Abstract
Mounting evidence suggests that inflammation is important in epileptogenesis. Particularly Interesting New Cysteine Histidine-rich (PINCH) protein is a highly conserved, LIM-domain protein known to interact with hyperphosphorylated Tau. We assessed PINCH expression in resected epileptogenic human hippocampi and further explored the relationships among PINCH, hpTau and associated kinases. Resected hippocampal tissue from 7 patients with mesial temporal lobe epilepsy (MTLE) was assessed by Western analyses to measure levels of PINCH and hyperphosphorylated Tau, as well as changes in phosphorylation levels of associated kinases AKT and GSK3β in comparison to normal control tissue. Immunolabeling was also conducted to evaluate PINCH and hpTau patterns of expression, co-localization and cell-type specific expression. Hippocampal PINCH was increased by 2.6 fold in the epilepsy cases over controls and hpTau was increased 10 fold over control. Decreased phospho-AKT and phospho-GSK3β in epilepsy tissue suggested involvement of this pathway in MTLE. PINCH and hpTau co-localized in some neurons in MTLE tissue. While PINCH was expressed by both neurons and astrocytes in MTLE tissue, hpTau was extracellular or associated with neurons. PINCH was absent from the serum of control subjects but readily detectable from the serum of patients with chronic epilepsy. Our study describes the expression of PINCH and points to AKT/GSK3β signaling dysregulation as a possible pathway in hpTau formation in MTLE. In view of the interactions between hpTau and PINCH, understanding the role of PINCH in MTLE may provide increased understanding of mechanisms leading to inflammation and MTLE epileptogenesis and a potential biomarker for drug-resistant epilepsy.
Keywords: PINCH protein, Hyperphosphorylated Tau, Mesial temporal lobe epilepsy, Hippocampal sclerosis
1. Introduction
Mesial temporal lobe epilepsy (MTLE) is the most common form of epilepsy in adults and can result in hippocampal sclerosis [11]. A large percentage of patients suffering from MTLE have a history of a prior injury such as prolonged febrile seizure, status epilepticus, head trauma, or meningitis in the first 4–5 years of life that may play a role in epileptogenesis [6,8,54]. Pathological studies of MTLE specimens typically reveal atrophy and gliosis within the hippocampus associated with loss of inhibitory neurons and aberrant sprouting of mossy fibers with granular cell axons in the dentate gyrus [9,46]. Tau is a microtubule-associated protein that is ubiquitously expressed in the brain where it assembles and stabilizes microtubules in neuronal axons. Accumulation of hyperphosphorylated (hp)Tau is implicated in more than 20 neuropathological diseases including Alzheimer’s disease (AD) [30], HIV encephalitis (HIVE) [2,40,42], chronic traumatic brain injury [45] and fronto-temporal dementia (FTD) [45,56]. The pathology associated with aberrant hpTau involves its dissociation from microtubules and accumulation of paired helical filaments (PHF) and neurofibrillary tangles (NFT) in neurons and in some cases astrocytes [7]. Recent studies in rodent and fruit fly models of epilepsy have also shown that reducing Tau decreases hyperexcitability in neural networks [15,23,41]. In a large post-mortem analysis of 138 patient with chronic epilepsy, neurofibrillary tangles were identified in mesial temporal structures with a density and distribution equivalent to Braak stages III/IV [49]. Similar findings of hyperphosphorylated tau were recently reported in resected human mesial temporal lobe tissue from patients with a prolonged history of drug-resistant epilepsy (39.5 years) [47].
Multiple kinases and phosphatases have been linked to abnormal phosphorylation of Tau [7] and may also play a role in epileptogenesis. One such protein is Particularly Interesting New Cysteine Histidine-rich protein or PINCH. PINCH is a highly conserved, LIM-domain only protein consisting of five double zinc finger LIM domains [28]. PINCH expression is significantly increased during development where it functions in cell migration, attachment to the extracellular matrix (ECM), and to maintain cellular polarity [32,33]. In healthy adult mammals, PINCH expression in the central nervous system is nearly undetectable [44]. However, like hpTau, PINCH is robustly expressed by neurons and to a lesser degree by astrocytes in neurodegenerative diseases that include HIV, AD, and FTD [27,40,44]. In fact, recent studies show that PINCH interacts with hpTau and may influence its clearance via interactions with the heat shock machinery [40]. Recent studies in HIV show that changes in CSF levels of hpTau are mirrored by changes in PINCH [1].
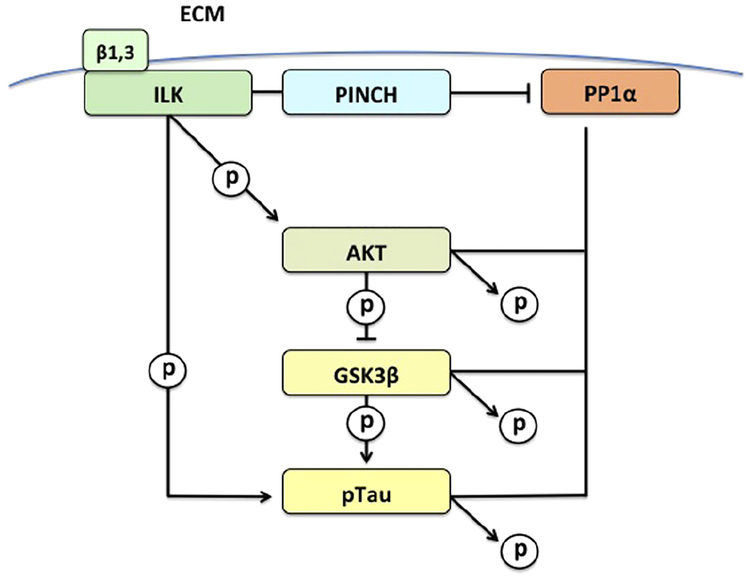
PINCH has been shown to regulate kinases such as integrin linked kinase (ILK), GSK3band AKT, and protein phosphatase 1α (PP1-α) [10,24,25,26], all of which are involved in Tau phosphorylation and de-phosphorylation (Fig. 1). PINCH-dependent AKT activation and GSK3β inhibition occurs when PINCH binds to and inhibits the activity of PP1α [10]. PP1α stabilizes microtubules by maintaining the balance of Tau phosphorylation and dephosphorylation, and is considered the rate-limiting enzyme in the regulation of microtubule associated protein-microtubule interactions. Moreover, PP1α can inhibit ILK-integrin signaling by associating with the β1subunit. When ILK is inactive, GSK3β becomes active and phosphorylates Tau at residues corresponding those detected in diseases including AD and HIVE [3,25,37,42]. Changes in expression of PINCH may impact kinase and phosphatase activities and Tau phosphorylation. Given these multiple levels at which hpTau and PINCH interact, the relationship between PINCH, hpTau, AKT and GSK3β were analyzed in hippocampus and para-hippocampal tissue from patients with intractable MTLE.
