Abstract
Loss-of-function mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) compromise epithelial HCO3− and Cl− secretion, reduce airway surface liquid (ASL) pH, and impair respiratory host defenses in people with cystic fibrosis (CF) 1–3. Here we report that apical addition of an unselective ion channel-forming small molecule, amphotericin B (AmB), restored HCO3− secretion and increased ASL pH in cultured human CF airway epithelia. These effects required the basolateral Na+/K+ ATPase, indicating that apical AmB channels functionally interfaced with this driver of anion secretion. AmB also restored ASL pH, viscosity, and antibacterial activity in primary cultures of airway epithelia from people with CF caused by different mutations, including ones that yield no CFTR, and increased ASL pH in CFTR-null pigs in vivo. Thus, unselective small molecule ion channels can restore CF airway host defenses via a mechanism that is CFTR-independent and therefore genotype-independent.
Increasing anion secretion through CFTR channels that bear specific mutations improves airway host defenses and lung function in people with CF1,4,5. However, not all CFTR mutations are amenable to this approach6. Small molecule ion channels that operate independent of CFTR to promote anion secretion might circumvent these limitations. Multiple studies have demonstrated that peptide or small molecule ion channels, transporters, or carriers can promote chloride transport in CFTR-deficient cells and/or changes in short circuit current or potential in CFTR-deficient epithelia7–10. It has remained unclear whether this approach can increase airway host defenses.
Recent studies have demonstrated that HCO3− secretion may enhance airway host defense2,3,11 by increasing ASL pH3, decreasing ASL viscosity3,12, increasing activity of antimicrobial factors3, maintaining ASL volume homeostasis13, counteracting local environment acidification by Pseudomonas aeruginosa14, and dissipating proton motive forces in bacteria15. The electrochemical gradient across the apical membrane favors HCO3− secretion; HCO3− is accumulated intracellularly through the integrated activity of Na+/K+ ATPase, H+/K+ ATPase, K+ channels, Na+/HCO3− transporters (NBC), and Na+/H+ antiporters, as well as carbonic anhydrase.3,16 Thus, when CFTR opens, HCO3− flows into the ASL, raising ASL pH. Without CFTR, intracellular HCO3− concentration is maintained17 and this gradient for HCO3− exit persists or likely increases as ASL pH falls. We reasoned that the electrochemical gradient for HCO3− across the apical membrane of CF epithelia at steady-state may permit even an unselective small molecule HCO3− transporter to increase basolateral-to-apical HCO3− flux and thus airway host defenses. An unselective small molecule iron transporter was sufficient to restore hemoglobinization in cells and animals that are deficient in iron-transport proteins, and this tolerance for lack of selectivity was linked to iron gradients across membranes that normally host the missing proteins18.
Amphotericin B (AmB) is a small molecule natural product that forms monovalent ion channels that are unselective for anions vs. cations. It is prescribed as an antifungal but has significant toxicity to humans19. We recently found that its cytotoxicity is primarily due to sterol extraction from membranes, not channel formation20,21. In the presence of lipid bilayers, AmB likely exists in at least two states that are in dynamic equilibrium.20,21. The primary state is a toxic extramembranous sterol sponge and a minor state is a membrane-embedded ion channel. Toxicity from the AmB sterol sponge only occurs when the molar ratio of AmB/sterol is > 120,21. These insights allowed the channel activity of AmB to be rationally separated from its cytotoxicity using either low concentrations of AmB that form ion channels but do not extract significant amounts of membrane sterol (generally < 0.5 μM in ergosterol-containing yeast, predicted to be generally < 5 μM in cholesterol-containing mammalian cells), or by pre-complexing AmB to excess sterols 20–22. Although AmB forms ion channels that are permeable to both cations and anions, it restored potassium transport and growth in yeast missing the potassium-selective Trk transporters22. In contrast, a synthetic single atom-deficient derivative that lacks ion channel activity (C35deOAmB) did not20,22. We hypothesized that in the alternative context of a favorable electrochemical gradient for HCO3− secretion, apical AmB channels would increase HCO3− secretion and thus ASL host defenses in CF epithelia.
AmB is known to transport monovalent anions and cations (Extended Data Fig. 1f-h,j), but not HCO3− specifically. We found that AmB, but not C35deOAmB, caused H13CO3− efflux in cholesterol-containing POPC liposomes (Extended Data Fig. 1a-e, i).
A low concentration of AmB increased ASL pH and H14CO3− secretion in CuFi-4 (G551D/∆F508) CF airway epithelia (Fig. 1a,b). pH-stat experiments indicated that HCO3− secretion, rather than proton absorption, primarily underlies the AmB-mediated increase in ASL pH (Extended Data Fig. 2a). For comparison, we tested ivacaftor, which increases the open state probability of CFTR23 and improves FEV1 in people with CF carrying a G551D or other residual function mutation4. The quantitative effects of AmB on ASL pH and H14CO3− secretion were similar to those of ivacaftor (Fig. 1a,b). AmB transports both anions and cations (Extended Data Fig. 1f-j), but ASL concentrations of Na+ and K+ were unchanged relative to vehicle-treated controls (Extended Data Fig. 2b,c). While AmB likely mobilizes ions other than HCO3−, we speculate that compensatory actions of other pumps, channels, carriers, and the paracellular pathway may help determine ASL ion concentrations at steady state.
Figure 1 |. AmB increased H14CO3− secretion and ASL pH in cultured CF airway epithelia.
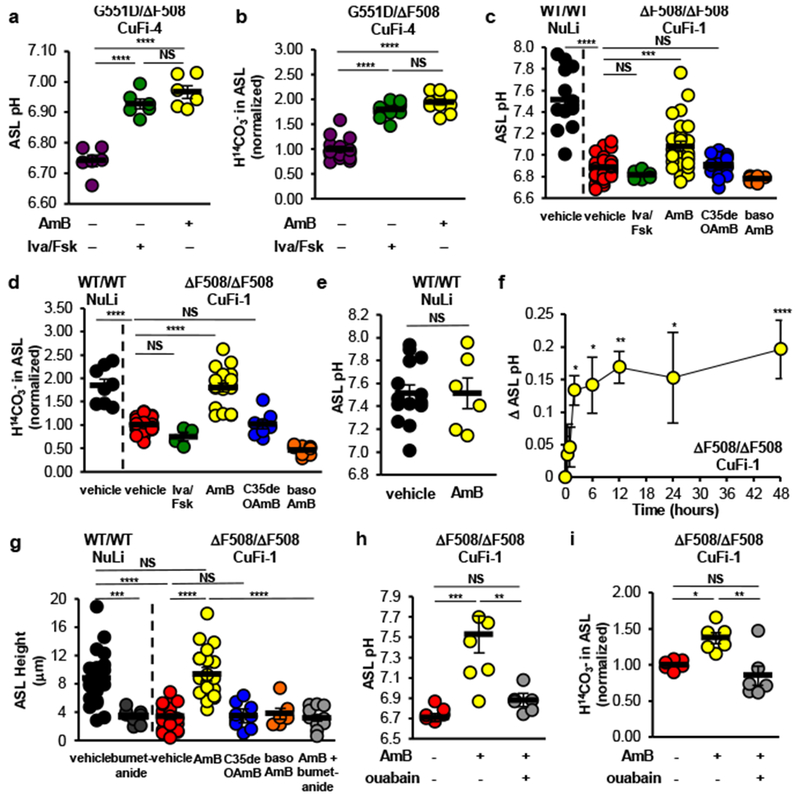
(a) Effects of ivacaftor/forskolin or AmB on ASL pH in CuFi-4 (G551D/∆F508) epithelia (n = 6). (b) Effect of ivacaftor/forskolin (n = 12) or AmB (n = 10) on basolateral-to-apical H14CO3− secretion (n = 16). (c) Effect of vehicle (FC-72), ivacaftor/forskolin (n = 6), AmB (n = 28), C35deOAmB (n = 14), or basolateral AmB (n = 6) on ASL pH in NuLi (CFTR+/+) (n = 14) or CuFi-1 (∆F508/∆F508) epithelia (n = 37). (d) Effect of vehicle, ivacaftor/forskolin (n = 4), AmB (n = 18), C35deOAmB (n = 8), or basolateral AmB (n = 8) on H14CO3− secretion in NuLi (n = 8) or CuFi-1 epithelia (n = 37). (e) Effect of AmB (n = 6) on ASL pH in NuLi epithelia (n = 16). (f) Average difference in ASL pH after AmB treatment in CuFi-1 epithelia as a function of time (0, 0.5, 1, 2, 12 hours, n = 6; 6 hours, n = 9; 24 hours, n = 12; 48 hours, n = 28). (g) ASL height in NuLi (n = 21) or CuFi-1 epithelia (n = 21) after treatment with apical AmB (n = 21), C35deOAmB (n = 9), basolateral AmB (n = 6), or AmB with bumetanide (n = 12). ASL pH (h) and H14CO3− secretion (i) in CuFi-1 epithelia (n = 6) after addition of vehicle, apical AmB, or AmB with ouabain. n are biologically independent samples from at least two independent experiments with similar results. Graphs depict means ± SEM. Panels (a-d, g-i): one-way ANOVA with Tukey test for multiple comparisons. Panels (e,f): two-sided unpaired Student’s t test. Panel (d): * indicate difference versus vehicle. NS, not significant; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Exact P values in Source Data.
Ivacaftor does not correct the non-membrane localized ∆F508-CFTR defect23, and as expected, it failed to increase ASL pH or H14CO3− secretion in CuFi-1 (∆F508/∆F508) epithelia (Fig. 1c,d). In contrast, AmB, which operates independently of the CFTR protein, increased both (Fig. 1c,d). No increase in ASL pH was observed with apical addition of AmB to non-CF (NuLi) epithelia (Fig. 1e). AmB was left on the epithelia throughout all the experiments described herein. AmB progressively increased ASL pH over 2 hours, and sustained the effect for at least 48 hours in these in vitro experiments (Fig. 1f). The AmB-mediated increase in H14CO3− secretion in CuFi-1 epithelia is sustained for at least 7 days (Extended Data Fig. 3a-c). These results contrast with the transient increase in pH (~15 minutes) produced by aerosolized NaHCO324. C35deOAmB and basolateral addition of AmB did not raise ASL pH or increase H14CO3− secretion, suggesting that these effects are specific to apically localized AmB channels (Fig. 1c,d).
