrating the effect ofIVM on ATP and BzATP-induced current amplitude (left Y-axis) and on current deactivation time (right Y-axis). Data illustrate fold-changes (IVM vs no IVM) and are presented as mean ± SEM (n = 3–5).
3.2. Ethanol inhibited P2X4 but not P2X7 channels in BV2 microglia
We next tested the effects of ethanol on currents evoked by 0.1 or 1.0 mM ATP and 0.1 mM BzATP in BV2 microglia. Pre-incubation of 100 mM and 200 mM ethanol for 1 min resulted in significant inhibition of 0.1 mM ATP-induced currents. Residual currents for 100 and 200 mM ethanol were respectively 88 ± 4.2% (P < 0.05) and 64.4 ± 3.8% (P < 0.01) from the control (Fig. 2A,E n = 7). In addition, there was a rebound current at the end of ethanol washout (Fig. 2B arrow), characteristic of an effect in P2X4Rs as reported earlier in HEK293 recombinant expression system (Ostrovskaya et al., 2011). As reported previously (Ostrovskaya et al., 2011), this rebound effect may be due to a result of faster ethanol washout compared to ATP which halts the inhibition returning current to its initial level.
Fig. 2. Effect of ethanol on ATP- and BzATP-induced currents in BV2 microglial cells.
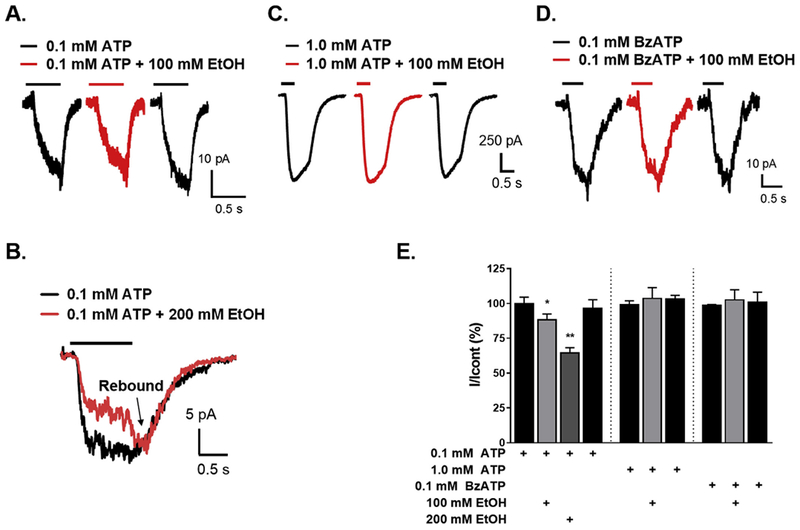
A-D. Representative current traces with (in red) and without ethanol (in black). Effect of 100 mM (A) and 200 mM (B) ethanol on 0.1 mM ATP-induced currents. Effect of 100 mM ethanol on 1.0 mM ATP- (C) and 0.1 mM BzATP- (D) induced currents. E. Bar graph of the data presented as mean ± SEM (n = 7). Ethanol significantly inhibited 0.1 mM ATP-induced currents, *P < 0.05; **P < 0.01 compared to control. There was no effect on BzATP- or 1.0 mM ATP-induced currents with ethanol. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Ethanol (100 mM) did not cause any changes in 1.0 mM ATP- or 0.1 mM BzATP-induced currents (Fig. 2C,D,E, n = 4). In this case 1.0 mM ATP was applied for 0.5 s to induce P2X7R channel activation only.
The use of BzATP did not differentiate between the activities of P2X4Rs and P2X7Rs as was found with IVM effect. In addition, in the current study, there was a better separation between specific P2XR subtype responses with the use of the 2 concentrations of ATP (0.1 and 1.0 mM). Despite the fact that 1.0 mM ATP initially activated both P2X4Rs and P2X7Rs (short-term effects), longer term responses were mostly due to P2X7R activation. This was also supported by the fact that extended applications of high ATP concentrations such as ≥ 0.1 mM caused sustained desensitization of P2X4Rs as demonstrated in recombinant expression systems (Ostrovskaya et al., 2011). We suggest that this effect is attributed to fast internalization of these receptors (Toulme and Khakh, 2012). Therefore based on these notions, we continued our functional studies with ATP only, assuming that 0.1 mM ATP activates P2X4Rs and that P2X7Rs largely contribute to the responses to 1.0 mM ATP.
3.3. Ethanol potentiated P2X7R-mediated pore activity
We used ethidium bromide (EB) uptake assay to test the effects of ethanol on P2XR-mediated pore activity in BV2 microglia. First, we tested the EB uptake induced by ATP differentially targeting P2X4Rs and P2X7Rs, i.e. 0.1 mM and 1.0 mM. ATP at 0.1 mM did not induce EB uptake (1.04 ± 0.3) compared to no ATP control set at 1, whereas 1.0 mM ATP induced a 10-fold dye uptake (10.13 ± 0.75) by BV2 microglia (Fig. 3A). Ethanol alone did not cause EB uptake (1.0 ± 0.06; 1.02 ± 0.063,1.04 ± 0.7 arbitrary units for respectively 0, 25 and 100 mM ethanol). Ethanol at 25 mM did not induce a change in EB uptake with 0.1 mM ATP. A small and non-significant dye uptake was observed at higher, 100 mM ethanol in the presence of 0.1 mM ATP (Fig. 3A).
Fig. 3. Ethanol effect on P2XR pore activity in BV2 cells.