Fig. 1.
Proposed PINCH signaling pathway involved in hpTau accumulation in MTLE.
2. Materials and methods
2.1. Human brain tissue
Human brain tissue was obtained during surgery from 7 patients suffering from drug-resistant MTLE, 6 with hippocampal sclerosis and 1 with a focal cortical dysplasia without hippocampal sclerosis. The studies were conducted in accordance with the University of Southern California’s and Temple University’s Human Subjects Protections and Institutional Review Boards. None of these patients had other known neurological disorders such as FTD, HIVE, or AD (see below). Patients provided written informed consent based on the research protocol approved by the IRB of the University of Southern California. All samples consisted of resected tissue from the mesial temporal lobe, including hippocampus and para-hippocampal gyrus. Approximately 15–20 mm3 of the hippocampal head and body was dissected en bloc, and immediately placed at −80 °C or fixed in formalin until analyses. All cases had detailed clinical histories. Control tissue was obtained from 4 post-mortem age-matched patients without a clinical history of CNS disorders, CNS infections or seizures from the California NeuroAIDS Tissue Network or Alzheimer’s disease Research Center tissue repositories at the University of California under the guidelines of UC San Diego Human Subjects Protections and Institutional Review Boards (Table 1).
Table 1.
Clinical information for cases in the study.
| Epilepsy | Sex, race | Neuropathology | Age at surgery | Age at first seisure | Years of seizure | Seizure type | Seizure burden | Risk factors | No of AEDS | Failed AEDS | Radiology results | Surgery type | Surgery outcome | Pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MH | Hippcampal sclerosis | 33 | 9 | 24 | Focal w aloe (CPS) | 1–3/day | Febrile szs | 3 depakote, lamictal, topamax | 1 unknown in mexico | RMTS | R temp selective | 1 yr post op sz free ILAE class 1 | Hippcampal sclerosis | |
| FH | Hippcampal sclerosis | 56 | 25 | 31 | Focal w aloe (CPS) | 1–3/day | None known | 2 trileptil, keppra | 3 vimpat, topamax, depakote | R cyst with mild edema | Small right hippocampal cyst, multiple areas of subtle flair including right insular, likely secondary to VPS, NCC | 1 yr post op class 1 | Hippcampal sclerosis | |
| MH | Hippcampal sclerosis | 50 | 17 | 33 | Focal w aloe (CPS) | 1–3/month | None known | 1 tegretol | 3 dilantin, keppra, depakote | LMTS | L temp selective | 1 yr post op class 1 | Hippcampal sclerosis | |
| MH | Focal cortical dysplasia.no sclerosis | 28 | 22 | 6 | Focal w aloe (CPS) | 1–3/day | None known | 2 keppra, depakote | 1 dilantin | R cortical dysplasia | R temp standard following phase2 | 1 yr post op class 3, one sz | Focal cortical dysplasia, no sclerosis | |
| MH | Left temporal sclerosis | 44 | 3 | 41 | Focal w aloe (CPS) +GTC | 1–6/week, +1–3 month | Head trauma aged 3 | 2 keppra, vimpat | 2, depakote, keppra | LMTS | L temp selective | 1 yr post op class 1 | Left temporal sclerosis | |
| MH | Hippcampal sclerosis | 51 | 9 | 42 | Focal w aloe (CPS) | 1–6/week | Head trauma aged 9, second head trauma no age noted | 2 depakote, vimpat | 6 phenobarbitol, dilantin, tegretol, vimpat, topamax, keppra | BMTS, R cortical dysplasia, R temp pole atrophy | R temp selective | 1 year post op class 2, only auras | Hippcampal sclerosis | |
| MH | Hippcampal sclerosis | 45 | 14 | 31 | Focal w aloe (CPS) | 1–6/week | None known | 1 keppra | 4, primidone, tegretol, depakote, topamax | RMTS, left bone enhancing lesion | R temp selective | 1 yr post op class 1 | Hippcampal sclerosis | |
| Controls | Age at death | |||||||||||||
| FH | None | 30 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| FH | None | 30 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| MW | None | 53 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| F | None | 26 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
M-Male, F-Female, H-Hispanic, W-White, B-Black.
cps: Complex partialszs: simple partial szs, generalized tonic clonic.
cps: Complete partial seizure.
ILAE classification: class1 sz free, class2 only auras, class 3 1–3 szs per year, may have auras.
2.2. Protein extraction from tissue
Frozen brain tissue was homogenized by mechanical disruption on ice in TNN buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.5% NP40, 1:200 protease inhibitor cocktail) (Calbiochem, San Diego, CA, USA). Once completely homogenized, samples were centrifuged at 13,000×g at 4 °C for 5 min. The supernatant containing the protein was collected and protein concentrations were determined via the Bradford assay.
2.3. Blood collection and analyses
Whole blood from 7 volunteers enrolled in the USC Comprehensive Epilepsy Center and 5 controls with no history of seizures was collected in accordance with the University of Southern California’s and Temple University’s Human Subjects Protections and Institutional Review Boards. Participants provided written informed consent based on the research protocol approved by the IRB of the University of Southern California. Seven milliliters of whole blood were collected into vacutainer tubes without anti-coagulants (Bec-ton Dickinson Vacutainer, Franklin Lakes, NJ, USA). Tubes containing whole blood were incubated upright at room temperature for 30 min to allow clotting. The serum was separated by centrifugation at 1500× g for 10 min at 20 °C. All samples were processed within 1 h after phlebotomy and serum was stored at −20 °C in 1 mL aliquots until analyses. Serum samples were subjected to Albumin/IgG depletion using the Pierce® Albumin/IgG Removal Kit according to manufacturer’s instructions (Pierce Scientific, Rockford, IL, USA). An equal volume of depleted serum samples were loaded per well and assessed by standard Western analyses as described below.
