Abstract
We report on the stereoselective synthesis of both molecular granny and square knots through the use of lanthanide-complexed overhand knots of specific handedness as three-crossing “entanglement synthons”. The composite knots are assembled by combining two entanglement synthons (of the same chirality for a granny knot; of opposite handedness for a square knot) in three synthetic steps: first, a CuAAC reaction joins together one end of each overhand knot. Ring-closing olefin metathesis (RCM) then affords the closed-loop knot, locking the topology. This allows the lanthanide ions necessary for stabilizing the entangled conformation of the synthons to subsequently be removed. The composite knots were characterized by 1H and 13C NMR spectroscopy and mass spectrometry and the chirality of the knot stereoisomers compared by circular dichroism. The synthetic strategy of combining building blocks of defined stereochemistry (here overhand knots of Λ- or Δ-handed entanglement) is reminiscent of the chiron approach of using minimalist chiral synthons in the stereoselective synthesis of molecules with multiple asymmetric centers.
Introduction
Most of the small-molecule knots1 synthesized to date are trefoil2 (31)3 knots, the simplest nontrivial knot topology. The few examples of more complex synthetic molecular knots4,5 are highly symmetrical,6 with the strand crossings assembled in one or two steps using frameworks designed to only tolerate the particular crossing pattern required. Unfortunately, such strategies are not extendable to many knot topologies, which often lack sufficient symmetry in their crossing arrangements. Alternative synthetic approaches will need to be developed in order to access at the molecular level the majority of knot topologies.1a
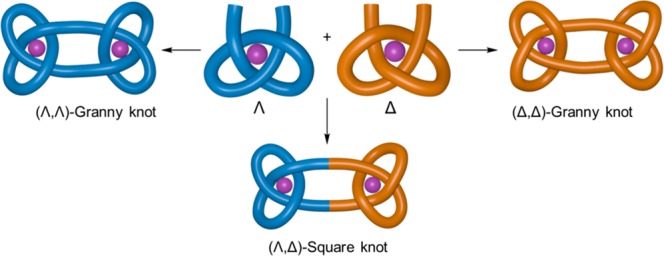
Knots are topologically distinct from each other in terms of the number of times a strand crosses itself, the arrangement (relative stereochemistry) of each crossing compared to other crossings, and the handedness (chirality) of the crossing sequence as a whole.1a,3 Constitutionally identical but differently knotted molecular strands are thus stereoisomers. The stereochemical relationship between strand crossings in knots is somewhat reminiscent of that between asymmetric centers in orthodox organic molecules (Figure 1): the topological stereoisomerism of knots is determined by the relative orientation (over, under) of each strand crossing; conventional organic stereoisomerism is determined by the relative handedness of stereogenic elements (centers, planes, axes, helices, etc.). For example, the dimerization of a molecular fragment with an (R)-asymmetric center gives an (R,R)-molecule, which is the enantiomer of the (S)-building block dimer and a diastereomer of the meso-(R,S)-compound (Figure 1a). In the case of knots, joining two trefoil tangles (a three-crossing entanglement with an overunder-overunder-overunder crossing sequence) of the same handedness (Λ- or Δ-) forms a single enantiomer of a granny knot (−31#–31 or +31#+31),1a,3 whereas combining trefoil tangles of opposite handedness forms the diastereomeric, topologically achiral, square knot (+31#–31)1a,3 (Figure 1b).
Figure 1.