AmB-treated CuFi-1 epithelia did not respond to chemical activation of CFTR, suggesting that AmB did not promote trafficking of ∆F508 CFTR to the apical membrane (Extended Data Fig. 3d-i). AmB addition did not disrupt membrane integrity, as there was no difference in transepithelial electrical resistance (Rt) between CuFi-1 epithelia treated with either vehicle, low (2 μM), or high (50 μM) doses of AmB over extended timeframes (Extended Data Fig. 3j). No toxicity was observed in CuFi-1 epithelia with the same AmB doses (Extended Data Fig. 3k).
Another model of CF links ASL height to pathology13. At baseline, CuFi-1 epithelia had decreased ASL height as compared to NuLi epithelia (Fig. 1g, Extended Data Fig. 4a,b). Apical addition of AmB increased ASL height to match that of NuLi epithelia (Fig. 1g, Extended Data Fig. 4c). Vehicle, C35deOAmB, and basolateral AmB did not increase ASL height (Fig. 1g, Extended Data Fig. 4d,e). These results suggest that AmB-based channels restore ASL volume homeostasis despite their lack of ion selectivity.
Secretion of ions through apical channels depends on an electrochemical gradient generated in large part by basolateral transport proteins. We showed that AmB-mediated growth rescue in Trk-deficient yeast is attenuated by chemical inhibition of H+ ATPases that drive secondary K+ influx22. Secretion of chloride through apical peptide channels in monolayers derived from a colonic cancer cell line was mitigated by blocking basolateral potassium channels 25. We predicted that inhibiting basolateral transport in CF airway epithelia would similarly prevent AmB-mediated anion secretion. Inhibiting the basolateral Na+/K+ ATPase with ouabain abolished the AmB-mediated increase in ASL pH and H14CO3− secretion in CuFi-1 epithelia (Fig. 1h,i). Inhibiting the basolateral Na+/K+/2Cl− co-transporter with bumetanide decreased ASL height in NuLi epithelia and abolished the AmB-mediated increase in ASL height in CuFi-1 epithelia (Fig. 1g, Extended Data Fig. 4f,g). Thus, apical AmB channels functionally interface with endogenous basolateral proteins that drive anion secretion.
We next tested whether AmB could restore key aspects of airway host defense in differentiated primary cultures of human airway epithelia. We studied epithelia from 9 donors with CF representing different CFTR mutations, including some that yield no CFTR (Fig. 2a, Extended Data Fig. 5a). Apical AmB increased ASL pH ~0.2 pH units (Fig. 2a) and this effect was sustained for at least 48 hours (Fig. 2b). C35deOAmB and basolateral AmB did not increase ASL pH (Extended Data Fig. 5b).
Figure 2 |. AmB improved host defenses in primary cultured airway epithelia derived from genetically diverse humans with CF.

(a) Effect of AmB on ASL pH on primary cultured airway epithelia derived from 9 humans with CF with different CFTR mutations (n = 9). (b) Average difference in ASL pH after AmB treatment as a function of time (t = 0, 0.5, 2 hours, n = 6; t = 6, 12, 24 hours, n = 3; t = 1, 48 hours, n = 9). Effect of apical AmB treatment on (c) ASL viscosity (τASL/τsaline) (n = 6) and (d) ASL antibacterial activity (n = 8) in primary CF epithelia. Graphs depict means ± SEM. Panels (a,b,d): each data point represents an average of 1-3 epithelia samples from each human donor. Panels (a,c,d): n are biologically independent samples from at least three independent experiments with similar results. Panel (b): the same samples for each donor were measured repeatedly over time and ** indicate differences versus vehicle; data is from at least three independent experiments with similar results. All panels: two-sided unpaired Student’s t test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Exact P values in Source Data.
ASL viscosity is increased and antibacterial activity is decreased in cultures of CF airway epithelia3,26. Non-CF lung epithelia has a viscosity 2.5 times that of saline3. Apical addition of AmB to genetically diverse primary cultures of CF epithelia decreased ASL viscosity (Fig. 2c, Extended Data Fig. 5d, h-j) to a degree that matched ivacaftor in primary CFTR-G551D sinonasal epithelia12. Antimicrobial activity of ASL is reduced by about 50% in CF3. AmB addition nearly doubled ASL bacterial killing in CF epithelia (Fig. 2d, Extended Data Fig. 5e,f). C35deOAmB had no effect (Extended Data Fig. 5f). AmB alone does not have antibacterial activity against S. aureus (Extended Data Fig. 5g).
In CuFi-1 epithelia ASL pH increased and then fell with increasing concentrations of AmB (Fig. 3a). Based on our previous findings in yeast20–22, we hypothesized that pre-forming a AmB:cholesterol complex would mitigate potential sterol binding-mediated effects that could contribute to reduced efficacy at higher concentrations of AmB. We found that a pre-formed AmB:cholesterol (1:5) complex increased ASL pH up to the maximum concentration of AmB tested (100 μM) (Fig. 3a).
Figure 3. AmBisome® increased ASL pH in cultured CF airway epithelia and in CFTR−/− pigs.

(a) Concentration-dependent effect of a pre-formed AmB:cholesterol complex on ASL pH (0 μM, n = 37; 0.25 μM, n = 15; 0.5, 50, 100 μM, n = 9; 2 μM, n = 28; 5, 10 μM, n = 16; 20, 25 μM, n = 12) in CuFi-1 (∆F508/∆F508) epithelia. The effect of AmBisome® on (b) ASL pH in CuFi-1 epithelia as a function of time (n = 9) and (c) H14CO3− secretion after 2 h (n = 20). (d) Effect of AmBisome® treatment on ASL pH of CFTR−/− pigs as compared to baseline (n = 4). n are biologically independent samples from at least three independent experiments with similar results. Graphs depict means ± SEM. Panels (a-c): two-sided unpaired Student’s t test (Welch’s correction used for a: 2, 5, 20 μM and b: 60 min). Panel (a), * indicate difference in AmB versus AmB:Chol. Panel (c): * indicate difference versus vehicle. Panel (d): the same pig was measured before and after AmBisome® as indicated by symbols joined by lines; paired Student’s t test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Exact P values in Source Data.
AmBisome® is an FDA-approved liposomal formulation that contains AmB and cholesterol in a 1:2.5 ratio27. AmBisome® caused H13CO3− efflux in liposomes (Extended Data Fig. 6a), increased ASL pH and H14CO3− secretion in CuFi-1 epithelia measured 2 and 48 hours after addition (Fig. 3b,c, Extended Data Fig. 6b-c), and increased ASL pH over a large range of AmBisome® concentrations from 6-2450 μg/mL, equivalent to 0.25-100 μM AmB (Extended Data Fig. 6c).
To assess whether AmBisome® can restore ASL pH in vivo, we used a porcine model of CF24. The ASL pH of CFTR−/− pigs does not increase without intervention with aerosolized HCO3− or tromethamine buffer24, and the ASL pH of non-CF pigs is about 7.253. Administering 60 μL of a 1 mg/mL AmBisome® solution through a tracheal window to 1 cm2 surface of airway increased ASL pH in CFTR−/− pigs (Fig. 3d).
Thus, small molecule ion channels can permeabilize the apical membrane of CF airway epithelia to HCO3− and restore ASL pH, viscosity, and antibacterial activity, key components of airway host defenses. CFTR selectively conducts anions, whereas the AmB channel conducts both monovalent anions and cations. AmB is therefore an imperfect substitute for CFTR. However, the mechanisms that create an electrochemical driving force for anion secretion establish a setting in which an unselective channel is sufficient to support anion secretion, the fundamental defect in CF airway epithelia. Other mechanisms may also contribute to AmB-mediated increase in transepithelial H13CO3− transport, such as the coupling of AmB-mediated chloride secretion to H13CO3− secretion by anion exchangers (e.g. SLC26A4) and other apical protein anion channels (e.g. SLC26A9).28 These findings reveal a CFTR-independent and thus genotype-independent approach for treating people with CF, including those with nonsense and premature termination codons that produce little or no CFTR. Because this mechanism is distinct, there is also potential for additive effects with CFTR modulators4,23. Moreover, AmB is an already clinically approved drug that might benefit people with CF, and AmBisome® is safely delivered to the lungs to treat pulmonary fungal infections without producing significant systemic exposure (Extended Data Table 1).
Methods
Cell lines and growth conditions.
NuLi, CuFi-1, and CuFi-4 cells29 (Welsh Laboratory, University of Iowa) were first grown from cryostock on Thermo Scientific BioLite Cell Culture Treated 75 cm2 flasks, seeded at 1.5 × 104 cells/cm2, 1 × 103 cells/cm2, and 1 × 104 cells/cm2 respectively. These flasks were previously coated with 3 mL of 60 μg/ml human placental collagen type IV (Sigma-Aldrich) for a minimum of 18 hours at room temperature, rinsed twice with PBS, and then dried prior to seeding. The cells were cultured with 12 mL of the Bronchial Epithelial Cell Growth Medium (BEGM) BulletKit (Lonza CC-3170), which includes the basal media and eight SingleQuots of supplements (BPE, 2 ml; Hydrocortisone, 0.5 ml; hEGF, 0.5 ml; Epinephrine, 0.5 ml; Transferrin, 0.5 ml; Insulin, 0.5 ml; Retinoic Acid, 0.5 ml; Triiodothyronine, 0.5 ml). The gentamycin-amphotericin B aliquot was discarded and the media was instead supplemented with 50 μg/ml penicillin-streptomycin (Corning Cellgro), 50 μg/ml gentamycin (Sigma-Aldrich G1397), and 2 μg/ml fluconazole (Sigma-Aldrich). The original CF transplant donors were genotyped by Integrated Genetics (Westborough, MA). Cell lines were secondarily confirmed by the ATCC repository to have the correct genotype and were free of mycoplasma contamination. MycoAlert Mycoplasma detection kit (Lonza LT07-418) was used to detect any RNA transcripts common to a broad spectrum of mycoplasma. Cell lines were confirmed to be mycoplasma-free.
Cells were grown to a 90% confluence at 37 °C and 5% CO2 with media changed every 2 days, and then trypsinized with 4 mL of 0.25% trypsin with 1 mM EDTA (Gibco 25200-056). Trypsin was inactivated with 10 mL of HEPES Buffered Saline Solution (Lonza CC-5024) with added 1% bovine calf serum. Cells were spun down in an Eppendorf Centrifuge 5430 R at 1500 rpm for 5 minutes and resuspended in BEGM media for passaging.