2.4. Western analyses
Equal amounts of protein from brain tissue (30 μg) or an equal volume of IgG/albumin depleted serum (10 μl) were loaded per well into 4–12% Bis- Tris pre-cast mini or midi-gels (Invitrogen, Carlsbad, CA, USA), separated by electrophoresis and transferred onto nitrocellulose membranes. Membranes were blocked in 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for 30 min before incubation with primary antibodies. Primary antibodies were used at 1:1,000 for Western analyses unless otherwise indicated. Antibodies included: PINCH1, (BD, Rockville, MD, USA), pAKT serine 473 (Cell Signaling, Danvers, MA, USA), total AKT (Cell Signaling), pGSK3β-S9, pGSK3β-Y216 (Cell Signaling), total GSK3β (SCBT, Santa Cruz, CA, USA), GAPDH (1: 5,000) (SCBT), or Grb-2 (Cell Signaling). Anti-Tau antibodies from Thermo-Fischer (Thermo-Fischer, Pittsburgh, PA, USA) included: HT7 against human total Tau, AT8 (detects phospho- S202, -T205, -S199, -S208) and AT100 (detects phospho-S121, -T214) against PHF-Tau. Other hpTau specific antibodies included phospho-S262 and -S396 (Abcam, Cambridge, MA, USA). Membranes were incubated with primary antibodies for either 2 h at room temperature or overnight at 4 °C, washed 3X in 1X TBST and incubated with the appropriate secondary anti-mouse or -rabbit antibodies (1:10,000; Abcam) for 1 h at room temperature, and developed with ECL or ECL PRIME (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Band intensities were calculated using ImageJ software [43] and normalized to loading controls, Grb-2 or GAPDH.
2.5. Immunohistochemical labeling
Formalin-fixed, paraffin-embedded hippocampal tissue from MTLE (n = 3) and control participants (n = 3) were processed for immunolabeling. Five μm serial tissue sections were rehydrated through ethanol to water, processed in citrate buffer for antigen retrieval and blocked with 5% normal horse serum in PBS, 0.1% BSA for 2 h. Slides were incubated with appropriate pairs of primary antibodies at 1:200 in PBS, 0.1% BSA overnight in a humidified chamber at room temperature. Primary antibodies included anti-PINCH (BD), anti-Tau S396 (Thermo-Fischer), MAP2 (Cell Signaling), neurofilament (Covance, San Diego, CA, USA), GFAP (Abcam), and Iba-1 (Abcam). After primary antibody incubation, slides were rinsed three times with 1X PBS, and then incubated with combined fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:500) and Texas-red isothiocyanate (TRITC)-tagged secondary antibodies (1:500) (Thermo-Fisher) for 2 h at room temperature in the dark. After washing 3 times with 1X PBS, sections were cover slipped with an aqueous based mounting media containing DAPI for nuclear labeling (Vector Laboratories), visualized with the Leica DMI6000 and analyzed with Leica Application Suite (LAS) deconvolution software.
2.6. Statistical analyses
Statistical analyses of band densities from Western blots measured with ImageJ were conducted using the Prism 4 GraphPad software program (GraphPad Software, Inc.). Data were analyzed either by one-way ANOVA with post hoc analyses or one-tailed paired student’s T-test where appropriate and results were considered significant if p < 0.05, n ≥ 3.
3. Results
3.1. PINCH and hpTau levels are significantly increased in resected hippocampal tissues from MTLE patients
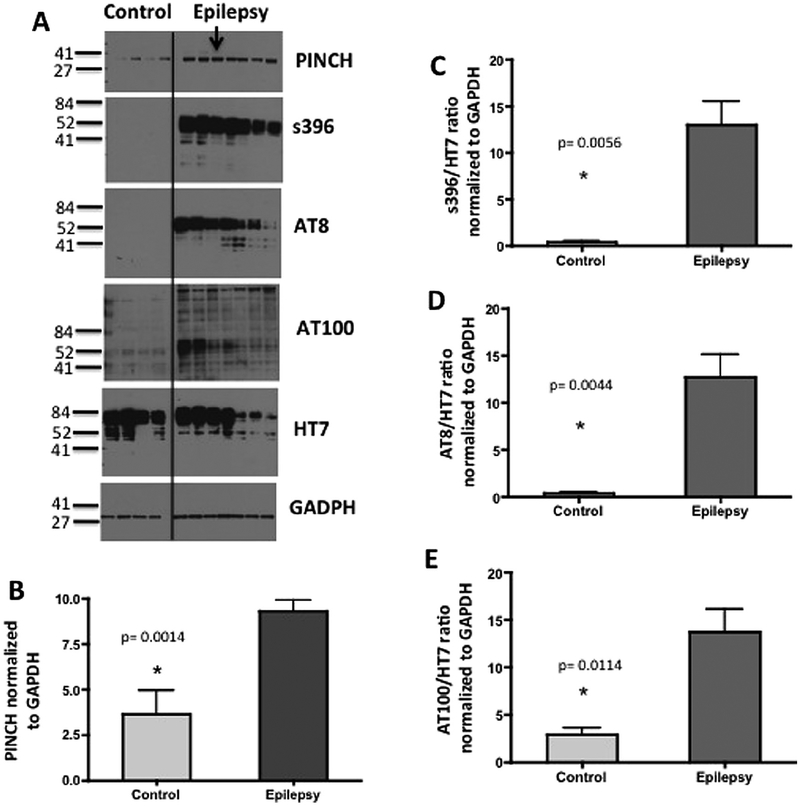
To assess if PINCH and hpTau are elevated in epilepsy, Western analyses were conducted on proteins collected from resected hippocampal tissue of patients undergoing surgical intervention for intractable temporal lobe epilepsy and compared to levels in hippocampal tissue from controls brains. Both PINCH (PINCH, p = 0.00014) and hpTau levels were increased significantly in epilepsy cases (n = 7) compared normal age-matched control tissues (n = 4), (Fig. 2A, C–E). Three different hpTau antibodies that recognize phosphorylation of specific amino acid residues were utilized and include S396 (p = 0.0056), AT8 (p = 0.0044) and AT100 (p = 0.0114), indicating that in MTLE tissues Tau is phosphorylated at sites specifically associated with pathology [4,52].
Fig. 2.
PINCH and hpTau levels are significantly increased in MTLE. (A) Representative Western blot shows increased PINCH and hpTau in epilepsy (n = 7) versus control (n = 4) tissues. Arrow indicates the case with out sclerosis. Membranes containing 20 μg/lane of total protein were probed with anti-PINCH antibody, and anti-Tau antibodies: S396, AT8 and AT100 and HT7 and GAPDH as a loading control. (B) Graphic representation of fold change of PINCH over GAPDH,*p = 0.0014 by unpaired t-test. (C) Graphic representation of fold change of hpTau (S396) over total Tau (HT7),*p = 0.0056 by unpaired t-test. (D) Graphic representation of fold change of hpTau (AT8) over total Tau (HT7), *p = 0.0044 by unpaired t-test. (E) Graphic representation of fold change of hpTau (AT100) over total Tau (HT7), *p = 0.0114 by unpaired t-test.