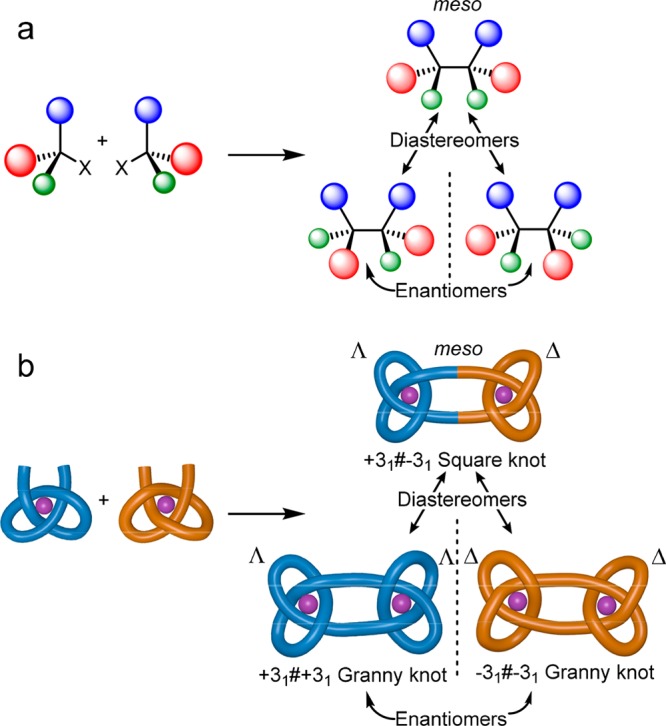
Parallels in the structural relationship between asymmetric centers in chiral molecules and strand crossings in knots. (a) Stereochemical consequences of combining two one-asymmetric-center synthons. (b) Topological consequences of combining two three-crossing entanglement synthons.
Granny and square knots are examples of composite knots, that is closed-loops consisting of two or more ring-opened prime knots joined together.3 The synthesis of composite knots5 is complicated by the difficulty in controlling knotting stereochemistry.1a In the sole reported attempt at the synthesis of composite knots for more than 20 years,5a Sauvage’s group carried out the cyclodimerization of linear Cu(I) helicates. However, there was no attempt to control stereochemistry, and the result was an inseparable mixture of granny and square knots, together with topologically trivial macrocycles.5a Recently, symmetrical interwoven grids5b,7 and circular helicates5c have been used to control the relative stereochemistry of strand crossings in the synthesis of a granny knot and a nine-crossing composite knot.5b,5c However, those strategies did not control absolute stereochemistry (chirality) either, nor are these approaches readily extendable to topologies with less symmetrical crossing patterns.1a,1f
Given the stereochemical parallels between asymmetric centers and strand crossings, we wondered whether strategies used in conventional asymmetric synthesis could be usefully applied to the stereoselective synthesis of different molecular knot topologies. The “chiron approach”8 is a simple but effective approach for constructing molecules with multiple asymmetric centers. Minimalist chiral synthons, usually derived from cheap and readily available natural products, are combined to form the target molecule stereoselectively. However, a complication in trying to extend such an approach from asymmetric centers to strand entanglements is that although most asymmetric carbon atoms are configurationally fixed, an entanglement is a conformation that can be undone by bond rotations in a strand with open ends.
It has previously been shown that tris(2,6-pyridinedicarboxamide) ligands can be tied into overhand knots5a,9 (three-crossing entanglements or “trefoil tangles”) upon coordination to lanthanide(III) ions.10 The handedness of the entanglement can be controlled by introducing asymmetry at the benzylic positions of the ligand backbone: sterics dictate that only the Λ-overhand knot conformation forms from the (R,R,R,R,R,R)-strand, while the (S,S,S,S,S,S)-enantiomer generates the Δ-overhand knot (Figure 2).11 Crucially, as long as a lanthanide ion remains coordinated, the strand entanglement remains in place with its handedness retained. This makes the coordinated unit a potential “entanglement synthon” with open ends for constructing knots by adding together crossings. Once a linear combination of such synthons is macrocyclized to form a closed loop, the crossing sequence will become topologically fixed and the metal ions can be safely removed without the entanglement being able to unravel.
Figure 2.