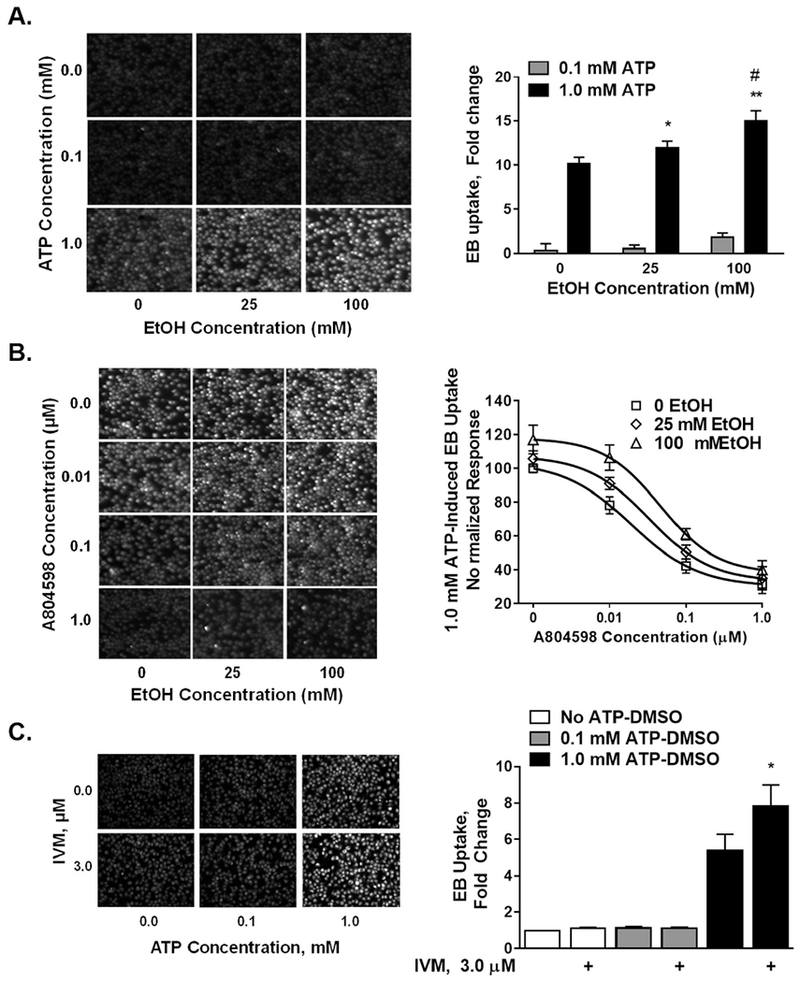
Left panels — representative EB fluorescence images, 10× magnification. Right panels — data analysis presented as mean ± SEM. A. ATP at 0.1 mM did not induce EB uptake, whereas at 1.0 mM caused a 10-fold EB uptake compared to the no ATP control. Ethanol increased 1.0 mM ATP-induced dye uptake in a concentration dependent manner (25 and 100 mM). One-way ANOVA - *P < 0.05, **P < 0.01 compared to 0 mM ethanol, #P < 0.05 compared to 1.0 mM ATP+25 mM ethanol. B. P2X7R antagonist A804598 inhibited 1.0 mM ATP-induced EB uptake. There was a right-shift of the antagonist curve with ethanol where the curve with 100 mM ethanol was significantly different from that at 0 mM ethanol. C. IVM at 3 mM potentiated 1 mM ATP-induced EB uptake. There was no effect on 0.1 mM ATP. *P < 0.05 compared to 1 mM ATP-induced dye uptake, 1-way ANOVA. Data presented from 3 to 5 individual assays performed in triplicates.
Ethanol enhanced 1.0 mM ATP-induced EB uptake in a concentration-dependent manner (Fig. 3A). Ethanol at 25 mM induced a small but significant increase in 1.0 mM ATP-induced EB uptake. Much larger increase in EB uptake was found at 100 mM ethanol which was significantly more compared to 1.0 mM ATP-induced EB uptake in the presence of 0 and 25 mM ethanol.
To verify whether 1.0 mM ATP-induced EB uptake was driven by P2X7Rs, we used a P2X7R-specific antagonist A804598. The antagonist inhibited 1.0 mM ATP-induced uptake in a concentration-dependent manner (Fig. 3B, 10 nM—1.0 mM). A804598 did not affect ethanol-potentiation of 1.0 mM ATP- induced EB uptake (Fig. 3B). Accordingly, 2-way ANOVA demonstrated significant effect for both the antagonist (F (3,116) = 127.5, P < 0.001) and ethanol (F (3, 116) = 12.34, P < 0.001) but no significant interaction between the 2 drugs (F (6, 116) = 0.62, P = 0.7134). Antagonist IC50 values for 0, 25 and 100 mM ethanol concentrations were 19.0; 36.3 and 46.0 nM.
We also tested the effect of IVM on ATP-mediated EB uptake. IVM (3.0 μM) did not exert any change in EB uptake when applied with 0.1 mM ATP (Fig. 3C). Interestingly, IVM significantly potentiated 1.0 mM ATP-induced EB uptake (Fig. 3C).
3.4. Ethanol inhibited P2X4-mediated migration of BV2 cells
BV2 migration was tested using the Transwell insert system. Incubation of cells with 0.1 mM ATP for 30 min significantly increased the number of migrated cells through the porous membranes (Fig. 4). Ethanol inhibited 0.1 mM ATP-induced migration in a concentration-dependent manner reaching statistical significance at 100 mM. We observed a trend (P = 0.085, two-tailed t-test) to increase 0.1 mM ATP-mediated migration of BV2 cells when the cells were pre-incubated with IVM (3.0 μM). P2X4R antagonist 5-BDBD (Balazs et al., 2013; Gofman et al., 2014) applied at 3.0 μM completely abolished 0.1 mM ATP-induced migration of the cells.