3.2. PINCH and hpTau co-localize in some neurons in MTLE tissues
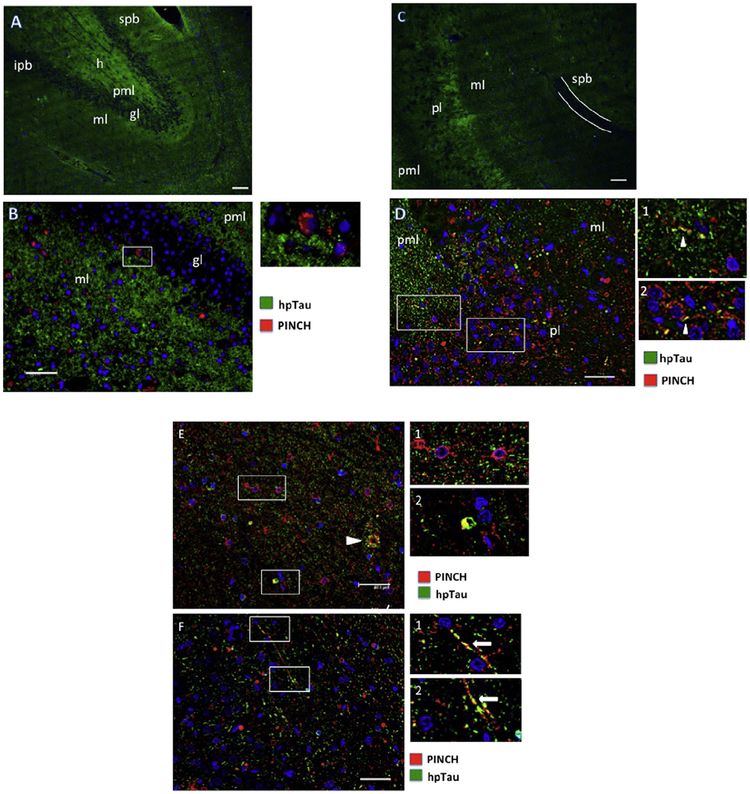
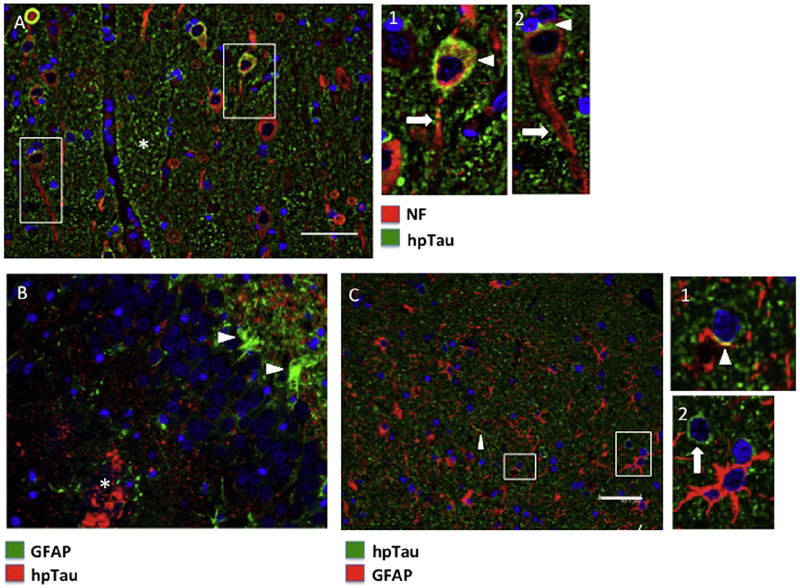
We next assessed patterns of hpTau and PINCH protein expression in resected mesial temporal lobe tissues, including hippocampus and para-hippocampal gyrus from MTLE patients. Double immunofluorescence labeling of tissues revealed both regional and cell-type specific patterns of expression. The dentate gyrus showed an even and ubiquitous distribution of hpTau (green), especially in the hilus, molecular and polymorphic layers, with the granular cell layer being largely spared (Fig. 3A). Double immunolabeling and higher magnification show regions where hpTau fills the extracellular space, with low levels of PINCH (red) detected in association with cell bodies in the molecular and granular layers (Fig. 3B), again with sparing of the dentate gyrus granular layer.
Fig. 3.
Patterns of PINCH and hpTau expression in the hippocampus from representative MTLE cases. (A) hpTau expression pattern (green) in the dentate gyrus. (B) Double immunolabeling for PINCH (red) and hpTau (green) in the dentate gyrus. Inset shows a PINCH immunoreactive cell at the junction of the molecular and granular layers. (C) hpTau pattern (green) in the CA1/2 region of the hippocampus. The white line indicates the suprapyramidal blade of the dentate gyrus. (D) Double immunolabeling of PINCH (red) and hpTau (green) in the CA2 region of the hippocampus. Insets indicate some areas of hpTau/PINCH co-localization (arrowheads) with cellular processes. (E) PINCH (red) and hpTau (green) double immunolabeling in the CA1/2 region of the hippocampus. Arrowhead indicates extracellular accumulation of PINCH and hpTau. Inset 1, PINCH labeling of small neurons. Inset 2, PINCH/hpTau co-localization in a small cell. (F) PINCH and hpTau co-localization in long cellular processes in the CA1/2 region, arrows, insets). Nuclei are labeled blue with DAPI. spb, suprapyramidal blade of dentate gyrus; h, hilus; ipb, infrapyramidal blade of dentate gyrus; pml, polymorphic layer; ml, molecular layer; gl, granular layer; pl, pyramidal layer. Scale bars ≈ 40 μm.
In CA1/2 regions, hpTau (green) is detected in molecular, pyramidal and polymorphic cell layers, again with sparing of the dentate gyrus granular layer as shown in the suprapyramidal blade outlined in white (Fig. 3C). Double immunolabeling of PINCH (red) and hpTau (green) indicate co-localization (arrowheads, Fig. 3D, insets 1, 2) in the CA1/2 region with PINCH being primarily cell-associated and hpTau both extracellular and intracellular. PINCH and hpTau co-localize and appear to associate with cellular bodies and processes of small cells morphologically similar to neurons (Fig. 3D, F, insets). In some cases, PINCH and hpTau co-localize with structures reminiscent of tangles (Fig. 3E, arrowhead). These results show that while both PINCH and hpTau are highly expressed in the hippocampus of MTLE patients, and may co-localize, distinct regional distribution patterns are observed.