For culturing onto membrane supports for differentiation, cells were resuspended after centrifugation in Ultroser G media. This is composed of a 1:1 ratio of DMEM:Ham’s F-12 supplemented with 2% v/v Ultroser G (Crescent Chemical). The membrane supports used were Millicell 0.4 μm PCF inserts (0.6 cm2) (Millipore PIHP01250) for Ussing chamber studies of candidate ionophores, Falcon® Permeable Support for 6 Well Plate with 0.4 μm Transparent PET Membranes (4.67 cm2) (Fisher 08-771) in 6-well companion plates (Fisher 08-771-24) for pH-stat studies, and the Corning Costar 0.4 μm 24-well plate Transwell Clear Polyester Membrane inserts (0.33 cm2) (Corning 3470) for all other studies. These membranes were coated with collagen in the same manner as the flasks detailed above. The Millicell inserts were seeded with 200,000 cells each, the Falcon inserts were seeded with 500,000 cells each, and the Transwell inserts were seeded with 115,000 cells each. These membranes were allowed to mature at an air-liquid interface for a minimum of 14 days to reach full differentiation as previously described29,30, with the Ultroser G media changed every other day. After maturation, media was changed every 7 days. For covariate control, membranes used in experiments were close in age and maturation as much as possible.
Primary cultures of airway epithelia.
Airway epithelial cells were obtained from human trachea and bronchi of CF and non-CF specimens obtained from the Iowa Donor Network, either as post-mortem specimens or from tissue deemed not fit for transplant. All of the corresponding genotypes are indicated in the manuscript (see Extended Data Fig. 5). All samples were de-identified in the Cell Culture Core Repository and patient identification information was not provided to the researchers doing the experiments. Patients were not recruited. Studies were approved by the University of Iowa Institutional Review Board. We have complied with all relevant ethical regulations and informed consent was obtained from all participants. After pronase enzymatic digestion, cells were seeded onto collagen-coated semi-permeable membranes (0.33 - 1.12 cm2, Corning 3470 polyester, 3460 polyester, 3413 polycarbonate) and grown at an air-liquid interface using previously described methods30. Airway epithelial cell cultures were analyzed after they had differentiated and at least 14 days post-seeding.
Statistics.
No data were excluded. All data depicts the means ± SEM with a minimum of 6 biological replicates. D’Agostino & Pearson normality test was used to confirm normal distribution of data. Statistical analysis represents P values obtained from one-way ANOVA or two-sided unpaired or paired student t-test where necessary. In cases where variance was not homogenous between comparison groups, parametric t-test with Welch’s correction was performed to account for differences in variance. NS, not significant. * P < 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001 unless otherwise noted. Based on pilot experiments, we chose sample sizes that adequately power each experiment to detect a difference in outcomes between groups. No statistical methods were used to predetermine sample size. Epithelial samples were manually assigned at random into control and experimental groups for each experiment. Animals served as their own controls.
Studies of Na+, K+, Cl−, and H13CO3− efflux from POPC liposomes (Extended Data Fig. 1a-j; Extended Data Fig. 6a).
General information.
Palmitoyl oleoyl phosphatidylcholine (POPC) was obtained as a 25 mg/mL solution in CHCl3 from Avanti Polar Lipids (850457C). The solution was stored at −20 ºC under an atmosphere of dry argon and used within 3 months. Cholesterol (Sigma Aldrich C8667) was purified by recrystallization from ethanol. NaH13CO3 was obtained as a white solid from Sigma Aldrich (372382). Sodium, potassium, and chloride measurements were obtained using a Denver Instruments (Denver, CO) Model 225 pH meter equipped with the appropriate ion selective probe inside a Faraday cage. Sodium selective measurements were obtained using an Orion micro sodium electrode (Thermo 9811BN). Potassium selective measurements were obtained with an Orion Potassium Sure-Flow Combination Electrode with Waterproof BNC connector (Thermo 9719BNWP). Chloride selective measurements were obtained using an Orion combination chloride electrode (Thermo 9617BNWP). For sodium efflux experiments, measurements were made on 1.5 mL solutions that were magnetically stirred in 7 mL vials incubated at 23 °C. For chloride and potassium efflux experiments, measurements were made on 4 mL solutions that were magnetically stirred in 20 mL vials incubated at 23 °C. For sodium, potassium, and chloride efflux experiments, the concentration of each ion was sampled every 10 seconds throughout the course of the efflux experiments. 13C NMR spectra for HCO3− efflux experiments were acquired on a Bruker Avance III HD 500 MHz NMR spectrometer equipped with a 5 mm BBFO CryoProbe (Extended Data Figure 1a-e), a Varian Inova 600MHz NMR spectrometer with a Varian 10 mm broadband probe (Extended Data Figure 1i), and a Varian Inova 600MHz NMR spectrometer with a Varian 5 mm broadband autox probe (Extended Data Figure 6a). The instrument was locked on D2O. The inverse-gated 13C spectra were collected.
The efflux data from each run was normalized to the percent of total ion release from 0 to 100%. For HCO3− efflux experiments, after lysis of the liposome suspension, the integration of the signal corresponding to extravesicular HCO3− relative to the integration of the 13C glucose standard was scaled to correspond to 100% efflux. For each experimental run with AmB addition, the signal corresponding to extravesicular HCO3− was integrated relative to the 13C internal standard for each free induction decay (FID). The scaling factor S was calculated for each experiment using the following relationship:
Each data point was then multiplied by S before plotting as a function of time.
Liposome preparation.
Prior to preparing a lipid film, the lipid solution was warmed to ambient temperature to prevent condensation from contaminating the solution and degrading the lipid film. 42 mg of solid cholesterol was added to a 20 mL scintillation vial (Fisher Scientific), followed by 14 mL of the POPC solution. The solvent was removed with a gentle stream of nitrogen, and the resulting lipid film was stored under high vacuum for a minimum of twelve hours prior to use. For sodium efflux experiments, the film was rehydrated with 2 mL of 250 mM NaHCO3, 40 mM HEPES buffer, pH 7.5 and vortexed vigorously for approximately 3 minutes to form a suspension of multilamellar vesicles (MLVs). For potassium efflux experiments, the film was rehydrated with 2 mL of 250 mM KHCO3, 40 mM HEPES buffer, pH 7.5. For chloride efflux experiments, the film was rehydrated with 2 mL of 250 mM NaCl, 40 mM HEPES buffer, pH 7.5. For HCO3− efflux experiments, the film was rehydrated with 2 mL of 250 mM NaH13CO3, 40 mM HEPES buffer, pH 7.5 (D2O). To obtain a sufficient quantity of large unilamellar vesicles (LUVs), at least two independent lipid film preparations were pooled together for the subsequent formation of LUVs. The lipid suspension was then subjected to 15 freeze-thaw cycles, where the suspension was alternatingly allowed to freeze in a liquid nitrogen bath, followed by thawing in a 50 °C water bath. The resulting lipid suspension was pulled into a Hamilton (Reno, NV) 1 mL gastight syringe and the syringe was placed in an Avanti Polar Lipids Mini-Extruder (610000). The lipid solution was then passed through a 5.00 μm pore size Whatman Nuclepore hydrophilic polycarbonate filter (VWR 28158-067) 35 times, the newly formed large unilamellar vesicle (LUV) suspension being collected in the syringe that did not contain the original suspension of MLVs to prevent the carryover of MLVs into the LUV solution. To obtain a sufficient quantity of LUVs, at least four independent 1 mL preparations were pooled together for the dialysis and subsequent efflux experiments. The newly formed LUVs were dialyzed using Thermo Scientific Slide-A-Lyzer G2 dialysis cassettes (3 mL, 3,500 MWCO, 87723). Unless otherwise described, the LUV suspension was dialyzed 3 times against 600 mL of 62.5 mM MgSO4, 40 mM HEPES buffer, pH 7.3. The first two dialyses were two hours long, while the final dialysis was performed overnight.
Determination of total phosphorus was adapted from a previous report31. The LUV suspension was diluted 40-fold with 62.5 mM MgSO4 in 40 mM HEPES buffer pH 7.3. Three 10 μL samples of the diluted LUV suspension were added to three separate 7 mL vials. Subsequently, the solvent was removed with a stream of N2. 450 μL of 8.9 M H2SO4 was added to each dried LUV film, including a fourth vial containing no lipids that was used as a blank. The four samples were incubated open to ambient atmosphere in a 225 °C aluminum heating block for 25 min and then moved to 23 °C and allowed to cool for 5 minutes at room temperature. After cooling, 150 μL of 30% w/v aqueous hydrogen peroxide was added to each sample, and the vials were returned to the 225 °C heating block for 30 minutes. The samples were then moved to 23 °C and allowed to cool for 5 minutes at room temperature before the addition of 3.9 mL water. Then 500 μL of 2.5% w/v ammonium molybdate was added to each vial, and the resulting mixtures were then vortexed briefly and vigorously five times. Subsequently, 500 μL of 10% w/v ascorbic acid was added to each vial, and the resulting mixtures were then vortexed briefly and vigorously five times. The vials were enclosed with a PTFE lined cap and then placed in a 100 °C aluminum heating block for 7 minutes. The samples were moved to 23 °C and allowed to cool for approximately 15 minutes at room temperature to 23 °C prior to analysis by UV/Vis spectroscopy. Total phosphorus was determined by observing the absorbance at 820 nm and comparing this value to a standard curve obtained through this method and a standard phosphorus solution of known concentration.
Efflux from LUVs.
13C NMR studies of H13CO3− efflux from POPC liposomes (Extended Data Fig. 1a-e).
Cholesterol-containing POPC liposomes were prepared as described above. The LUV suspension was dialyzed 10 times against 300 mL of 87 mM Na2SO4 in 40 mM HEPES buffer pH 7.3 (H2O) with stirring. The first dialysis was four hours long, while the subsequent nine dialyses were performed for 1 hour. Determination of phosphorous content was performed as described above.
The pooled LUV suspension was diluted to 70 mM lipid with 87 mM Na2SO4, 40 mM HEPES buffer, pH 7.3 (D2O), and 0.025% (w/v) 13C D-glucose (1-13C) (Sigma Aldrich 297046) was added as an internal standard. 13C NMR spectra were acquired on a Bruker Avance III HD 500 MHz NMR spectrometer equipped with a 5 mm BBFO CryoProbe. The 13C frequency was set to 125.83 MHz, and spectral width was 31,512 Hz. The instrument was locked on D2O. Experimental conditions were: acquisition time, 0.93 s; 30° pulse width, 3.3 μs; relaxation delay, 0.2 s; number of scans, 256; temperature 23 °C.