Fig. 4. Ethanol effect on BV2 cell migration.
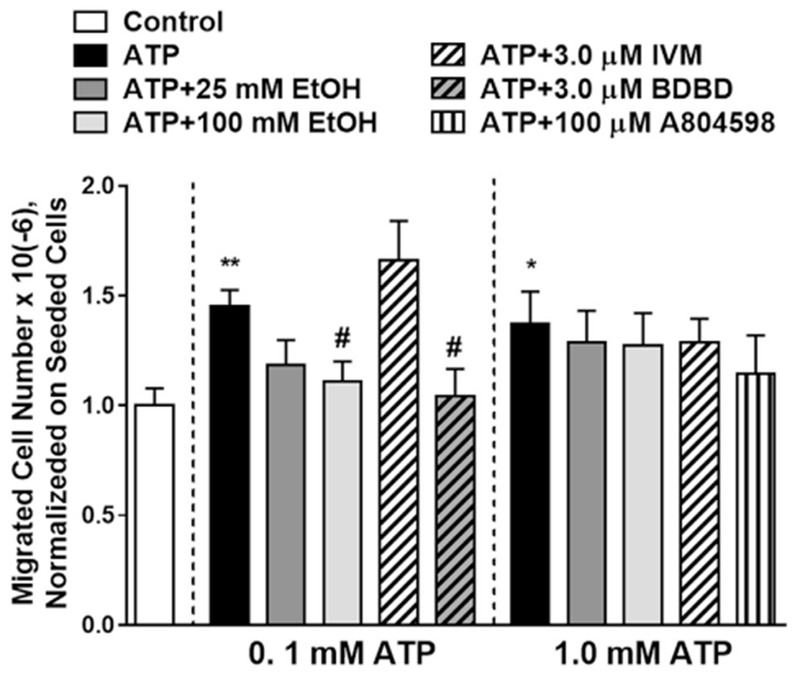
ATP at 0.1 mM induced migration of microglia that was inhibited by ethanol in a concentration-dependent manner. There was a trend to potentiate migration of the cells induced by 0.1 mM ATP by pretreatment with IVM (P = 0.085, two-tailed t-test). P2X4R antagonist 5-BDBD abolished 0.1 mM ATP-induced migration. ATP at 1.0 mM induced cell migration to a similar degree as seen with 0.1 mM ATP. Ethanol, IVM and P2X7R selective antagonist A804598 did not affect the migration of the cells induced by 1.0 mM ATP. Data presented as mean ± SEM from 4 separate experiments run in duplicates with analysis of 5—15 different fields on each filter. One-way ANOVA - *P < 0.05, **P < 0.01 compared to control. #P < 0.05 compared to 0.1 mM ATP.
There was also a significant increase in the number of migrated microglia when incubated with 1.0 mM ATP for 30 min. On the other hand, there was no significant effects of ethanol, IVM or the P2X7R antagonist A804598 on 1.0 mM ATP-mediated BV2 migration (Fig. 4).
3.5. Ethanol potentiated LPS-induced and P2X7R-mediated secretion of IL-1β; no involvement of P2X4Rs
Incubation of BV2 cells with LPS for 3 h induced secretion of pro-inflammatory cytokine IL-1β reaching to 1.03 ± 0.01 pg/mg protein from an undetectable level without LPS. Further application of 0.1 or 1.0 mM ATP for 30 min significantly potentiated IL-1β secretion (Fig. 5A). Ethanol (25 and 100 mM) applied for 30 min after LPS treatment period concentration dependently enhanced the cytokine release. Ethanol applied with 0.1 mM ATP did not affect (25 mM) or showed a trend to inhibit (100 mM) IL-1β secretion (LPS+0.1 mM ATP vs LPS, Fig. 5A). In contrast, ethanol applied with 1.0 mM ATP potentiated the IL-1β secretion in a concentration dependent manner (25 and 100 mM) to a higher extent than the release of the cytokine by LPS only. Two-way ANOVA demonstrated significant effects of ATP (F (2, 20) = 10.4, P < 0.001) and ethanol treatment (F(2, 20) = 6.58, P < 0.01) and their interaction (F (4,20) = 2.85, P = 0.05). Individual comparisons demonstrated significant differences between LPS+1.0 mM ATP and LPS or LPS+1.0 mM ATP at 100 mM ethanol (Fig. 5A).
Fig. 5. Ethanol effect on LPS-induced IL-1β (A) and TNFα (B) secretion from BV2 microglial cells.