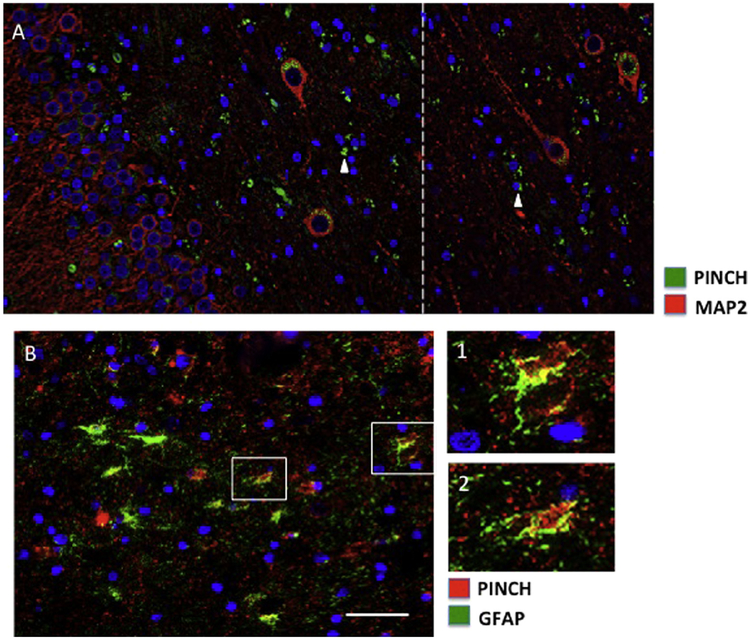
3.3. PINCH is expressed by neurons and astrocytes in MTLE
Previous studies in other neurodegenerative diseases including HIVE and AD confirm hpTau and PINCH expression primarily by neurons and to a lesser extent, by astrocytes [27,40,44]. Cell-type specific immunolabeling of PINCH and hpTau in MTLE indicated that PINCH is expressed by both neurons (Fig. 4A) and astrocytes (Fig. 4B). PINCH was detected in the soma of large MAP2 positive neurons located in the hilus region of the dentate gyrus, with labeling of smaller cells that are not MAP2 positive (Fig. 4A, arrowheads). PINCH was robustly expressed by GFAP positive astrocytes in the CA1/2 region with significant co-localization with hpTau (Fig. 4B, insets). These data show clear evidence of PINCH production by both neurons and astrocytes in MTLE.
Fig. 4.
PINCH expression in neurons and astrocytes in the hippocampus from representative MTLE cases. (A) Double immunolabeling of PINCH (green) and MAP2 (red) in large neurons in the hilus region of dentate gyrus. The white-hatched line indicates the junction of image stitching. PINCH is detected in MAP2 negative smaller cell bodies and processes (arrowheads). (B) PINCH (red) and GFAP (green) co-localize in the CA1/2 region of the hippocampus (insets 1 and 2). Nuclei are labeled blue with DAPI. Scale bars ≈ 40 μm.
3.4. hpTau is detected largely in the extracellular space and in association with neurons
Neurons appear to be the primary cell type expressing hpTau (Fig. 5A), with little hpTau co-localizing with astrocytes (Fig. 5B, C). Pyramidal neurons positive for both hpTau and neurofilament are observed in the CA1/2 region. As expected, hpTau is not detected robustly in neurofilament positive axons (Fig. 5A, insets arrow), but is associated with the neuronal soma (Fig. 5A, insets arrowhead). However, some hpTau associated with axonal processes is observed in damaged/dystrophic axons (Fig. 5A, inset 1, arrow). In addition, a large portion of hpTau appears to be extracellular (Fig. 5A, asterisk). hpTau is also detected in regions with highly reactive astrocytes (Fig. 5B, arrowheads) in the molecular layer of the dentate gyrus. Accumulated hpTau is detected the polymorphic layer of the dentate gyrus as well (Fig. 5B, asterisk). Less common were hpTau associations with cellular processes positive for GFAP (Fig. 5C, inset 1, arrowhead). In the CA1/2, hpTau appears largely extracellular, with few hpTau positive cell bodies (Fig. 5C, inset 2, arrow) and processes (Fig. 5C, arrowhead).
Fig. 5.
hpTau expression in neurons and astrocytes in the hippocampus from representative MTLE cases. (A) Double immunolabeling of hpTau (green) and neurofilament (red) in the CA1/2 region. Asterisk indicates largely extracellular hpTau. Insets indicate hpTau in neuronal soma (arrowheads) with little to no detection in the axon (arrow). (B) Double immunolabeling of astrocytes (GFAP, green) and hpTau (red). Reactive astrocytes (arrowheads) are adjacent to hpTau, but no co-localization is observed. Asterisk indicates accumulation of hpTau in the polymorphic layer of the dentate gyrus. (C) In the CA1/2 region, GFAP (red) and hpTau (green) co-localization is minimal (inset 1, arrowhead) and two clearly distinct cell populations are labeled (inset 2). Nuclei are labeled blue with DAPI. Scale bars ≈ 40 μm.