For each experiment, 1.4 μL of vehicle, AmB, or C35deOAmB (17.5 μM final concentration, 100X stock solution in DMSO) was added to 140 μL of the liposome suspension. The liposome suspension was immediately transferred to a New Era (NE-H5/4) micro NMR sample tube (3 mm lower/5 mm upper), and 8 consecutive FIDs were obtained as described above. For experimental runs with MnCl2, 5 μL of a 50 mM MnCl2 solution was added after the addition of AmB. To effect complete ion release, 10 μL of a 30% (v/v) solution of triton X-100 (Sigma Aldrich X100) was added to the liposome suspension before data acquisition32–35.
Na+, K+, and Cl− efflux from POPC liposomes (Extended Data Fig. 1f-h).
For sodium, potassium, and chloride efflux experiments, the pooled LUV suspension was diluted to 70 mM lipid with 62.5 mM MgSO4, 40 mM HEPES buffer, pH 7.3. The LUV suspension (1.5 mL for sodium, and 4 mL for chloride and potassium) was added to either a 7 mL (sodium) or 20 mL vial (chloride and potassium) and magnetically stirred. The appropriate probe was inserted, and data were collected for one minute prior to addition of AmB. For sodium efflux experiments, 15 μL of either vehicle or AmB (70 μM final concentration, 100X stock solution in DMSO) was added to 1.5 mL of LUV suspension, and data were collected for 10 minutes. To effect complete ion release, 15 μL of a 30% v/v solution of Triton X-100 was added, and data were collected for an additional five minutes. For chloride and potassium efflux experiments, 40 μL of either vehicle or AmB (70 μM final concentration, 100X stock solution in DMSO) was added to 4 mL of LUV suspension, and data were collected for 10 minutes. To effect complete ion release, 40 μL of a 30% v/v solution of triton X-100 was added, and data were collected for an additional five minutes.
H13CO3− efflux from POPC liposomes with rapid injection NMR (Extended Data Fig. 1i).
Cholesterol-containing POPC liposomes were prepared as described above. The LUV suspension was dialyzed 3 times against 600 mL of 62.5 mM MgSO4 in 40 mM HEPES buffer pH 7.3 (9:1 H2O:D2O) with stirring. The first two dialyses were two hours long, while the final dialysis was performed overnight. Determination of phosphorous content was performed as described above. H13CO3− efflux was measured using rapid injection NMR36,37. 13C NMR spectra were acquired using a Varian Inova 600MHz NMR spectrometer with a Varian 10 mm broadband probe. The 13C frequency was set to 150.83 MHz, and spectral width was 37037 Hz. Experimental conditions were: acquisition time, 0.50 s; 45° pulse width, 14 μs; relaxation delay, 1.5 s; number of scans, 1; temperature 23 °C. 2.5 mLs of the LUV suspension were added to an oven dried 10-mm NMR tube (New Era Enterprises Inc. NE-H10-7). The uncapped tube was placed into the probe of the NMR spectrometer. 70 μM AmB (100X stock in DMSO) was injected at a rate of 500 μL per second. The injector was used to mix the sample followed by data collection. The efflux of bicarbonate was monitored by 13C NMR by collecting 300 consecutive spectra over the course of 10 minutes after addition. To effect complete ion release, 80 μL of a 10% (v/v) solution of triton X-100 (Sigma Aldrich) was added to the liposome suspension before data acquisition32–35.
Studies of H+ efflux from POPC liposomes (Extended Data Fig. 1j).
Proton efflux from POPC/10% cholesterol liposomes was determined as previously described38.
H13CO3− efflux from POPC liposomes with AmBisome® and AmB:cholesterol (Extended Data Fig. 6a).
The LUV suspension was dialyzed 3 times against 600 mL of 62.5 mM MgSO4 in 40 mM HEPES buffer pH 7.3 (H2O) with stirring. The first two dialyses were two hours long, while the final dialysis was performed overnight. Determination of phosphorous content was performed as described above.
The pooled LUV solution was diluted to 100 mM lipid with 62.5 mM MgSO4, 40 mM HEPES buffer, pH 7.3 (D2O), and 0.025% (w/v) 13C D-glucose (1-13C) (Sigma Aldrich) was added as an internal standard. 13C NMR spectra were acquired on a Varian Inova 600MHz NMR spectrometer with a Varian 5 mm broadband autox probe. The 13C frequency was set to 125.83 MHz, and spectral width was 31,512 Hz. The instrument was locked on D2O. Experimental conditions were: acquisition time, 0.93 s; 30° pulse width, 3.3 μs; relaxation delay, 0.2 s; number of scans, 256; temperature 23 °C.
For each experiment, 5 μL of DMSO or sterile water vehicle, AmBisome® in sterile water, or AmB:cholesterol in DMSO (100 μM final AmB concentration, 100X stock solution) was added to 500 μL of the liposome suspension in an oven dried New Era (Vineland, NJ) 5 mm NMR sample tube. 12 consecutive FIDs were obtained as described above. To effect complete ion release, 40 μL of a 10% (v/v) solution of triton X-100 (Sigma Aldrich) was added to the liposome suspension before data acquisition32–35.
Measurement of ASL pH in cell line and primary cultures of airway epithelia (Fig. 1a,c,e,f; Fig. 2a,b; Fig. 3a-b, Extended Data Fig. 5b-c, Extended Data Fig. 6c).
Small diameter NuLi, CuFi, and primary cultured epithelia were used for this experiment (0.33 cm2). The ratiometric pH indicator SNARF-conjugated dextran (Molecular Probes) was used to measure ASL pH. SNARF powder was suspended via sonication in perfluorocarbon (FC-72, Sigma) and distributed onto the apical surface. ASL pH was measured 2 hr later 3,12,39. SNARF was excited at 488 nm and emission was recorded at 580 nm and 640 nm using a Zeiss LSM 800 microscope at 40X water immersion for cell line cultures and a Zeiss LSM 510 microscope for primary cultures. To generate a standard curve for pH determination, SNARF was dissolved in colorless pH standards and fluorescence ratios were converted to pH.
Agents tested in this assay were first lyophilized into powder and then suspended in the appropriate volume of perfluorocarbon (FC-72, Sigma) and sonicated for 1 minute to suspend. AmBisome® should not be sonicated; instead, the fine powder was suspended by vortexing. 20 μL of this suspension was administered onto the surface of cultured airway epithelia (0.33 cm2) at the following approximate concentrations in suspension:
amphotericin B 0.25-100 μM
amphotoricin B-cholesterol complex 0.5-100 μM
C35deOamphotericin B 2 μM 20
10 μM forskolin/10 μM ivacaftor 12
AmBisome® 0.25-2450 μg/mL
In all experiments, ASL pH of compound-treated epithelia was measured compared the results to vehicle-treated epithelia.
For apical AmB administration, cultured airway epithelia were incubated for 30 min – 48 hrs at 37°C before measurement of ASL pH. For AmB-cholesterol complex and C35deOAmB administration, cultured airway epithelia were incubated for 48 hrs at 37°C before measurement of ASL pH. For 10 μM ivacaftor/10 μM forskolin administration, cultured airway epithelia were incubated for 2 hrs at 37°C before measurement of ASL pH12. For basolateral AmB administration, a 2 mM stock of AmB in DMSO was diluted 1000-fold to a final concentration of 2 μM in USG media. The basolateral media of cultured airway epithelia was replaced with the AmB-containing USG media and incubated for 48 hrs at 37°C before measurement of ASL pH.
Specific conditions for experiments in Figures 1–3 not otherwise described in legends:
1a: ivacaftor/forskolin (10 μM, 2 hours); apical AmB (2 μM, 48 hours)
1c: ivacaftor/forskolin (10 μM, 2 hours), AmB (2 μM, 48 hours), C35deOAmB (2 μM, 48 hours), basolateral addition of AmB (2 μM, 48 hours)
1e: AmB (2 μM, 48 hours)
1f: AmB (2 μM)
2a: AmB (2 μM, 48 hours)
2b: AmB (2 μM)
3a: AmB (48 hours)
3b: AmBisome® (1 mg/mL)
pH-stat titration of NuLi and CuFi monolayers (Extended Data Fig. 2a). Large diameter NuLi and CuFi-1 cultured epithelia were used for this experiment (4.67 cm2). These cultures were mounted in a dual-channel Ussing chamber (Warner U2500) using the culture cup insert for Transwell adapter, 24mm (U9924T-24). The membranes were bathed at 37°C on the apical side with a buffer-free solution (140 mM NaCl, 2 mM KCl, 2 mM CaCl2, and 1 mM MgCl2, 15 mM dextrose, gassed with air) and on the basolateral side with either a HCO3− buffer (120 mM NaCl, 25 mM NaHCO3, 5 mM KCl, 2 mM CaCl2, 1.2 mM MgCl2, 13.75 NaH2PO4, 5.6 mM dextrose, pH adjusted to 7.0) or a HCO3−-free buffer (140 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 5 mM dextrose, pH adjusted to 7.0). A microdiameter pH electrode (89231-590) and temperature probe (Radiometer Analytical T201 Temperature Sensor, E51M001) and titration burette attached to a Hach TIM856 NB pH/EP/Stat pH-STAT Titrator (R41T028) were inserted into the apical chamber. The basolateral chamber was covered with the chamber lid to prevent gas exchange. The pH electrode was then calibrated using known pH solutions (Hach, S11M002, S11M004, S11M007).
The apical pH was titrated to a target pH of 6.0 using 1 mM HCl as titrant (min speed 0.25 mL/min, max speed 0.35 mL/min)40–43. Acid titration was measured over 20 minutes to establish a baseline value for the cultured epithelia (max speed 2 mL/min). Both apical and basolateral bathing solutions were then removed. A stock solution of AmB in DMSO was added to a final concentration of 0.5, 1, or 2 μM in an aliquot of buffer-free solution and added to the apical chamber, and the basolateral chamber was replaced with fresh HCO3− or HCO3−-free buffer. The apical pH was once again titrated to a target pH of 6.0 using 1 mM HCl as titrant. Acid titration was then measured over another 20 minutes to evaluate AmB-mediated pH change in the apical solution.