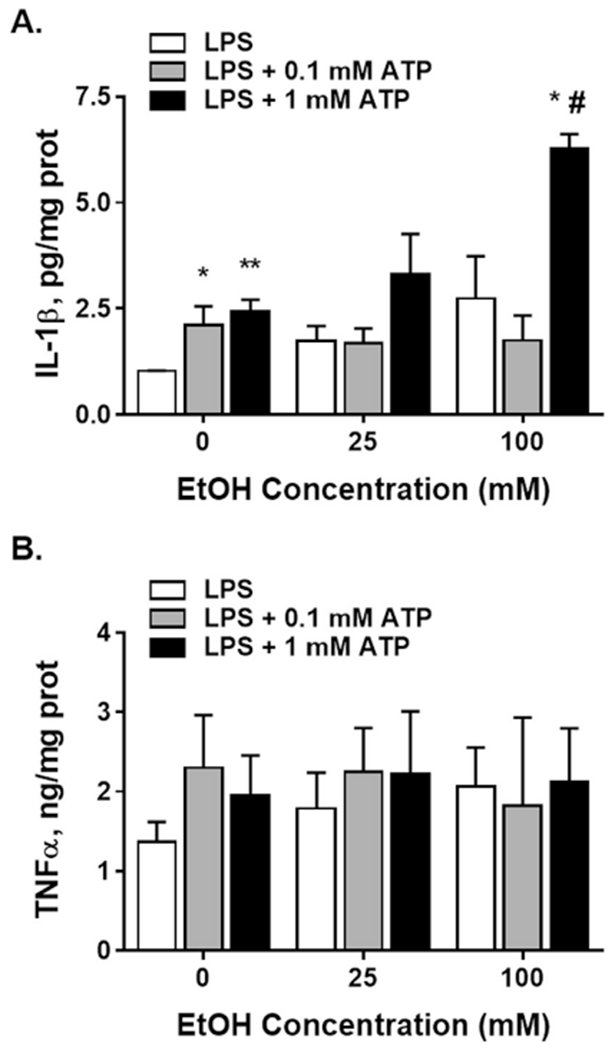
A. Activation of P2X4Rs and P2X7Rs significantly increased LPS-induced IL-1β secretion. Ethanol alone enhanced the cytokine release in a concentration dependent manner (25 and 100 mM). The effect on P2X7Rs but not P2X4Rs was further potentiated by ethanol reaching significance at 100 mM. B. Ethanol did not modulate LPS-induced TNFα secretion from BV2 cells in the absence or presence of 0.1 and 1.0 mM ATP. Data presented as mean ± SEM from 4 separate experiments. Two-way ANOVA - *P < 0.05, compared to LPS-100 mM ethanol; #P < 0.05, versus LPS+0.1 mM ATP-100 mM ethanol.
LPS treatment of BV2 cells also induced TNFα secretion reaching to 1.37 ± 0.25 ng/mg protein from undetectable levels in cellular supernatants which was dramatically more compared to that of IL-1β (Fig. 5A and B). In contrast to the changes in IL-1β, there were no significant effects of ATP (0.1 and 1.0 mM) or ethanol (25 and 100 mM) on LPS-induced TNFα secretion from BV2 cells.
3.6. Ethanol up-regulated expression of P2X4Rs and P2X7Rs in BV2 cells
After 24 h incubation with BV2 microglia, ethanol induced an increase in protein expression of both P2XR subtypes in a concentration-dependent manner from 25 to 100 mM (Fig. 6). The effect of 25 mM ethanol was not statistically significant. The effects of 50 and 100 mM ethanol reached statistical significance (See Fig. 6 legend).
Fig. 6. Effect of ethanol on the protein expression of P2X4Rs (A) and P2X7Rs (B) in BV2 cells.
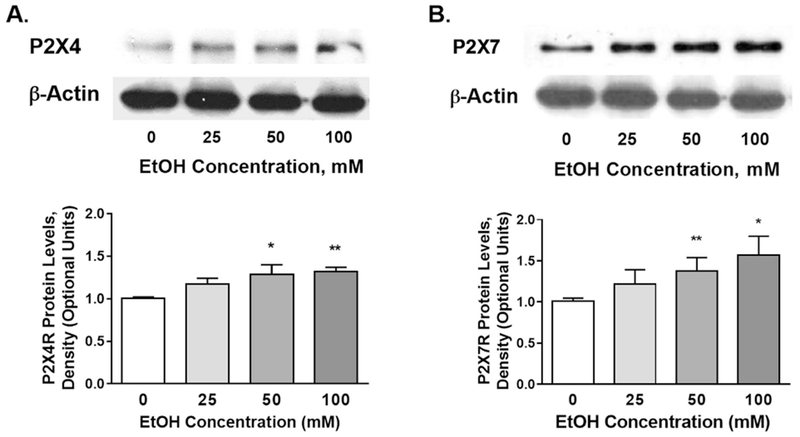
Upper panels — representative WBs demonstrating protein bands for the P2X4R (left), P2X7R (right) and Actin. Lower panels - densitometry analyses of the P2X4 (left) or P2X7 (right) levels normalized to their corresponding actin levels. Changes were compared to the values for 0 ethanol which were arbitrarily adjusted to 1. Incubation of cells with ethanol for 24 h increased the expression of both P2XRs in a concentration-dependent manner. Significant differences were noted at 50 and 100 mM ethanol for both P2X4 and P2X7Rs. Data are presented as mean ± SEM (n = 5—7). *P < 0.05, **P < 0.01 compared to 0 ethanol.
4. Discussion
In the current work, we tested the effects of ethanol on P2X4R and P2X7R activity, expression and functional consequences using murine BV2 cultured cells. The findings of the current work demonstrate differential modulation of microglial P2X4Rs and P2X7Rs in regards to their pharmacology and functional consequences. Ethanol inhibited P2X4Rs but was inactive on P2X7R channel activity. Ethanol potentiated pore formation in P2X7Rs whereas it inhibited P2X4R-mediated microglia migration. Furthermore, ethanol concentration-dependently potentiated IL-1 3 release mediated via P2X7Rs and did not modulate P2X4R-mediated IL-1β secretion. In regards to the effect on the expression of the two P2XR subtypes, ethanol upregulated both P2X4Rs and P2X7Rs expression in a concentration-dependent manner.