3.5. Key kinases in hpTau formation are dysregulated in MTLE
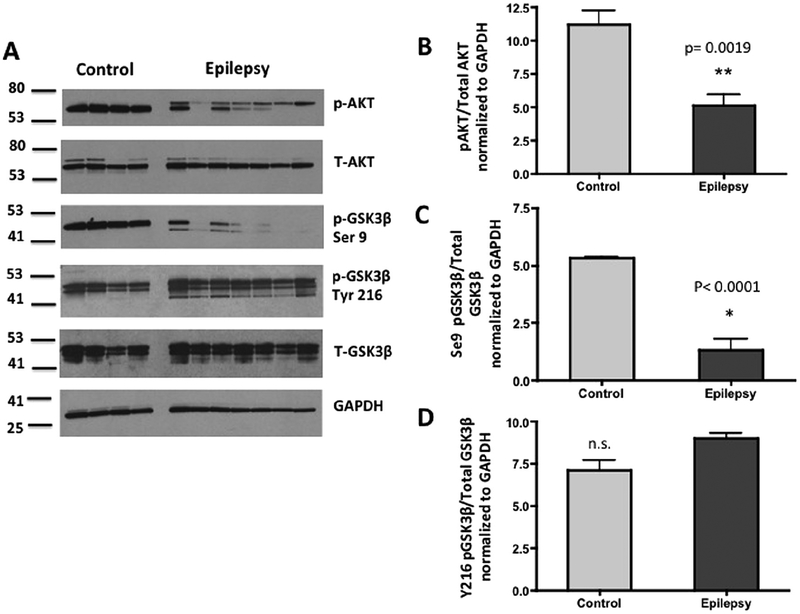
Next, we explored the potential relationships among levels of PINCH, hpTau and phosphorylation states of AKT and GSK3β, two important kinases involved in Tau hyperphosphorylation (Fig. 1). AKT is active when it is phosphorylated at serine 273 and phosphorylates GSK3β at serine 9, rendering the latter inactive. Levels of pAKT at serine 473 and pGSK3β at serine 9 in epilepsy tissues are significantly reduced (p = 0.0019 and p < 0.0001, respectively) (Fig. 3A–C). Thus, GSK3β that is not phosphorylated at serine 9 is active and coincides with increased hpTau levels (Fig. 2C–E). On the other hand, phosphorylation of GSK3β at tyrosine 216 activates it, but levels are not significantly different in epilepsy cases compared to control (Fig. 6A, D). Taken together these results show that hpTau and PINCH are increased significantly in hippocampal tissues from MTLE patients and that AKT/GSK3β signaling pathways are altered as well. These data link for the first time, PINCH and hpTau in epilepsy and illustrate dysregulation of the AKT/GSK3β pathway.
Fig. 6.
Levels of phosphorylated AKT and GSK3β are significantly decreased in MTLE compared to control. (A) Representative Western blot shows decreased pAKT, pGSK3β-S9 in epilepsy (n = 7) versus control (n = 4) tissues. Arrow indicates the case with out sclerosis. Membranes containing 20 μg/lane of total protein were probed with anti-pAKT serine 473 (ser273), total AKT, pGSK3β serine 9 (ser9), pGSK3β tyrosine 216 (tyr216), total GSK3β antibodies and GAPDH antibody as a loading control. (B) Graphic representation of the ratio of pAKT to total AKT (**p = 0.0019), and pGSK3β-S9 to total GSK3β (p, 0.0001) normalized to GAPDH by unpaired t-test. No significant differences (n.s.) were observed in levels of pGSK3β at tyrosine 216 (Tyr216).
3.6. PINCH is detected in the serum from epilepsy patients but not in control participants
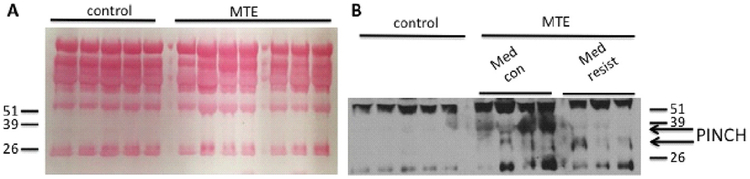
Compromise of the blood brain barrier (BBB) in seizures has been extensively studied although there is debate as to whether seizures cause BBB disruption or if BBB disruption leads to seizures (for review see [38]). Western analyses of the serum of 7 well-characterized patients with MTLE (4 medically controlled with AED and 3 with medically intractable epilepsy) enrolled at the USC Comprehensive Epilepsy Center tested positive for PINCH immunoreactivity in blood (Fig. 7A, B); whereas, controls (n = 5) were negative.
Fig. 7.
PINCH is detectable in serum from epilepsy patients. (A) Ponceau red staining of the membrane shows even loading. (B) Western blot shows PINCH levels in epilepsy serum samples (n = 7) versus control (n = 4). Membranes containing 10 μl/lane of IgG/Albumin depleted serum were probed with anti-PINCH antibody.
Taken together these results show that PINCH is increased in the brains and blood of MTLE patients but is absent in normal controls.
4. Discussion
By analyzing mesial temporal lobe structures resected from a relatively young cohort of patients with drug-resistant epilepsy, we have demonstrate that PINCH and hpTau levels are significantly increased in epileptogenic tissues and co-localize in neurons. Accumulation of hpTau is a common feature of many neurodegenerative diseases including HIVE, AD, and FTD, and has only recently been reported in mesial temporal tissues of post-mortem studies or patient with a prolonged course of drug-resistant epilepsy [49,47]. The present study confirms and extends the finding of hpTau to a younger cohort of patients with a shorter duration of epilepsy, and demonstrates the correlation of hpTau with PINCH and associated kinases such as phospho-AKT and phospho-GSK3β in epileptogenic mesial temporal tissues. Our data show that both hpTau and PINCH are significantly increased in MTLE tissues compared to controls and are accompanied by changes in AKT/GSK3 phosphorylation levels that have been shown to promote Tau hyperphosphorylation [7]. Finally, we have also shown that while PINCH is not present in the serum of control individuals, it is readily detectable in serum of patients with chronic epilepsy.
Recently identified as a binding partner of hpTau, the PINCH protein [1,27,40,44] is involved in protein recruitment, protein complex assembly and shuttling throughout the cell (for review, see [29]). During brain development, PINCH is expressed at high levels and functions in cytoskeletal organization and communication between the cell and the extracellular matrix [32,33]. PINCH is nearly undetectable in healthy adult brain tissue, and appears to be induced by injury or inflammation [27,44]. PINCH is a regulator in part of Tau phosphorylation since in vitro studies showed that shRNA knockdown of PINCH decreased the levels of hpTau even after prior treatment with okadaic acid, a well-known inducer of hpTau [40]. Further studies revealed that PINCH and hpTau bind one another, and that this interaction may be important for the regulation of hpTau’s interaction with heat shock machinery [40]. In other studies, changes in the levels of PINCH and hpTau in CSF of HIV patients were shown to correlate directly with one another [1]. Taken together, results from this study and others support an important role for PINCH in tau phosphorylation.
PINCH is a key modulator of several kinases involved in Tau phosphorylation and de-phosphorylation such as integrin linked kinase (ILK), AKT and protein phosphatase 1α (PP1-α) [10,24,25,26], and is therefore critical for maintaining neuronal structure and fitness (Fig. 1). For example, the LIM1 domain of PINCH binds to ILK in the intracellular compartment and to β1, β3 integrin at the cell surface to ensure proper communication among the cytoskeleton, focal adhesions and the ECM. In the absence of PINCH, ILK cannot localize to focal adhesions [13,17,31,55,57,58], thereby disrupting the cytoskeleton, cell migration and attachment.