Data was plotted as nmoles of H+ titrated in per minute, and the slope of this curve was divided by the area of the culture (4.67 cm2) to obtain the rate of acid titration (nmoles H+/min/cm2).
Determination of ASL Na+, K+, Mg2+, and Ca2+ concentrations in CuFi-1 monolayers (Extended Data Fig. 2b-e).
Small diameter CuFi-1 cultured epithelia were used for this experiment (0.33 cm2). 24 hours prior to the start of experiment, the apical side of all cultured epithelia was rinsed three times with 200 μL warm PBS to remove excess mucus. Fresh USG media was first added to the basolateral membrane. CuFi-1 epithelia were treated with either perfluorocarbon (FC-72) vehicle or 2 μM AmB suspended in FC-72, and incubated at 37 °C for 48 hours. 0.1 μL capacity microcapillary tubes (Drummond Scientific NC1453214) were placed into 200 μL pipette tips (Denville Scientific P1122). 48 hours after AmB addition, the microcapillary tubes were gently touched around the edge of the apical membrane of each epithelial culture insert until completely filled with ASL via capillary action. After collecting 0.1 μL of ASL, a p200 pipette was used to push the entire sample into 15 μL of molecular biology grade water (Corning 46-000-CM). The sample was then quantitatively transferred to a 15 mL capacity conical vial by washing 3 times with 50 μL molecular biology grade water.
Quantification of sodium, magnesium, potassium, and calcium was accomplished using inductively coupled plasma mass spectrometry (ICP-MS) of acidified samples. Each sample was diluted to a final volume of 5 mL with 1.0% HNO3 (v/v) in double distilled water. Quantitative standards were made using a mixed Na, Mg, K, and Ca standard at 100 μg/mL of each element (Inorganic Ventures, Christiansburg, VA, USA) which were combined to create a 100 ng/mL mixed element standard in 1.0% nitric acid (v/v).
ICP-MS was performed on a computer-controlled (QTEGRA software) Thermo iCapQ ICP-MS (Thermo Fisher Scientific, Waltham, MA, USA) operating in KED mode and equipped with a ESI SC-2DX PrepFAST autosampler (Omaha, NE, USA). Internal standard was added inline using the prepFAST system and consisted of 1 ng/mL of a mixed element solution containing Bi, In, 6Li, Sc, Tb, Y (IV-ICPMS-71D from Inorganic Ventures). Online dilution was also carried out by the prepFAST system and used to generate calibration curves consisting of 5000, 1000, 500, 100, and 50 ng/mL Na, Mg, K, Ca. Each sample was acquired using 1 survey run (10 sweeps) and 3 main (peak jumping) runs (40 sweeps). The isotopes selected for analysis were 23Na, 24Mg, 39K, 44Ca, and 89Y (chosen as internal standards for data interpolation and machine stability). Instrument performance is optimized daily through autotuning followed by verification via a performance report (passing manufacturer specifications).
H14CO3− transport across NuLi and CuFi monolayers (Fig. 1b,d; Fig. 3c; Extended Data Fig. 3a-c; Extended Data Fig. 6b).
Small diameter NuLi and CuFi cultured epithelia were used for this experiment (0.33 cm2). 14C-labeled sodium bicarbonate was obtained as a sterile 35.7 mM aqueous solution pH 9.5 (MP Biomedicals 0117441H). All experiments were run less than 2 months post seeding. Fresh USG media was added to the basolateral side prior to experimentation. The apical membrane was treated with 20 μL of vehicle, AmB, or ivacaftor/forskolin as a suspension in perfluorocarbon-72 (Sigma Aldrich), and the cultured epithelia were incubated for 48 hours, 7 days, 14 days, or 28 days at 37 °C in a 5% CO2 atmosphere. After the end of the treatment period, 5 μL of a 1.4 mM H14CO3− stock solution in USG media was added to the basolateral media. The cultured epithelia were then incubated at 37 °C for 10 minutes. After 10 minutes, the apical membrane of the cultured epithelia was immediately washed with 200 μL of PBS. The ASL wash and a 200 μL aliquot of the basolateral media were diluted in scintillation cocktail (Perkin Elmer 6013199) and analyzed via liquid scintillation counting18. The measured rate of basolateral-to-apical H14CO3− secretion was then normalized to the vehicle-treated control.
Specific conditions for experiments in Figures 1–3 not otherwise described in legends:
1b: ivacaftor/forskolin (10 μM, 2 hours); apical AmB (2 μM, 48 hours)
1d: ivacaftor/forskolin (10 μM, 2 hours), AmB (2 μM, 48 hours), C35deOAmB (2 μM, 48 hours), basolateral addition of AmB (2 μM, 48 hours)
3c: AmBisome® (1 mg/mL)
ASL pH and H14CO3− studies with basolateral ouabain (Figure 1h,i).
Small diameter CuFi-1 cultured epithelia were used for this experiment (0.33 cm2). All experiments were run less than 2 months post seeding. Fresh USG media was added to the basolateral side prior to experimentation. The apical membrane was treated with 20 μL of vehicle or 2 μM AmB as a suspension in perfluorocarbon-72 (Sigma Aldrich), and the cultured epithelia were incubated for 47 hours at 37 °C in a 5% CO2 atmosphere. The basolateral media was then removed and replaced with 10 mM ouabain44 (Sigma Aldrich O3125) dissolved in a low sodium Ringer’s buffer (125 mM choline chloride, 10 mM NaCl, 14 mM KHCO3, 5 mM HEPES, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgCl2, 5 mM dextrose, pH 7.4 with 10 N NaOH) at 37˚ C. The epithelia were then incubated at 37 °C for 1 hour. After 1 hour, ASL pH and H14CO3− were measured as described above.
Ussing Chamber studies of NuLi and CuFi monolayers (Extended Data Fig. 3d-i).
To assess the presence of membrane-expressed CFTR, differentiated cultures of NuLi and CuFi-1 epithelia grown on Corning Costar 0.4 μm 24-well plate Transwell Clear Polyester Membrane inserts were used. NuLi and CuFi-1 epithelia were treated with 20 μL of perfluorocarbon (FC-72, Sigma) vehicle or 2 μM amphotericin B (AmB) sonicated into a suspension in FC-72. After 48 hours of incubation, the epithelia were mounted in a dual-channel Ussing chamber (Warner U2500) using the culture cup insert for Transwell adapter, 6.5 mm (Warner U9924T-06) and bathed on both the apical and basolateral sides with a HCO3− solution (120 mM NaCl, 25 mM NaHCO3, 5 mM KCl, 2 mM CaCl2, 1.2 mM MgCl2, 13.75 mM NaH2PO4, pH 7.0) at 37°C and gassed with compressed air. Dextrose was added to this solution immediately prior to experiments to a final concentration of 5.6 mM. Epithelial sodium channel (ENaC) and calcium-activated chloride channel (CaCC) were inhibited by apical addition of 100 μM amiloride and 100 μM DIDS (4,4’-disothiocyanostilbene-2,2’-disulfonic acid), respectively, to achieve a baseline for permeabilization. 10 μM forskolin/100 μM IBMX (3-isobutyl-1-methylxanthine) added apically was used to activate CFTR, and 1 μM CFTRinh-172 was used to inhibit CFTR. Each successive addition of reagent was allowed approximately 10 minutes to equilibrate before the addition of the next reagent29.
Measurement of transepithelial electrical resistance (Rt) (Extended Data Fig. 3j).
Small diameter CuFi-1 cultured epithelia were used for this experiment (0.33 cm2). Cultured epithelia were treated with FC-72 vehicle, 2 and 50 μM AmB administered in perfluorocarbon (FC-72, Sigma) as previously described for 48 hours, 7 days, or 28 days. 200 μL of fresh USG media was placed on the apical side of the epithelia. Transepithelial electrical resistance (Rt) was then measured using a Millicell® ERS-2 Voltohmmeter across the apical and basolateral sides of the epithelia in a snaking pattern for two technical replicates per biological replicate.
Lactate dehydrogenase (LDH) assay (Extended Data Fig. 3k).
Small diameter CuFi-1 cultured epithelia were used for this experiment (0.33 cm2). An LDH Cytotoxicity Assay Kit (Cayman Chemical) was used to determine if AmB is toxic to CuFi-1 airway epithelia. Prior to treatment, media was removed from the basolateral side of epithelia and replaced with 500 μL of fresh USG media. Cultured epithelia were then treated with FC-72 vehicle, 2 and 50 μM AmB administered in perfluorocarbon (FC-72, Sigma) as previously described for 48 hours, 7 days, or 28 days. 48 hours prior to the end of each experiment time frame, basolateral media was changed again and 20 μL of 10% Triton X-100 solution was added to the apical surface to elicit maximum release. On the day of the experiment, assay reagents were prepared according to kit instructions and 500 μL of USG media was added to three empty wells in a 24-well plate for background control. Culture inserts were removed from the wells and 250 μL of LDH Reaction Solution was added to each well. The plate was then gently shaken on an orbital shaker for 30 minutes at 37°C. Absorbance was read at 490 nm using a plate reader. % cytotoxicity was calculated as follows:
% Cytotoxicity of test sample =
Airway surface liquid (ASL) height assay (Fig. 1g; Extended Data Fig. 4).
ASL height was studied using an established fluorescent dye-based assay45,46. Small diameter NuLi and CuFi-1 cultured epithelia were used for this experiment (0.33 cm2). 24 hours prior to the start of experiment, the apical side of all cultured epithelia was rinsed three times with warm PBS to remove excess mucus. NuLi epithelia were treated with perfluorocarbon (FC-72) vehicle or 500 μM basolateral bumetanide in DMSO vehicle applied to the media, and CuFi epithelia were treated with 20 μL vehicle, 0.5 μM AmB, or 0.5 μM C35deOAmB suspended in perfluorocarbon (FC-72, Sigma), with or without 500 μM basolateral bumetanide in DMSO vehicle applied to the media and incubated for 24 hours at 37 °C. For basolateral AmB administration, a 2 mM stock of AmB in DMSO was diluted 1000-fold to a final concentration of 2 μM in USG media. The basolateral media of cultured airway epithelia was replaced with the AmB-containing USG media. After 24 hours, 2.5 μL of a 2 mg/mL 70kDa Texas Red-dextran conjugate (Molecular Probes) solution in PBS was added to the apical side of the epithelia, followed by 100 μL of FC-770 to prevent evaporation. Then the culture support was placed on top of 100 μL of PBS on a 10 mm glass bottom Fluorodish for imaging (World Precision Instruments). Epithelia were imaged immediately after dye addition and again at 24 hours to examine dye absorption. Three Z-stack images per membrane were taken on an Zeiss LSM700 confocal microscope at 40x oil immersion. These images were analyzed using ImageJ 47 to determine the average ASL height in the center 1300 pixels of each image. Images were smoothed, converted to 8-bit, and thresholded to most accurately represent the red area. The parameters for Analyze Particles were particles from 1-Infinity μm2 in size and from 0%−100% circularity. Height was determined by dividing the area output in pixels by the known 1300 pixel width and converted to microns using the known scaling factor of 0.49 μm/pixel.