In line with published work, the electrophysiological studies demonstrated the presence of P2X4Rs and P2X7Rs with differential pharmacological properties in BV2 microglia. Application of lower concentration of ATP (0.1 mM) induced a fast desensitizing current typical for P2X4R activation whereas P2X7Rs predominantly contributed to the large currents induced by either short-term (0.5 s) or long-term (10 s) application of higher concentration of ATP (1.0 mM). Synthetic ATP analog BzATP, which is known to preferentially activate P2X7Rs (Donnelly-Roberts et al., 2009), induced smaller currents which were pharmacologically comparable to those evoked via both P2X7 as well as P2X4 channels. Furthermore, the application of IVM potentiated currents evoked by both 0.1 mM ATP and BzATP. While it is apparent that P2X4Rs contributed to the potentiating effect of IVM, these findings suggest that IVM is also able to modulate P2X7Rs in BV2 cells. In agreement, though to a lesser degree, IVM significantly potentiated the P2X7R pore activity. Our findings are consistent with the data of Raouf et al. (2007) who previously reported that IVM modulates BzATP induced currents in BV2 cells. In contrast, Norenberg et al. reported that IVM allosterically modulated human but not murine P2X7 channel activity (Norenberg et al., 2012). This second study did not find modulation of the P2X7R pore activity by IVM (Norenberg et al., 2012). The disparities between these reports may be due to differences in the used cellular systems. That is, work performed by Norenberg et al. used a recombinant expression system in HEK 293 cells, whereas our study and the investigation of Raouf et al. used BV2 cells. Since BV2 cells endogenously express both P2XR subtypes, it is possible that there are other processes, such as potential physical interaction between the 2 receptors reported recently (Perez-Flores et al., 2015), which can affect responses to IVM.
Our work further demonstrated differential responses of P2X4Rs and P2X7Rs to ethanol. Ethanol significantly inhibited currents in P2X4Rs at both 100 and 200 mM concentrations. The findings with P2X4Rs are consistent with earlier observations from our lab on the inhibitory effect of ethanol in recombinant rat P2X4Rs. Previously, we reported that ethanol inhibited rat P2X4Rs expressed in Xenopus oocytes in a concentration dependent manner (Asatryan et al., 2010; Davies et al., 2005; Popova et al., 2010), HEK 293 cells as well as hippocampal neurons (Ostrovskaya et al., 2011). In the current investigation we demonstrated an expected rebound in P2X4 channel activation during ethanol application which was also reported in our previous study with the use of recombinant expression system expressing rat P2X4Rs (Ostrovskaya et al., 2011). However, in contrast to our previous study where we observed sensitivity of P2X4Rs to ethanol concentrations as low as 10 mM, in the current study P2X4R-mediated responses were only observed when using higher concentrations of ethanol (i.e. 100 mM). This may be related to the fact that BV2 cells are less sensitive to extracellular challenges than the primary cells (Henn et al., 2009). On the other hand, high ethanol concentrations are relevant to alcohol abuse conditions such as alcohol binge and/or chronic use. For example, in chronic alcoholics ethanol levels can reach as high as 400 mg% which equals to 100 mM in the absence of significant clinical symptoms (Olson et al., 2013).
In contrast to the effects in P2X4Rs, ethanol did not affect P2X7R channel activity based on the electrophysiological findings. However, ethanol potentiated the pore activity of P2X7Rs. Ethanol concentration-dependently (25 and 100 mM) up-regulated the EB dye uptake induced by 1.0 mM ATP. We found that the pore activity was specific to P2X7Rs as the pretreatment of BV2 cells with a P2X7R selective and competitive antagonist A804598 inhibited the 1.o mM ATP-induced EB uptake into the cells in a concentration-dependent manner. In addition, the parallel right shift in the antagonist inhibition curves with ethanol combined with the statistical analysis, which demonstrated no significant interaction between the effect of ethanol and antagonist, suggested the allosteric nature of the ethanol effect. No pore activity was observed for P2X4Rs using the EB uptake. This was in agreement with previous findings demonstrating the lack of P2X4R pore dilation when tested in the presence of the physiological buffer (Khadra et al., 2012; Ostrovskaya et al., 2011). Overall, these are the first findings on the effects of ethanol in P2X7Rs which suggest that ethanol potentiates P2X7R pore activity while being inactive at P2X7 channel.
The current work also demonstrated differences in P2X4Rs and P2X7Rs in regards to their functional responses to ethanol. We studied the involvement of the 2 receptors in microglial migration. It is now well accepted that microglia are highly dynamic and motile. While motility plays a key role in the intrinsic functional role of microglia for constant surveillance of neurons and involves microglial processes, the somatic movements of microglia results in migration of these cells (Eyo and Dailey, 2013). Migration is an important part of microglia activation process in response to physiological signals, such as during brain development, plasticity and maintenance, and to pathological insults during aging and CNS disorders (Eyo and Dailey, 2013).
Results from the present work suggests that P2X4Rs but not P2X7Rs predominantly mediated BV2 migration. This conclusion is based on the fact that channel activity was abolished when tested in the presence of the P2X4R antagonist 5-BDBD but not when tested in the presence of the P2X7R antagonist A804598. The enhancement of microglia migration upon application with P2X4R allosteric modulator IVM also supports a role for P2X4Rs. Furthermore, ethanol concentration-dependently inhibited migration of P2X4R-mediated microglial migration. These findings parallel the findings on the ability to inhibit P2X4R channel activity. On the other hand, there was no effect of ethanol or IVM on the migration in the presence of P2X7R activation. Albeit using somewhat different experimental strategies, these findings add further support to the previous findings suggesting that migration of microglia is controlled by P2X4Rs (Gofman et al., 2014). More importantly, our findings now suggest that P2X7R functioning does not play an important role in microglia migration and ethanol modulation of this function.