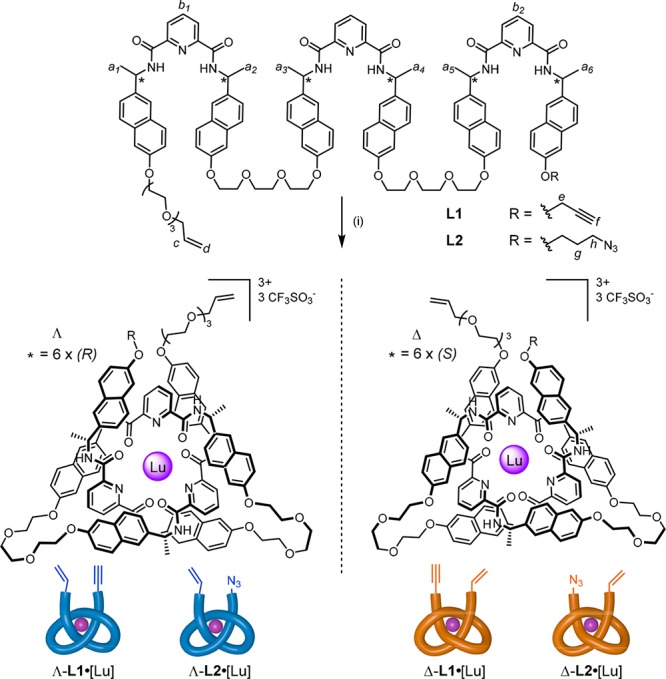
Lanthanide coordination induces folding and threading of L1/L2 to form entanglement synthons: alkene and alkyne- or azide-bearing overhand knots of single handedness (Λ or Δ). Reagents and conditions: (i) Lu(CF3SO3)3, MeCN, 80 °C, 16 h. Yields: 81% (Λ-L1•[Lu]), 82% (Δ-L1•[Lu]), 85% (Λ-L2•[Lu]), and 73% (Δ-L2•[Lu]).
Accordingly, we decided to use lanthanide-coordinated overhand knots as entanglement synthons to selectively construct composite knots with different stereochemistries. A CuAAC coupling step followed by ring closing metathesis (RCM) was used to combine two overhand knots to form open-ended and closed-loop six-crossing strands of precise stereochemical composition.12 The approach was exemplified through the stereoselective synthesis of a topologically achiral square knot, Λ,Δ-1, and the enantioselective synthesis of granny knots, Λ,Λ-1 and Δ,Δ-1 (Figure 2).
Results and Discussion
Suitable ligand strands containing a terminal alkene at one end of the tris(2,6-pyridinedicarboxamide) strand and either alkyne (L1) or azide (L2) groups at the other were prepared as outlined in the Supporting Information. The lengths of the spacers linking the alkene, alkyne, and azide groups to the rest of the strand proved important for achieving efficient macrocyclization to the closed-loop composite knots.13 Each synthon was treated with Lu(CF3SO3)3 in MeCN at 80 °C to generate the corresponding overhand knots, Λ-L1•[Lu], Δ-L1•[Lu], Λ-L2•[Lu], and Δ-L2•[Lu] in 73–85% yields. In all cases, the folding-threading process to form the overhand knot was found to be complete after 16 h, as evidenced by electrospray ionization mass spectrometry (ESI-MS, Figures S4 and S5) and 1H NMR spectroscopy (Spectra S19–S22). Signals diagnostic of the entangled conformations of the L1•[Lu] and L2•[Lu] complexes include large upfield shifts of the pyridine Hb1,b2 protons due to their proximity to aromatic rings in the overhand knots. The handedness of the entanglements, dictated by point chirality in the ligand strands, was confirmed by circular dichroism (Spectrum S35).
To generate the square knot, Λ-L1•[Lu] was first connected to Δ-L2•[Lu] through a CuAAC reaction, giving the double overhand knot (Λ,Δ)-L3•[Lu]2 in 70% yield after purification by size exclusion chromatography (Scheme 1). 1H NMR spectroscopy showed the absence of an alkyne proton Hf at ∼3.0 ppm, together with shifts in the resonances corresponding to the azide α-protons Hh (3.65–4.81 ppm) and β-protons Hg (2.19–2.53 ppm), and the propargylic α-protons He (3.94–4.98 ppm) (Figure 3b, i-iii).
Scheme 1. Synthesis of Double Overhand Knot (Λ,Δ)-L3•[Lu]2 and Square Knot (Λ,Δ)-1.