Viscosity of AmB-treated primary cultures of airway epithelia (Fig. 2c, Extended Data Fig. 5h-j).
ASL viscosity in airway epithelial cultures was determined as previously described12,48. Small diameter primary cultured epithelia were used for this experiment (0.33 cm2). The apical surface was not washed for at least 2 weeks before study. Cultured epithelia were treated with 2 μM AmB administered in perfluorocarbon (FC-72, Sigma) as previously described for 48 hours. FITC-dextran (70 kD, Sigma) was then administered to the apical surface of epithelia as a dry powder 2 hrs before measurement of viscosity. FRAP was assayed in a humidified chamber at 37°C using a Zeiss LSM 510 META microscope. Images were acquired until maximal recovery was reached. At least 6 recovery curves from different locations in each culture were acquired and averaged to obtain data for one epithelial culture. The time constant (τsaline) was calculated by regression analysis from fluorescence recovery curves. Viscosity is expressed relative to the time constant of saline (τASL/τsaline).
Antibacterial activity of AmB-treated primary cultures of airway epithelia (Fig. 2d, Extended Data Fig. 5f,g).
Staphylococcus aureus-coated gold grids were used to measure antibacterial activity of airway epithelial cultures as previously described3,39. Small diameter primary cultured epithelia were used for this experiment (0.33 cm2). Bacteria-coated gold TEM grids were placed onto the apical surface of airway epithelia for 1 min after 48 hours of perfluorocarbon (FC-72, Sigma), 2 μM AmB, or 2 μM C35deOAmB treatment. As controls, bacteria-coated grids were also placed in saline or AmB in FC-72 laid over saline to simulate the administration method for 1 minute. After removal, bacteria on the grids were assessed for viability using Live/Dead BacLight Bacterial Viability assay (Invitrogen). Viability was determined in 4-6 fields to determine the percentages of dead bacteria.
Animals for study (Fig. 3d).
We studied female and male newborn pigs with targeted disruption of the CFTR gene CFTR−/−, generated from mating CFTR+/− pigs. Pigs were obtained from Exemplar Genetics. The University of Iowa Animal Care and Use Committee approved all animal studies, and we have complied with all relevant ethical regulations.
Measurement of ASL pH in CFTR−/− pigs (Fig. 3d).
ASL pH was measured in pigs in vivo as previously described24,39. To administer AmBisome® in pig trachea, pigs were initially sedated with ketamine (Ketaject, Phoenix; 20 mg/kg, i.m. injection) and anesthetized using propofol (Diprivan, Fresenius Kabi; 2 mg/kg, i.v. injection). The trachea was surgically exposed and accessed anteriorly, and a small anterior window was cut through the tracheal rings. To mimic physiologic conditions, data was obtained in a 100% humidified chamber at 37°C and constant 5% CO2.
For the first CFTR−/− pig, a baseline ASL pH measurement was taken for about 8 minutes before 60 μL of 100 μg/mL AmBisome® in FC-72 was administered to the tracheal window. ASL pH was continually measured for 60 minutes. Then, 60 μL of 1 mg/mL of AmBisome® was administered to the tracheal window and pH was continually measured for another 60 minutes. For the second CFTR−/− pig, a baseline ASL pH measurement was taken for about 8 minutes before 60 μL of FC-72 vehicle was administered to the tracheal window as an internal control. ASL pH was continually measured for another 30 minutes. Then, 60 μL of 1 mg/mL of AmBisome® was administered to the tracheal window and continuous measurements were then taken for 106 minutes. For the third and fourth CFTR−/− pigs, a baseline ASL pH measurement was taken for about 10 minutes. Then, 60 μL of 1 mg/mL of AmBisome® in FC-72 was administered to the tracheal window and continuous measurements were taken for 120 minutes.
Extended Data
Extended Data Figure 1 |. AmB can transport potassium, sodium, chloride, protons and bicarbonate across a lipid membrane.
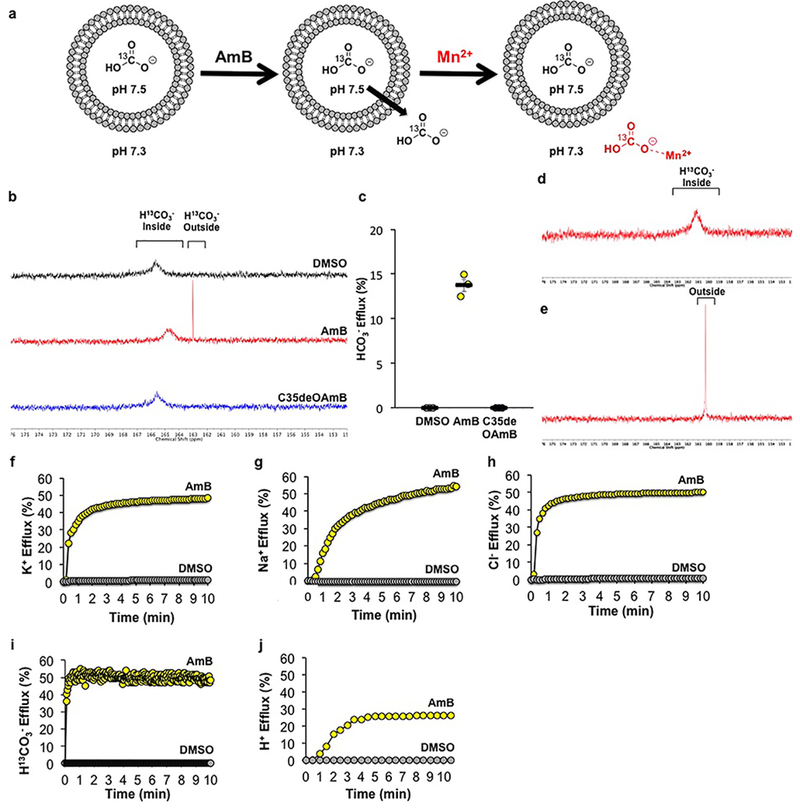
Traces indicate percent of maximum ion efflux after Triton-X addition. a) Schematic for the 13C-NMR bicarbonate efflux experiment. (b) 13C-NMR spectra of H13CO3−-loaded POPC/10% cholesterol liposomes treated with AmB, C35deOAmB, or DMSO vehicle. NaH13CO3 was loaded inside the liposomes and the intravesicular solution was buffered to pH 7.5, while the extravesicular solution was buffered to pH 7.3. Due to this pH difference, intravesicular HCO3− displays a more downfield chemical shift relative to extravesicular HCO3−. Addition of AmB (1:4,000 AmB:POPC) produces an upfield 13C signal corresponding to extravesicular HCO3−, while the addition C35deOAmB or DMSO vehicle does not, demonstrating that AmB is able to facilitate HCO3− efflux. (c) Percent efflux of bicarbonate mediated by DMSO, AmB, or C35deOAmB quantified 10 minutes after addition to POPC liposomes (n = 3 biologically independent samples). The data from each run was normalized to the percent of total ion release from 0 to 100%. After lysis of the liposome suspension, the integration of the signal corresponding to extravesicular HCO3− relative to the integration of a 13C glucose standard was scaled to correspond to 100% efflux. (d) To confirm that the upfield signal corresponds to extravesicular HCO3−, Mn2+, which binds to HCO3− and quenches the observed 13C signal via paramagnetic relaxation enhancement (PRE), was added to the extravesicular solution. Because Mn2+ is impermeable to the POPC bilayer, Mn2+ can only affect the signal corresponding to HCO3− outside of the liposomes. Addition of Mn2+ quenched the upfield signal produced with the addition of AmB but not the signal corresponding to intravesicular HCO3− confirming that AmB causes efflux of HCO3−. (e) To effect complete ion release, the POPC liposomes were lysed with Triton X-100 at the conclusion of the experiment. (f) Potassium efflux from POPC/10% cholesterol liposomes after addition of [AmB] equivalent to 1:1000 AmB:lipid, or DMSO vehicle. (g) Sodium efflux from POPC/10% cholesterol liposomes after addition of [AmB] equivalent to 1:1000 AmB:lipid, or DMSO vehicle. (h) Chloride efflux from POPC/10% cholesterol liposomes after addition of [AmB] equivalent to 1:1000 AmB:lipid, or DMSO vehicle. (i) Bicarbonate efflux from POPC/10% cholesterol liposomes after addition of [AmB] equivalent to 1:1000 AmB:lipid, or DMSO vehicle. Kinetics of efflux were measuring using rapid injection NMR to add AmB to liposomes. (j) Proton efflux from POPC/10% cholesterol liposomes after addition of [AmB] equivalent to 1:1000 AmB:lipid, or DMSO vehicle. In (b,d-j), a representative spectrum or graph from at least three independent experiments is shown. In all panels, measurements were taken from distinct samples. In panel (c), graph depicts mean ± SEM.
Extended Data Figure 2 |. AmB-mediated pH changes are HCO3−-dependent and do not alter major cation concentrations in the ASL.