We also studied the involvement of the 2 P2XR subtypes in the secretion of pro-inflammatory cytokines from microglia. IL-1β is a known pro-inflammatory mediator that together with other mediators contributes to neuroinflammation and neurodegeneration (Zhou et al., 2016). The role of P2X7Rs in inflammatory processes and specifically in the pathological release of IL-1β secretion is well-established (Matute et al., 2007; Mingam et al., 2008). P2X7Rs are directly involved in inflammasome formation and mediate the release of IL-1β via their pore (Skaper et al., 2010; Volonte et al., 2012). Participation of P2X4Rs in IL-1β release is not completely understood. Their involvement in inflammasome-mediated IL-1β secretion has been shown during the spinal cord injury (de Rivero Vaccari et al., 2012). Another recent report suggests a novel physical interaction between P2X4Rs and P2X7Rs via the P2X7R C-terminus which also affects the IL-1β release (Perez-Flores et al., 2015).
Consistent with these aforementioned findings, the present work found that there was activation of both P2X4Rs and P2X7Rs mediated IL-1β secretion in LPS-primed BV2 cells. Of note, there was no significant release of IL-1β from microglia cells. In agreement, Burm et al. recently reported a substantially lower ability of microglia to secrete IL-1β compared to that of bone-marrow derived macrophages which is apparently due to relatively lower levels of expression of P2XRs (Burm et al., 2016). They also suggest the participation of both, P2X7Rs and P2X4Rs (Burm et al., 2016). Furthermore, when applied with ATP, ethanol did not significantly affect (25 mM) or showed a trend to inhibit (100 mM) P2X4R- mediated IL-1β secretion, whereas it potentiated P2X7R-mediated cytokine release from BV2 cells. These data are consistent with our previous (Asatryan et al., 2010; Davies et al., 2005; Popova et al., 2010) and current findings on the inhibition of ATP-evoked currents via P2X4Rs. In addition, findings with P2X7Rs strongly agreed with our findings on ethanol potentiation of the P2X7R pore activity. Interestingly, these effects were specific to IL-1β as P2X4Rs or P2X7Rs did not significantly contribute to the release of another pro-inflammatory cytokine, TNFα. Overall, these findings add to the notion that ethanol differentially affects the activities of P2X4R and P2X7R subtypes which also translates into differential functional consequences for both receptors.
P2X4Rs and P2X7Rs are often expressed simultaneously in immune cells (Skaper et al., 2010), however their expression profile is different. P2X7Rs are readily expressed on the cell plasma membrane whereas P2X4Rs are mainly expressed intracellularly. P2X4Rs are localized to endosomes but tend to traffic quickly to and from plasma membrane (Boumechache et al., 2009). The two P2XR subtypes therefore may respond differently to cell activators, modulators, etc. For example, differential regulation of the expression of the two P2XR subtypes have been found with LPS treatment in BV2 microglia (Boumechache et al., 2009; Raouf et al., 2007). LPS increased the expression of P2X4Rs but not P2X7Rs. It is thought that LPS causes stabilization of the receptor on the cell surface. In contrast to these reports, in our studies ethanol caused increases in the expression of both P2X4Rs and P2X7Rs in a concentration-dependent manner. This was an overall expression of the receptors and did not differentiate between plasma membrane and intracellular pools. Our findings on P2X4R expression is consistent with the recently published data on ethanol (100 mM)-induced upregulation of P2X4Rs in embryonic stem cell derived microglial cells (Gofman et al., 2014). There are no other reports on the effect of ethanol on P2X7R expression in isolated or cultured microglia. However, findings from the current study with microglia are in agreement with our recent report on increased P2X7R expression in brain regions of C57BL/6J mice exposed to intragastric ethanol and high diet (Asatryan et al., 2015). Up-regulation of both P2X4R subtypes with longer-term (24 h) ethanol exposure suggests that both receptors play a role in ethanol-induced functional responses of microglia. Significant previous work in our laboratory have demonstrated a role of P2X4Rs in ethanol induced behaviors reviewed in (Franklin et al., 2014) and a role for P2X7Rs in neuro-immune consequences induced by ethanol exposure (Asatryan et al., 2015).
In conclusion, our findings demonstrate differential effects of ethanol on P2X4R and P2X7R activity and function in microglia. These differences are obvious in regards to the activity and functional consequences of microglia. Ethanol inhibited P2X4 channel activity and P2X4R-mediated migration of microglia, whereas it potentiated P2X7R-mediated pore activity and pro-inflammatory IL-1β secretion from microglial cells. Microglia may have diverse responses to ethanol depending on the extent and length of ethanol exposure. In this context, our findings suggest that P2X4Rs and P2X7Rs play differential roles in ethanol effects on microglia, ranging from behavioral to pathologic states. These differences may be explained by the different signaling processes downstream of activation of each P2XR subtype leading to diverse outcomes. Microglial P2X4Rs may be involved in behavioral effects of ethanol and ethanol inhibition of these receptors may result in reduced microglial motility and neuronal surveillance. This is consistent with recent findings demonstrating initial decreases in microglial markers during acute ethanol challenge (Walter and Crews, 2017). Ethanol inhibition of P2X4Rs may also contribute to processes leading to alcohol addiction (Khoja et al., 2016; Tsuda et al., 2013; Yardley et al., 2012). On the contrary, via increased pore activity and release of the pro-inflammatory cytokine ILl-β microglial, P2X7Rs may participate in the advanced disease stages characterized by microglia activation, neuroinflammation and degeneration (Asatryan et al., 2015; Bhattacharya and Biber, 2016). Future correlation of these differences to the diverse microglial phenotypes may help in the diagnostics of the extent of ethanol exposure and in strategies to ameliorate these effects by targeting specific P2XRs.