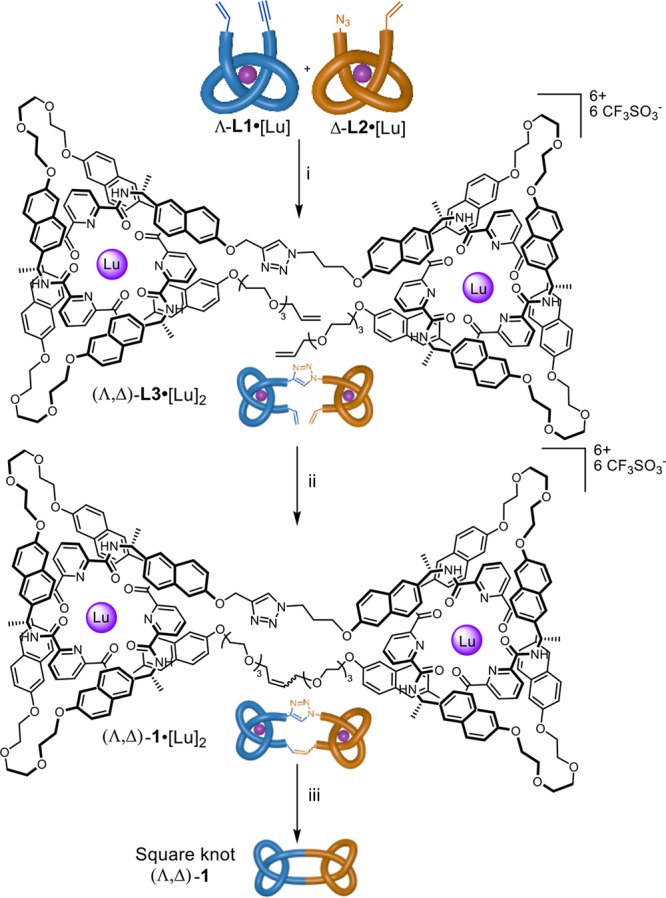
Reagents and conditions: (i) Cu(MeCN)4(CF3SO3), Tentagel-TBTA, MeCN/MeOH 1:1, RT, 16 h, 70%. (ii) Hoveyda-Grubbs second generation catalyst, MeNO2/CH2Cl2 1:1, 30%. (iii) Et4NF, MeCN, RT, 5 min, 95%.
Figure 3.
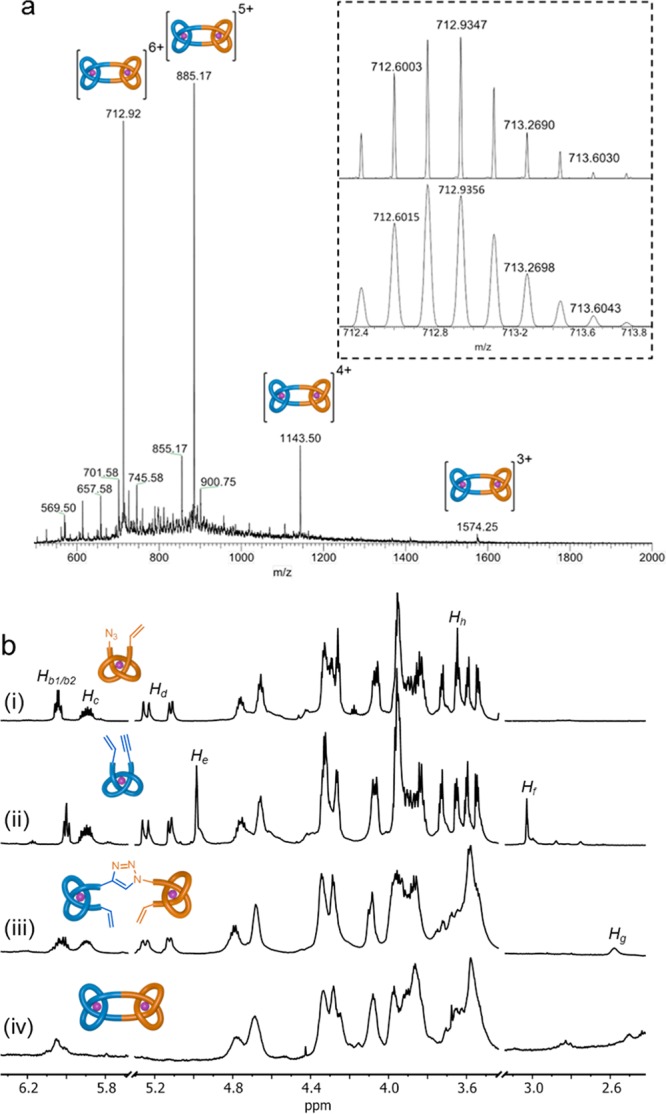
(a) ESI-MS (positive mode) of square knot complex (Λ,Δ)-1•[Lu]2 (inset: isotopic distribution from HR-MS). (b) Partial 1H NMR spectra (600 MHz, MeCN-d3, 298 K) of (i) entanglement synthon Δ-L2•[Lu], (ii) entanglement synthon Λ-L1•[Lu], (iii) double overhand knot (Λ,Δ)-L3•[Lu]2, and (iv) square knot coordination complex (Λ,Δ)-1•[Lu]2. For full assignments, see the Supporting Information.