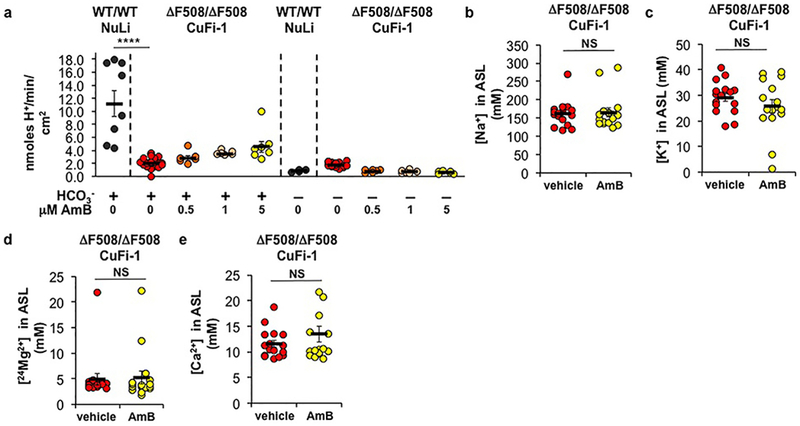
(a) Base secretion and acid absorption rates in NuLi (HCO3− +: n = 8 biologically independent samples; HCO3− -: n = 4 biologically independent samples) or CuFi-1 (ΔF508/ΔF508) epithelia (HCO3− +: n = 23 biologically independent samples, p < 0.0001; HCO3− -: n = 18 biologically independent samples) over 20 minutes after acute addition of increasing [AmB], as measured by pH-stat titration (All n are biologically independent samples. HCO3− +: 0.5,1 μM, n = 6; 5 μM, n = 7. 0.5 μM p = 0.9663, 1 μM p = 0.7328, 5 μM p = 0.1459. HCO3− -: 0.5,1,5 μM, n = 6). The apical pH was titrated to a target pH of 6.0. The effect of AmB (2 μM) or FC-72 vehicle after 48 hours on (b) Na+ (p = 0.7855), (c) K+ (p = 0.2892), (d) 24Mg2+ (p = 0.8339) and (e) Ca2+ (p = 0.2708 with Welch’s correction) concentrations in the ASL in CuFi-1 (ΔF508/ΔF508) as measured by ICP-MS (n = 16 biologically independent samples). In panel (a), ANOVA was used to assess statistical significance. In panels (b-d), two-sided unpaired Student’s t test was used to assess statistical significance. In panel (e), two-sided unpaired Student’s t test with Welch’s correction was used. Graph depicts means ± SEM; NS, not significant; ****P ≤ 0.0001. In all panels, measurements were taken from biologically independent samples.
Extended Data Figure 3 |. AmB treatment is sustained, ineffective on wild type, not due to increased CFTR activity, does not disturb membrane integrity, and is non-toxic.
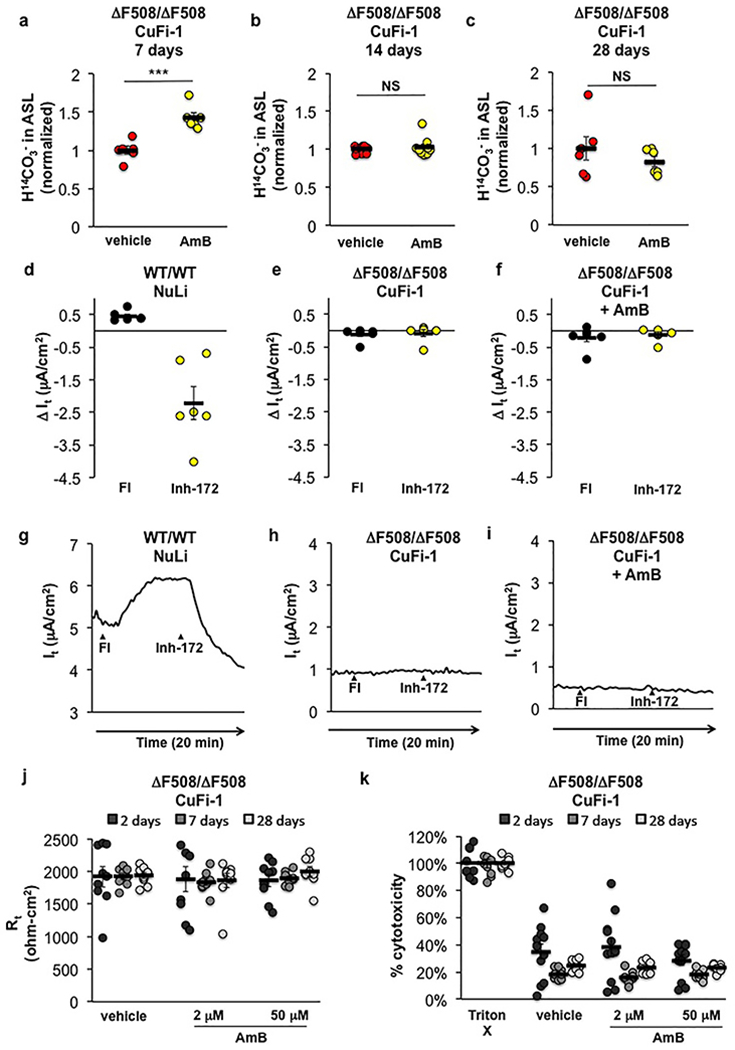
The effect of AmB (2 μM) or FC-72 vehicle left on the surface of CuFi-1 (ΔF508/ΔF508) epithelia for (a) 7 (n = 6 biologically independent samples, p = 0.0004), (b) 14 (n = 9 biologically independent samples, p = 0.5138), or (c) 28 days (n = 6 biologically independent samples, p = 0.3421) on H14CO3− movement from the basolateral buffer to the ASL over 10 minutes post-radiolabel addition, as normalized to FC-72 vehicle addition. Changes in transepithelial current (It) after treatment with 10 μM forskolin/100 μM IBMX (FI) to activate CFTR and 1 μM CFTRinh-172 to inhibit CFTR in (d,g) NuLi (CFTR+/+) epithelia, (e,h) CuFi-1 (ΔF508/ΔF508) epithelia, and (f,i) CuFi-1 epithelia treated with AmB (2 μM; 48 hours) (n = 6 biologically independent samples). In (g) to (i), a representative graph from 6 independent experiments repeated with similar results is shown. (j) Transepithelial electrical resistance (Rt) in CuFi-1 epithelia did not differ between treatment with vehicle or increasing doses of AmB over increasing time periods after a single treatment (n = 9 biologically independent samples). (k) Cytotoxicity as measured by detection of lactase dehydrogenase in CuFi-1 epithelia over increasing time periods after a single AmB or vehicle treatment, represented as percent of total cellular lysis by Triton X-100. AmB treatment did not cause increased cytotoxicity as compared to vehicle (n = 12 biologically independent samples). In (a) to (c), two-sided unpaired Student’s t test was used to assess statistical significance. In (a) to (f) and (j) to (k), graphs depict means ± SEM; NS, not significant; ***P ≤ 0.001 relative to vehicle control. In all panels, measurements were taken from biologically independent samples.
Extended Data Figure 4 |. AmB increases ASL height.

ASL height, as imaged by confocal microscopy, in (a) NuLi (CFTR+/+) epithelia, (b) CuFi-1 epithelia, (c) CuFi-1 epithelia with apical addition of AmB, (d) CuFi-1 epithelia with apical addition of C35deOAmB, (e) CuFi-1 epithelia with basolateral addition of AmB, and (f) NuLi and (g) AmB-treated CuFi-1 epithelia with basolateral addition of bumetanide (500 μM). Representative images from at least 6 independent experiments are shown. In all panels, measurements were taken from biologically independent samples. Scale bar represents 10 microns.
Extended Data Figure 5 |. AmB restores ASL pH and antibacterial activity in primary cultures of human airway epithelia from donors with CF.

(a) Genotypes and Δ pH measurements of patient donors in ASL pH assay. (b) The effects of AmB (2 μM, 48 hours; n = 9 biologically independent samples, p = 0.446), C35deOAmB (2 μM, 48 hours; n = 5 biologically independent samples, p = 0.9994) and basolateral addition of AmB (2 μM, 48 hours; n = 3 biologically independent samples, p = 0.6359) on the average ASL pH of primary cultured airway epithelia derived from CF humans with different CFTR mutations. (c) The effect of AmB (2 μM, 48 hours; n = 7 biologically independent samples, p = 0.4866) on ASL pH in non-CF epithelia. (d) Genotypes of patient donors in ASL viscosity assay. (e) Genotypes of patient donors in ASL antibacterial activity assay. (f) The effect of AmB (2 μM, 48 hours; n = 8 biologically independent samples, p = 0.0042) and C35deOAmB (2 μM, 48 hours; n = 5 biologically independent samples, p = 0.9626) on the average ASL antibacterial activity of primary cultured airway epithelia derived from CF humans with different CFTR mutations. Antibacterial activity is measured by the % of S. aureus killed after exposure to ASL. (g) The ability of AmB (2 μM) alone to kill S. aureus as compared to saline (n = 36 biologically independent samples, p = 0.1569). Representative FRAP traces for measuring ASL viscosity from 6 independent experiments repeated with similar results are shown in (h) non-CF, (i) CF, and (j) AmB-treated CF epithelia. In panels (b) and (f), ANOVA was used to assess statistical significance. In panel (c), two-sided unpaired Student’s t test with Welch’s correction was used. In panel (g), two-sided unpaired Student’s t test was used to assess statistical significance. Graphs depict means ± SEM; NS, not significant; *P ≤ 0.05; **P ≤ 0.01. In all panels, measurements were taken from biologically independent samples.
Extended Data Figure 6 |. AmBisome® increases transepithelial H14CO3− secretion and ASL pH in a time and dose-dependent manner.

(a) The effect of AmBisome® (1:1000 AmB:lipid ratio), AmB:Chol (1:1000 AmB:lipid ratio in DMSO), and sterile water or DMSO vehicle on H13CO3− transport across a POPC/10% cholesterol lipid membrane. (b) The effect of AmBisome® (1 mg/mL, 48 hours; n = 16 biologically independent samples, p = 0.0477) or FC-72 vehicle on H14CO3− movement from the basolateral buffer to the ASL over 10 minutes post-radiolabel addition in CuFi-1 (ΔF508/ΔF508) as normalized to FC-72 vehicle addition. (c) The effect of increasing [AmBisome®] (1 mg/mL, 48 hours; all concentrations, n = 9 biologically independent samples. 0.25 μM p = 0.0106, 2 μM p < 0.0001, 25 μM p = 0.0002, 50 μM p < 0.0001, 100 μM p < 0.0001) on ASL pH in CuFi-1 epithelia as compared to vehicle control. In panel (a), a representative graph from at least three independent experiments is shown. In panel (b), two-sided unpaired Student’s t test was used to assess statistical significance. In panel (c), ANOVA was used to assess statistical significance; delta and statistics are compared to FC-72 vehicle control. Graphs depict means ± SEM; NS, not significant; *P ≤ 0.05; ****P ≤ 0.0001 relative to vehicle control. In (a), the same sample for each replicate was measured repeatedly over time. In (b) and (c), measurements were taken from biologically independent samples.