Acknowledgements
This work was supported in part by National Institutes of Alcohol Abuse and Alcoholism (NIAAA) AA017243 Award, Integrative Neuroscience Initiative on Alcoholism (INIA) West Pilot Project, Zumberge Individual Research Award (to L.A.), NIAAA AA022448 Award (to D.L.D.), and the support from USC School of Pharmacy.
Footnotes
Conflict of interest
None.
References
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C, 2010. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci 30, 8285–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Khoja S, Rodgers KE, Alkana RL, Tsukamoto H, Davies DL, 2015. Chronic ethanol exposure combined with high fat diet up-regulates P2X7 receptors that parallels neuroinflammation and neuronal loss in C57BL/6J mice. J. Neuroimmunol 285, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Perkins DI, Trudell JR, Alkana RL, Davies DL, 2010. Ivermectin antagonizes ethanol inhibition in P2X4 receptors. J. Pharmacol. Exp. Ther 334, 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs B, Danko T, Kovacs G, Koles L, Hediger MA, Zsembery A, 2013. Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptors by two complementary methods. Cell. Physiol. Bio-chem 32, 11–24. [DOI] [PubMed] [Google Scholar]
- Bartlett R, Stokes L, Sluyter R, 2014. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev 66, 638–675. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Biber K, 2016. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 64, 1772–1787. [DOI] [PubMed] [Google Scholar]
- Boumechache M, Masin M, Edwardson JM, Gorecki DC, Murrell-Lagnado R, 2009. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J. Biol. Chem 284, 13446–13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burm SM, Zuiderwijk-Sick EA, Weert PM, Bajramovic JJ, 2016. ATP-induced IL-1 beta secretion is selectively impaired in microglia as compared to hematopoietic macrophages. Glia 64, 2231–2246. [DOI] [PubMed] [Google Scholar]
- Burnstock G, 2016. Purinergic mechanisms and pain. Adv. Pharmacol 75, 91–137. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, 2005. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114, 386–396. [DOI] [PubMed] [Google Scholar]
- Choi M, Cho K, Shin S, Ko H, Kwon K, Shin C, Ko K, 2010. ATP induced microglial cell migration through non-transcriptional activation of matrix metalloproteinase-9. Arch. Pharm. Res 33, 257–265. [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, Marchand F, Kaan TKY, McMahon SB, Malcangio M, 2010. P2X7-dependent release of interleukin-1b and nociception in the spinal cord following lipopolysaccharide. J. Neurosci 30, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, 2009. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 44, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP, 2015. Neuro-immune function and the consequences of alcohol exposure. Alcohol Res 37, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, 2014. Neuroimmune basis of alcoholic brain damage. Int. Rev. Neurobiol 118, 315–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL, 2005. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacol 49, 243–253. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S, 2012. P2X4 receptors influence inflam- masome activation after spinal cord injury. J. Neurosci 32, 3058–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF, 2009. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol 157, 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Dailey ME, 2013. Microglia: key elements in neural development, plasticity, and pathology. J. Neuroimmun. Pharmacol 8, 494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A, Miller-Fleming L, Pais TF, 2014. Microglia and inflammation: conspiracy, controversy or control? Cell. Mol. Life Sci 71, 3969–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Asatryan L, Jakowec MW, Trudell JR, Bell RL, Davies DL, 2014. P2X4 receptors (P2X4Rs) represent a novel target for the development of drugs to prevent and/or treat alcohol use disorders. Front. Neurosci 8, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron FP, Chalimoniuk M, Strosznajder J, Shen S, Gonzalez FA, Weisman GA, Sun GY, 2003. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J. Neurochem 87, 344–352. [DOI] [PubMed] [Google Scholar]
- Gofman L, Cenna JM, Potula R, 2014. P2X4 receptor regulates alcohol-induced responses in microglia. J. Neuroimmun. Pharmacol 9, 668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong QJ, Li YY, Xin WJ, Zang Y, Ren WJ, Wei XH, Li YY, Zhang T, Liu XG, 2009. ATP induces long-term potentiation of C-fiber-evoked field potentials in spinal dorsal horn: the roles of P2X4 receptors and p38 MAPK in microglia. Glia 57, 583–591. [DOI] [PubMed] [Google Scholar]
- Graeber MB, 2010. Changing face of microglia. Science 330, 783–788. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT, 2008. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol 210, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjarn M, Schrattenholz A, Porzgen P, Leist M, 2009. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altex 26, 83–94. [DOI] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, Zhu C, Carroll W, Perez-Medrano A, Iwakura Y, Jarvis MF, 2009. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1[alpha][beta] knockout mice. Behav. Brain Res 204, 77–8 . [DOI] [PubMed] [Google Scholar]