Macrocyclization of (Λ,Δ)-L3•[Lu]2 occurred over 14 h at 50 °C in a MeNO2/CH2Cl2 (1:1) solvent mixture using the second generation Hoveyda-Grubbs catalyst.14 After workup, size exclusion chromatography afforded the metalated molecular square knot, (Λ,Δ)-1•[Lu]2, in 30% yield. The isolated pure material is only a fraction of the amount actually formed. The closed knot is difficult to separate from closely running bands that contain products resulting from incomplete olefin metathesis and the presence of other anions (1H NMR analysis of the reaction mixture indicates that olefin metathesis proceeds in ∼80% conversion per alkene).
The closed-loop structure of (Λ,Δ)-1•[Lu]2 was confirmed by 1H NMR spectroscopy (e.g., loss of terminal alkene protons Hd; Figure 3b), ESI-MS (molecular ion mass corresponds to loss of C2H4, Figure 3a), and high-resolution mass spectrometry (HR-MS; the isotope distribution of the molecular ion confirming the molecular formula, Figure 3a inset). Diffusion-ordered spectroscopy (DOSY) shows that the composite knots diffuse with a larger hydrodynamic radius than the overhand knot building block, Λ-L1•[Lu] (Spectras S1 and S2).
Coordination complex (Λ,Δ)-1•[Lu]2 was smoothly demetalated with tetraethylammonium fluoride in MeCN, affording the metal-free square knot (Λ,Δ)-1 in 95% yield within 5 min at RT (Supporting Information, Section 4.2). In line with other complex intertwined organic compounds,4d,4e,5b,5c the 1H NMR of (Λ,Δ)-1 is broad (Figure S2), presumably due to conformational dynamics of the strand (e.g., reptation15) being significantly impeded by the entanglement. Slight sharpening of the 1H NMR spectrum occurs at elevated temperatures (Spectrum S34). This may be a result of the loosening of intrastrand amide–amide hydrogen bonding as well as increased reptation. Remetalation of the wholly organic knot could be accomplished through treatment with excess lutetium trifluoromethanesulfonate (MeCN, 80 °C, 18 h; Figure S3).
Enantioselective syntheses of each enantiomer of the granny composite knot were achieved in a similar fashion to the square knot (Figure 4). Starting from the three-crossing entanglement synthons with Λ-handedness, Λ-L1•[Lu] and Λ-L2•[Lu], granny knot (Λ,Λ)-1•[Lu]2 was isolated in 26% yield over two steps (Figure 4a). Its enantiomer (Δ,Δ)-1•[Lu]2 was synthesized from the two Δ-entanglement synthons in 16% overall yield (Figure 4b). The identity of each composite knot was confirmed by NMR spectroscopy and HR-MS. Demetalation of the granny knot (Λ,Λ)-1•[Lu]2, under similar conditions to those used for the square knot, afforded (Λ,Λ)-1 in 92% yield (Supporting Information, Section 4.2), with no appreciable difference in rate between the two topoisomers. The topology-based stereochemical differences between (Λ,Λ)-1 and (Λ,Δ)-1 are insufficient to significantly influence the reactivity of the labile lanthanide-ligand interactions.
Figure 4.