Studies of resected tissues have provided valuable information regarding cellular networks involved in MTLE [9,11,12,46,50,51,34]. However, the advanced stage of neuropathology in these tissues limits their use to address mechanisms that may contribute to seizure onset and epileptogenesis.
Of the 7 cases studied by Western analyses, 1 case (Figs. 2A, 3A, arrow) showed focal cortical dysplasia with no evidence of hippocampal sclerosis. Incidentally, this participant also represented the case with fewest years living with seizure disorder (6 years) (Table 1). Even in the absence of sclerosis, levels of PINCH and hpTau were elevated at levels similar to cases with sclerosis. These results suggest that changes in PINCH and hpTau may be generally related to the process of epileptogenesis rather than the development hippocampal sclerosis per se. Moreover, decreased levels of phosphorylated AKT (ser273) indicating decreased activity, and of phosphorylated GSK3β (ser9) indicating increased activity (Fig. 3) are observed in both sclerotic and non-sclerotic cases pointing to ongoing dysregulation of these pathways that may contribute to increased hpTau.
Many studies have addressed possible connections between chronic seizure disorder and rapid brain aging that may increase risk for AD or other dementias [5,14,16,18,19,20,21,22,35,36,39,48]. One large post-mortem study by Thom et al. reported that hippocampal sclerosis in chronic epilepsy was linked to moderate asymmetrical Tau accumulation rather than changes associated with Braak staging [49]. More recently, a pattern of Tau pathology similar to both Alzheimer’s dementia and chronic traumatic encephalopathy was observed in study of resected hippocampi from “elderly” patients with chronic epilepsy who underwent mesial temporal lobe resection between 50 and 65 years of age [47]. Interestingly, the extent of Tau pathology correlated with post-operative verbal learning measures. In this context, previous studies addressing PINCH in neurodegenerative disease such as HIV, have proposed that along with hpTau accumulation, PINCH may also contribute to rapid brain aging. Results from our study support these findings, but also demonstrate similar patterns of Tau/PINCH pathology earlier in the course of chronic epilepsy. Given the multiple levels at which hpTau and PINCH interact, our findings that PINCH is highly expressed in MTLE may lead to increased understanding of underlying cellular mechanisms leading to neuronal injury and epileptogenesis.
Given the correlation between PINCH and hpTau, our finding that PINCH is readily detectable in serum derived from patients with epilepsy, yet absent from the serum of control subjects, is particularly interesting. Most intriguing is the possibility that PINCH may serve as a biomarker in epilepsy, similar to other proposed molecules shown to be elevated in patient with epilepsy such as matrix metalloproteinase-3 (MMP-3) and circulating micro-RNAs [53].
Several limitations exist within the current study. First, as with all studies conducted on resected surgical tissues, transoperative variables such as anesthesia duration and drugs, hemodynamic changes, time of hippocampal vascular supply coagulation were uncontrolled. Second, times between last seizure and surgery may have been variable among the MTLE cases. Third, times between tissue collection and freezing and biochemical assays were different. Finally, tissues from controls were collected post-mortem and differences in tissue preservation may exist. However, given the consistency across groups our data provide strong evidence of dysregulation of the PINCH-AKT-GSK3β signaling pathway that may have contributed to significantly increased hpTau levels in MTLE.
Our study provides evidence of PINCH’s involvement in MTLE and points to dysregulation of AKT/GSK3β signaling as likely contributing pathways in hpTau formation in these cases. Changes in blood levels of PINCH may provide a surrogate marker for PINCH levels in the brain and, by correlation, hpTau levels. Given the interactions between hpTau and PINCH, understanding the role of PINCH in MTLE may provide increased understanding of inflammatory mechanisms leading epileptogenesis.
Acknowledgments
This work was supported by Athole G. Jacobi, MD and the Marianne Garman Burton Foundation for Caregivers (to D. Langford) and the Rudi Schulte Research Institute.
References
- [1].Adiga R, Ozdemir Ahmet Y, Carides Alexandra, et al. Changes in PINCH levels in the CSF of HIV patients correlate with CD4 count and with hpTau. J Neurovirol 2014;20:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. Int Rev Psychiatry 2008;20:15–24. [DOI] [PubMed] [Google Scholar]
- [3].Anthony IC, Ramage SN, Carnie FW, et al. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 2006;111:529–38. [DOI] [PubMed] [Google Scholar]
- [4].Augustinack JC, Schneider A, Mandelkow EM, et al. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol 2002;103:26–35. [DOI] [PubMed] [Google Scholar]
- [5].Breteler MM, de Groot RR, van Romunde LK, et al. Risk of dementia in patients with Parkinson’s disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol 1995;142:1300–5. [DOI] [PubMed] [Google Scholar]
- [6].Bruton CJ. The neuropathology of temporal lobe epilepsy. New York: Oxford; 1988. [Google Scholar]
- [7].Buee L, Bussiere T, Buee-Scherrer V, et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 2000;33:95–130. [DOI] [PubMed] [Google Scholar]
- [8].Curia G, Lucchi C, Vinet J, et al. Pathophysiogenesis of mesial temporal lobe epilepsy: is prevention of damage antiepileptogenic? Curr Med Chem 2014;21:663–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Lanerolle NC, Brines ML, Kim JH, et al. Neurochemical remodelling of the hippocampus in human temporal lobe epilepsy. Epilepsy Res Suppl 1992;9:205–19. discussion 220. [PubMed] [Google Scholar]
- [10].Eke I, Koch U, Hehlgans S, et al. PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha. J Clin Investig 2010;120:2516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Engel J Jr Mesial temporal lobe epilepsy: what have we learned? Neuroscientist 2001;7:340–52. [DOI] [PubMed] [Google Scholar]
- [12].Engel J Jr, Dichter M, Schwartzkroin P. Basic mechanisms of human epilpepsy In: Engel J Jr, Pedley T, editors. Epilespy: A comprehensive textbook. Philadelphia: Lippincott-Raven; 1997. p. 499–512. [Google Scholar]
- [13].Fukuda T, Chen K, Shi X, et al. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J Biol Chem 2003;278:51324–33. [DOI] [PubMed] [Google Scholar]
- [14].Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia 2004;45:1613–22. [DOI] [PubMed] [Google Scholar]