- Horvath RJ, Romero-Sandoval EA, Leo JAD, 2010. Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and [mu] opioid receptor protein expression while enhancing perivascular microglial ED2. Pain 150, 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K, 2009. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT.J. Neurochem 108,115–125. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Dantzer R, 2011. Alcoholism and inflammation: neuroimmunology of behavioral and mood disorders. Brain Behav. Immun (1), S13–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadra A, Yan Z, Coddou C, Tomic M, Sherman A, Stojilkovic SS, 2012. Gating properties of the P2X2a and P2X2b receptor channels: experiments and mathematical modeling. J. Gen. Physiol 139, 333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA, 1999. Allosteric control of gating and kinetics at P2X(4) receptor channels. J. Neurosci 19, 7289–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoja S, Shah V, Garcia D, Asatryan L, Jakowec MW, Davies DL, 2016. Role of purinergic P2X4 receptors in regulating striatal dopamine homeostasis and dependent behaviors. J. Neurochem 139, 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K, 2013. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: the importance of microglia phenotype. Neurobiol. Dis 54, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, Mak TW, Tamura T, Tsuda M, Inoue K, 2014. Transcription factor IRF5 drives P2X4R+- reactive microglia gating neuropathic pain. Nat. Commun 5, 3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Torre I, Perez-Cerda F, Perez-Samartin A, Alberdi E, Etxebarria E, Arranz AM, Ravid R, Rodriguez-Antiguedad A, Sanchez-Gomez M, Domercq M, 2007. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci 27, 9525–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K, 2011. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav. Immun 25 (1), S120–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingam R, De Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, Laye S, 2008. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav. Immun 22, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monif M, Burnstock G, Williams DA, 2010. Microglia: proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol 42, 1753–1756. [DOI] [PubMed] [Google Scholar]
- Monif M, Reid CA, Powell KL, Smart ML, Williams DA, 2009. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J. Neurosci 29, 3781–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg W, Sobottka H, Hempel C, Plotz T, Fischer W, Schmalzing G, Schaefer M, 2012. Positive allosteric modulation by ivermectin of human but not murine P2X7 receptors. Br. J. Pharmacol 167, 48–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S, 2007. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemo-taxis. Glia 55, 604–616. [DOI] [PubMed] [Google Scholar]
- Olson KN, Smith SW, Kloss JS, Ho JD, Apple FS, 2013. Relationship between blood alcohol concentration and observable symptoms of intoxication in patients presenting to an emergency department. Alcohol Alcohol L. 48, 386–389. [DOI] [PubMed] [Google Scholar]
- Ostrovskaya O, Asatryan L, Wyatt L, Popova M, Li K, Peoples RW, Alkana RL, Davies DL, 2011. Ethanol is a fast channel inhibitor of purinergic P2X4 receptors. J. Pharm. Exp. Ther 337, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R, 2003. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s Disease. J. Biol. Chem 278, 13309–13317. [DOI] [PubMed] [Google Scholar]
- Perez-Flores G, Levesque SA, Pacheco J, Vaca L, Lacroix S, Perez-Cornejo P, Arreola J, 2015. The P2X7/P2X4 interaction shapes the purinergic response in murine macrophages. Biochem. Biophys. Res. Comm 467, 484–490. [DOI] [PubMed] [Google Scholar]
- Popova M, Asatryan L, Ostrovskaya O, Wyatt RL, Li K, Alkana RL, Davies DL, 2010. A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J. Neurochem 112, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf R, Chabot-Dore AJ, Ase AR, Blais D, Seguela P, 2007. Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology 53, 496–504. [DOI] [PubMed] [Google Scholar]
- Shin S, Cho K, Choi M, Lee S, Han S, Kang Y, Kim H, Cheong J, Shin C, Ko K, 2010. Urokinase-type plasminogen activator induces BV-2 microglial cell migration through activation of matrix metalloproteinase-9. Neurochem. Res 35, 976–985. [DOI] [PubMed] [Google Scholar]
- Sierra A, Tremblay ME, Wake H, 2014. Never-resting microglia: physiological roles in the healthy brain and pathological implications. Front. Cell. Neurosci 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Debetto P, Giusti P, 2010. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 24, 337–345. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Illes P, 2014. P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol. Sci 35, 537–547. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y, 2004. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J. Neurosci 24, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Iwamaru Y, Sugama S, Sato M, Hashimoto M, Kitani H, 2008. Lysophospholipids and ATP mutually suppress maturation and release of IL-1β in mouse microglial cells using a Rho-dependent pathway. J. Immunol 180, 7827–7839. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sekiyama K, Sekigawa A, Fujita M, Waragai M, Sugama S, Iwamaru Y, Kitani H, Hashimoto M, 2010. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch. Immunol. Ther. Exp 58, 91–96. [DOI] [PubMed] [Google Scholar]
- Tay TL, Savage J, Hui CW, Bisht K, Tremblay ME, 2017. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J. Physiol 595, 1929–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulme E, Khakh BS, 2012. Imaging P2X4 receptor lateral mobility in microglia: regulation by calcium and p38 MAPK. J. Biol. Chem 287, 14734–14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K, 2013. P2X4 receptors and neuropathic pain. Front. Cell. Neurosci 7, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ., Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F, 2008. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J. Neurosci 28, 11263–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM, 2011. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol. Learn. Mem 96, 596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte C, Apolloni S, Skaper SD, Burnstock G, 2012. P2X7 receptors: channels, pores and more. CNS Neurol. Dis. Drug Targets 11, 705–721. [DOI] [PubMed] [Google Scholar]
- Walter TJ., Crews, F.T., 2017. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J. Neuroinflamm 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Wyatt L, Khoja S, Asatryan L, Ramaker MJ, Finn DA, Alkana RL, Huynh N, Louie SG, Petasis NA, Bortolato M, Davies DL, 2012. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacology 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Chen M-L, Zhang Y-Q, Zhao Z-Q, 2010. Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J. Neurosci 30, 8042–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Shi L, Wang Y, Chen S, Zhang J, 2016. Recent advances of the NLRP3 inflammasome in central nervous system disorders. J. Immunol. Res 2016, 9238290. [DOI] [PMC free article] [PubMed] [Google Scholar]