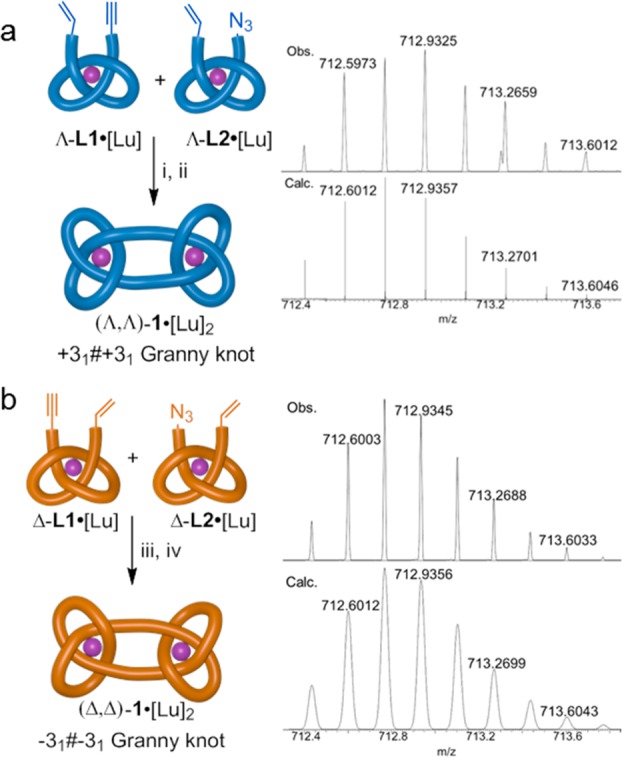
(a) Synthesis and HR-MS of granny knot complex (Λ,Λ)-1•[Lu]2. Conditions: (i) Cu(MeCN)4(CF3SO3), Tentagel-TBTA, MeCN/MeOH 1:1, RT, 16 h, 79%. (ii) Hoveyda-Grubbs 2nd generation catalyst, MeNO2/CH2Cl2 1:1, 33%. (b) Synthesis and HR-MS of granny knot complex (Δ,Δ)-1•[Lu]2. Conditions: (iii) Cu(MeCN)4(CF3SO3), Tentagel-TBTA, MeCN/MeOH 1:1, RT, 16 h, 52%. (iv) Hoveyda-Grubbs 2nd Gen, MeNO2/CH2Cl2 1:1, 31%. For each of the ion isotopic distributions, the experimentally observed spectrum is shown above the theoretically calculated spectrum.
The 1H and 13C NMR spectra of the granny and square knot are essentially indistinguishable (Spectra S27–S30). However, the composite knot diastereomers differ significantly in circular dichroism measurements (Figure 5). The CD spectra of the lanthanide complexes of the granny knot enantiomers (Λ,Λ)-1 and (Δ,Δ)-1 are symmetrical in terms of curve shape and have exciton couplings of equal and opposite sign with maxima at 253 nm, demonstrating the chirality (of opposite handedness) of their structures.16 The exciton couplets are consistent with the absolute configuration of the entanglement helicities of the synthons.10 The CD response of the square knot coordination complex is virtually, but not quite, baseline (Figure 5, green trace). The very small Cotton effect likely stems from the triazole ring in the composite knot strand connecting to the two entanglement synthons in nonidentical ways: through a carbon atom of what was the alkyne group in Λ-L1•[Lu] and a nitrogen atom of what was the azide group in Δ-L2•[Lu]. The resulting break in the symmetry in the chemical constitution of the knot strand results in the molecule not having a perfect plane of symmetry between its two halves: The square knot prepared from Λ-L1•[Lu] and Δ-L2•[Lu] would not be superimposable on one prepared from the enantiomeric building blocks, Δ-L1•[Lu] and Λ-L2•[Lu]. Although (Λ,Δ)-1 is topologically achiral, because of the chemical make up of the strand it is not a true meso-compound. Accordingly, its lanthanide complex elicits a very small, but finite, CD response.
Figure 5.