- [15].Gheyara AL, Ponnusamy R, Djukic B, et al. Tau reduction prevents disease in a mouse model of Dravet syndrome. Ann Neurol 2014;76:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gouras GK, Relkin NR, Sweeney D, et al. Increased apolipoprotein E epsilon 4 in epilepsy with senile plaques. Ann Neurol 1997;41:402–4. [DOI] [PubMed] [Google Scholar]
- [17].Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 1996;379:91–6. [DOI] [PubMed] [Google Scholar]
- [18].Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain 2009;132:2822–30. [DOI] [PubMed] [Google Scholar]
- [19].Hermann B, Seidenberg M. Epilepsy and cognition. Epilepsy Curr/American Epilepsy Society 2007;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hermann B, Seidenberg M, Jones J. The neurobehavioural comorbidities of epilepsy: can a natural history be developed? Lancet Neurol 2008;7:151–60. [DOI] [PubMed] [Google Scholar]
- [21].Hermann B, Seidenberg M, Sager M, et al. Growing old with epilepsy: the neglected issue of cognitive and brain health in aging and elder persons with chronic epilepsy. Epilepsia 2008;49:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hermann BP, Seidenberg M, Dow C, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol 2006;60:80–7. [DOI] [PubMed] [Google Scholar]
- [23].Holth JK, Bomben VC, Reed JG, et al. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J Neurosci 2013;33:1651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ishii T Role of integrin-linked kinase in neuronal cells. Curr Enzym Inhib 2005;1:3–10. [Google Scholar]
- [25].Ishii T, Furuoka H, Muroi Y, et al. Inactivation of integrin-linked kinase induces aberrant tau phosphorylation via sustained activation of glycogen synthase kinase 3beta in N1E–115 neuroblastoma cells. J Biol Chem 2003;278:26970–5. [DOI] [PubMed] [Google Scholar]
- [26].Ishii T, Satoh E, Nishimura M. Integrin-linked kinase controls neurite outgrowth in N1E–115 neuroblastoma cells. J Biol Chem 2001;276:42994–3003. [DOI] [PubMed] [Google Scholar]
- [27].Jatiani A, Pannizzo P, Gualco E, et al. Neuronal PINCH is regulated by TNF-alpha and is required for neurite extension. J Neuroimmune Pharmacol 2010;6:330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol 2004;5:920–31. [DOI] [PubMed] [Google Scholar]
- [29].Kovalevich J, Tracy B, Langford D. PINCH: more than just an adaptor protein in cellular response. J Cell Physiol 2011;226:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee VM, Brunden KR, Hutton M, et al. Developing therapeutic approaches to tau, selected kinases, and related neuronal protein targets. Cold Spring Harb Perspect Med 2011;1:a006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li F, Zhang Y, Wu C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci 1999;112(Pt 24):4589–99. [DOI] [PubMed] [Google Scholar]
- [32].Li S, Bordoy R, Stanchi F, et al. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci 2005;118:2913–21. [DOI] [PubMed] [Google Scholar]
- [33].Liang X, Zhou Q, Li X, et al. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol 2005;25:3056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu J, Reeves C, Michalak Z, et al. Evidence for mTOR pathway activation in a spectrum of epilepsy-associated pathologies. Acta Neuropathol Commun 2014;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol 1994;87:504–10. [DOI] [PubMed] [Google Scholar]
- [36].Marques CM, Caboclo LO, da Silva TI, et al. Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsy Behav: E&B 2007;10:477–85. [DOI] [PubMed] [Google Scholar]
- [37].Mills J, Digicaylioglu M, Legg AT, et al. Role of integrin-linked kinase in nerve growth factor-stimulated neurite outgrowth. J Neurosci 2003;23:1638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia 2006;47:1761–74. [DOI] [PubMed] [Google Scholar]
- [39].Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology 2004;62:1736–42. [DOI] [PubMed] [Google Scholar]
- [40].Ozdemir AY, Rom I, Kovalevich J, et al. PINCH in the cellular stress response to tau-hyperphosphorylation. PLoS ONE 2013;8:e58232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Palmio J, Suhonen J, Keranen T, et al. Cerebrospinal fluid tau as a marker of neuronal damage after epileptic seizure. Seizure 2009;18:474–7. [DOI] [PubMed] [Google Scholar]
- [42].Patrick C, Crews L, Desplats P, et al. Increased CDK5 expression in HIV encephalitis contributes to neurodegeneration via tau phosphorylation and is reversed with Roscovitine. Am J Pathol 2011;178:1646–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rasband WS. ImageJ. Bethesda: NIH; 1997. [Google Scholar]
- [44].Rearden A, Hurford R, Luu N, et al. Novel expression of PINCH in the central nervous system and its potential as a biomarker for human immunodeficiency virus-associated neurodegeneration. J Neurosci Res 2008;86:2535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stern RA, Riley DO, Daneshvar DH, et al. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PMR 2011;3:S460–7. [DOI] [PubMed] [Google Scholar]
- [46].Sutula T, Cascino G, Cavazos J, et al. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 1989;26:321–30. [DOI] [PubMed] [Google Scholar]
- [47].Tai XY, Koepp M, Duncan JS, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain 2016;139:2441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tellez-Zenteno JF, Matijevic S, Wiebe S. Somatic comorbidity of epilepsy in the general population in Canada. Epilepsia 2005;46:1955–62. [DOI] [PubMed] [Google Scholar]
- [49].Thom M, Liu JY, Thompson P, et al. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain 2011;134:2969–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Toth K, Magloczky Z. The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy. Front Neuroanatomy 2014;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Velasco M, Velasco F, Velasco AL, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia 2000;41:158–69. [DOI] [PubMed] [Google Scholar]
- [52].Wang JZ, Xia YY, Grundke-Iqbal I, et al. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis 2013;33(Suppl 1):S123–39. [DOI] [PubMed] [Google Scholar]
- [53].Wang R, Zeng GQ, Liu X, et al. Evaluation of serum matrix metalloproteinase-3 as a biomarker for diagnosis of epilepsy. J Neurol Sci 2016;367:291–7. [DOI] [PubMed] [Google Scholar]
- [54].Wieser HG, Epilepsy ICoNo. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2004;45:695–714. [DOI] [PubMed] [Google Scholar]
- [55].Wu C Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J Cell Sci 1999;112(Pt 24):4485–9. [DOI] [PubMed] [Google Scholar]
- [56].Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007;53:337–51. [DOI] [PubMed] [Google Scholar]
- [57].Zhang Y, Chen K, Tu Y, et al. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci 2002;115:4777–86. [DOI] [PubMed] [Google Scholar]
- [58].Zhang Y, Guo L, Chen K, et al. A critical role of the PINCH-integrin-linked kinase interaction in the regulation of cell shape change and migration. J Biol Chem 2002;277:318–26. [DOI] [PubMed] [Google Scholar]