Extended Data Table 1 |.
Published studies demonstrating safety of AmBisome® delivered to the airway via inhalation.
| Clinical studies with aerosolized AmBisome |
|---|
| Ringden, O., Meunier, F., Tollemar, J., Ricci, P., Tura, S., Kuse, E., … & Prentice, H. G. (1991). Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. Journal of Antimicrobial Chemotherapy, 28(suppl_B), 73-82. |
| Palmer, S. M., Drew, R. H., Whitehouse, J. D., Tapson, V. F., Davis, R. D., McConnell, R. R., … & Perfect, J. R. (2001). Safety Of Aerosolized Amphotericin B Lipid Complex In Lung Transplant Recipients 12. Transplantation, 72(3), 545-548. |
| Drew, R. H., Ashley, E. D., Benjamin Jr, D. K., Davis, R. D., Palmer, S. M., & Perfect, J. R. (2004). Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipients. Transplantation, 77(2), 232-237. |
| Perfect, J. R., Ashley, E. D., & Drew, R. (2004). Design of aerosolized amphotericin B formulations for prophylaxis trials among lung transplant recipients. Clinical infectious diseases, 39(Supplement_4), S207-S210. |
| Lowry, C. M., Marty, F. M., Vargas, S. O., Lee, J. T., Flumara, K., Deykin, A., & Baden, L. R. (2007). Safety of aerosolized liposomal versus deoxycholate amphotericin B formulations for prevention of invasive fungal infections following lung transplantation: a retrospective study. Transplant infectious disease, 9(2), 121-125. |
| Castagnola, E., Moresco, L., Cappelli, B., Cuzzubbo, D., Moroni, C., Lanino, E., & Faraci, M. (2007). Nebulized liposomal amphotericin B and combined systemic antifungal therapy for the treatment of severe pulmonary aspergillosis after allogeneic hematopoietic stem cell transplant for a fatal mitochondrial disorder. Journal of Chemotherapy, 19(3), 339-342. |
| Slobbe, L., Boersma, E., & Rijnders, B. J. (2008). Tolerability of prophylactic aerosolized liposomal amphotericin-B and impact on pulmonary function: data from a randomized placebo-controlled trial. Pulmonary pharmacology & therapeutics, 21(6), 855-859. |
| Rijnders, B. J., Cornelissen, J. J., Slobbe, L., Becker, M. J., Doorduijn, J. K., Hop, W. C., … & de Marie, S. (2008). Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clinical infectious diseases, 46(9), 1401-1408. |
| Monforte, V. et al. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: pharmacokokinetics and safety. J Heart Lung Transplant 28, 170-175, doi:10.1016/j.healun.2008.11.004 (2009). |
| Monforte, V., Ussetti, P., Gavaldà, J., Bravo, C., Laporta, R., Len, O., … & Román, A. (2010). Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. The Journal of Heart and Lung Transplantation, 29(5), 523-530. |
| Hullard-Pulstinger, A., Holler, E., Hahn, J., Andreesen, R., & Krause, S. W. (2011). Prophylactic application of nebulized liposomal amphotericin B in hematologic patients with neutropenia. Oncology Research and Treatment, 34(5), 254-258. |
| Monforte, V., López-Sánchez, A., Zurbano, F., Ussetti, P., Solé, A., Casals, C., … & Román, A. (2013). Prophylaxis with nebulized liposomal amphotericin B for Aspergilus infection in lung transplant patients does not cause changes in the lipid content of pulmonary surfactant. The Journal of Heart and Lung Transplantation, 32(3), 313-319. |
| Stone, N. R., Bicanic, T., Salim, R., & Hope, W. (2016). Liposomal amphotericin B (AmBisome®): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs, 76(4), 485-500. |
Supplementary Material
Acknowledgments.
We gratefully acknowledge assistance from M. Sivaguru and the Carl R. Woese IGB with confocal microscopy, S.E. Denmark and C. Delaney with rapid injection NMR, and D.J. Blair for helpful discussions. Support was provided by NIH (5R35GM118185 to M.D.B. and HL091842 to M.J.W.), and by Emily’s Entourage. M.J.W. is an HHMI Investigator. K.A.M. is a Medical Scholars Fellow.
Footnotes
Data availability. All data generated or analyzed during this study are included in this published article (and in the linked Source Data files).
References
- 1.Ratjen F et al. Cystic fibrosis. Nat. Rev. Dis. Primers 1, 15010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinton PM The neglected ion: HCO3−. Nat. Med. 7, 292–293 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Shah VS et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351, 503–507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsey , B. W. et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 365, 1663–1672 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wainwright CE et al. Lumacaftor–Ivacaftor in patients with cystic fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 373, 220–231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver KE, Han ST, Sorscher EJ & Cutting GR Transformative therapies for rare CFTR missense alleles. Curr. Opin. Pharmacol. 34, 76–82 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Etri M & Cuppoletti J Metalloporphyrin chloride ionophores: induction of increased anion permeability in lung epithelial cells. Am. J. Physiol. 270, L386–392 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Jiang C et al. Partial correction of defective Cl(−) secretion in cystic fibrosis epithelial cells by an analog of squalamine. Am. J. Physiol. Lung Cell Mol. Physiol 281, L1164–1172 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Koulov AV et al. Chloride transport across vesicle and cell membranes by steroid-based receptors. Angew. Chem. Int. Ed. Engl. 42, 4931–4933 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Shen B, Li X, Wang F, Yao X & Yang D A synthetic chloride channel restores chloride conductance in human cystic fibrosis epithelial cells. PLoS One 7, e34694 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulsen JH, Fischer H, Illek B & Machen TE Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. U.S.A. 91, 5340–5344 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang EH et al. Medical reversal of chronic sinusitis in a cystic fibrosis patient with ivacaftor. Int. Forum Allergy Rhinol. 5, 178–181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garland AL et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc. Natl. Acad. Sci. U.S.A. 110, 15973–15978 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garnett JP et al. Hyperglycaemia and Pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate-H+ secretion. Sci. Rep 6, 37955 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farha MA, French S, Stokes J & Brown ED Bicarbonate alters bacterial susceptibility to antibiotics by targeting the proton motive force. ACS Infect. Dis. 4, 382–390 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Peckham D, Holland E, Range S & Knox AJ Na+/K+ ATPase in lower airway epithelium from cystic fibrosis and non-cystic-fibrosis lung. Biochem. Biophys. Res. Commun. 232, 464–468 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Widdicombe J, Welsh M & Finkbeiner W Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc. Natl. Acad. Sci. U.S.A. 82, 6167–6171 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillo AS et al. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 356, 608–616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ermishkin LN, Kasumov KM & Potseluyev VM Properties of amphotericin B channels in a lipid bilayer. Biochim. Biophys. Acta 470, 357–367 (1977). [DOI] [PubMed] [Google Scholar]
- 20.Gray KC et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U.S.A. 109, 2234–2239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson TM et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 10, 400–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cioffi AG, Hou J, Grillo AS, Diaz KA & Burke MD Restored physiology in protein-deficient yeast by a small molecule channel. J. Am. Chem. Soc. 137, 10096–10099 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Goor F et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U.S.A. 106, 18825–18830 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou Alaiwa MH et al. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight 1, e87535 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DP et al. A synthetic channel-forming peptide induces Cl(−) secretion: modulation by Ca(2+)-dependent K(+) channels. Biochim. Biophys. Acta 1464, 69–82 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Derichs N, Jin B-J, Song Y, Finkbeiner WE & Verkman A Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J. 25, 2325–2332 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monforte V et al. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: pharmacokinetics and safety. J. Heart Lung Transplant 28, 170–175 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Saint-Criq V & Gray MA Role of CFTR in epithelial physiology. Cell Mol. Life Sci. 74, 93–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods References
- 29.Zabner J et al. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am. J. Physiol. Lung Cell Mol. Physiol 284, L844–854 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Karp PH et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol. Biol. 188, 115–137 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Chen PS, Toribara TY & Warner H Microdetermination of Phosphorus. Anal. Chem. 28, 1756–1758 (1956). [Google Scholar]
- 32.Andrews NJ et al. Structurally simple lipid bilayer transport agents for chloride and bicarbonate. Chem. Sci. 2, 256–260 (2011). [Google Scholar]
- 33.Busschaert N et al. Tripodal transmembrane transporters for bicarbonate. Chem. Commun. (Camb.) 46, 6252–6254 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Davis JT et al. Using small molecules to facilitate exchange of bicarbonate and chloride anions across liposomal membranes. Nat. Chem 1, 138–144 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Busschaert N et al. Synthetic transporters for sulfate: a new method for the direct detection of lipid bilayer sulfate transport. Chem. Sci 5, 1118–1127 (2014). [Google Scholar]
- 36.Denmark SE, Williams BJ, Eklov BM, Pham SM & Beutner GL Design, Validation, and Implementation of a Rapid-Injection NMR System. J. Org. Chem. 75, 5558–5572 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Thomas AA & Denmark SE Pre-transmetalation intermediates in the Suzuki-Miyaura reaction revealed: The missing link. Science 352, 329–332 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Davis SA et al. C3-OH of Amphotericin B Plays an Important Role in Ion Conductance. J. Am. Chem. Soc. 137, 15102–15104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pezzulo AA et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho DY, Hajighasemi M, Hwang PH, Illek B & Fischer H Proton secretion in freshly excised sinonasal mucosa from asthma and sinusitis patients. Am. J. Rhinol. Allergy 23, e10–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho DY, Hwang PH, Illek B & Fischer H Acid and base secretion in freshly excised nasal tissue from cystic fibrosis patients with DeltaF508 mutation. Int. Forum Allergy Rhinol. 1, 123–127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer H & Widdicombe JH Mechanisms of Acid and Base Secretion by the Airway Epithelium. J. Membr. Biol. 211, 139–150 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer H Function of Proton Channels in Lung Epithelia. Wiley Interdiscip. Rev. Membr. Transp. Signal 1, 247–258 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devor DC et al. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J. Gen. Physiol. 113, 743–760 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myerburg MM et al. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 42, 676–684 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worthington EN & Tarran R Methods for ASL measurements and mucus transport rates in cell cultures. Methods Mol. Biol. 742, 77–92 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Schneider CA, Rasband WS & Eliceiri KW NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang XX et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Invest. 126, 879–891 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