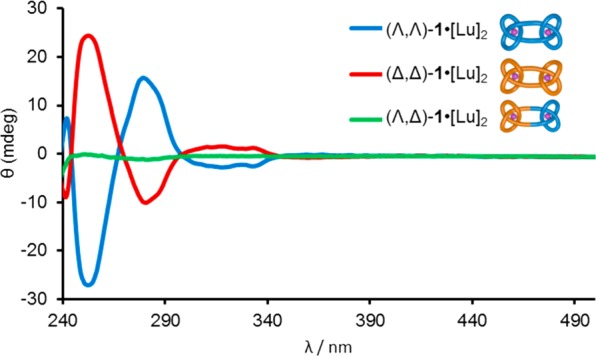
Circular dichroism spectra (1.0 × 10–4 M, MeCN, 298 K) of granny knots (Λ,Λ)-1•[Lu]2 (blue) and (Δ,Δ)-1•[Lu]2 (red) and square knot (Λ,Δ)-1•[Lu]2 (green). Normalized for absorbance.
Conclusions
Three molecular six-crossing composite knots of different topologies, −31#–31 (granny), +31#+31 (granny), and +31#–31 (square), were synthesized stereoselectively through a strategy involving joining together entanglement synthons of particular handedness to form a linear strand with multiple tangles that are each held in place by coordination to lanthanide cations. Subsequent macrocyclization of each strand locks its topology, forming a closed loop, 199 atoms long, from which the metal ions can be removed without the knot being able to unravel. Tris(2,6-pyridinedicarboxamide) ligands were shown to be suitable 31 entanglement synthons of either handedness (chirality predetermined by choice of asymmetric centers on the ligand strand). The resulting granny knots are enantiomers (each with six alternating crossings) that exhibit pronounced equal and opposite CD spectra. The diastereomeric square knot is topologically achiral (two nonalternating crossings and four alternating crossings), but a lack of symmetry in the chemical constitution of the knot strand means this example is not a true meso-compound.
The synthetic strategy is related to both the chiron approach8 of conventional asymmetric synthesis and tangle theory,1a,3 a way that mathematicians understand and (de)construct complex knot topologies through simpler fragments termed “tangles”. The new approach may prove useful for the preparation of other complex molecular knots and entangled materials that lack high symmetry in their crossing patterns.
Acknowledgments
We thank the Engineering and Physical Sciences Research Council (EPSRC; EP/P027067/1), the EU (Marie Curie Individual Postdoctoral Fellowship to FS; EC 746993), and the School of Chemistry Mass Spectrometry Service Centre for high-resolution mass spectrometry. We are grateful to Guzman Gil-Ramírez and Gen Zhang for preliminary studies on this general concept, David August for assistance with the DOSY experiments, and Joakim Halldin Stenlid for modeling studies. D.A.L. is a Royal Society Research Professor.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b01819.
Synthetic procedures; NMR, MS, CD, UV/vis data; and molecular models (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Fielden S. D. P.; Leigh D. A.; Woltering S. L. Molecular knots. Angew. Chem., Int. Ed. 2017, 56, 11166–11194. 10.1002/anie.201702531. [DOI] [PMC free article] [PubMed] [Google Scholar]; For other reviews on molecular knots, see:; b Fenlon E. E. Open problems in chemical topology. Eur. J. Org. Chem. 2008, 2008, 5023–5035. 10.1002/ejoc.200800578. [DOI] [Google Scholar]; c Beves J. E.; Blight B. A.; Campbell C. J.; Leigh D. A.; McBurney R. T. Strategies and tactics for the metal-directed synthesis of rotaxanes, knots, catenanes, and higher order links. Angew. Chem., Int. Ed. 2011, 50, 9260–9327. 10.1002/anie.201007963. [DOI] [PubMed] [Google Scholar]; d Forgan R. S.; Sauvage J.-P.; Stoddart J. F. Chemical topology: complex molecular knots, links, and entanglements. Chem. Rev. 2011, 111, 5434–5464. 10.1021/cr200034u. [DOI] [PubMed] [Google Scholar]; e Amabilino D. B.; Sauvage J.-P. The beauty of knots at the molecular level. Top. Curr. Chem. 2011, 323, 107–126. 10.1007/128_2011_292. [DOI] [PubMed] [Google Scholar]; f Ayme J.-F.; Beves J. E.; Campbell C. J.; Leigh D. A. Template synthesis of molecular knots. Chem. Soc. Rev. 2013, 42, 1700–1712. 10.1039/C2CS35229J. [DOI] [PubMed] [Google Scholar]; g Horner K. E.; Miller M. A.; Steed J. W.; Sutcliffe P. M. Knot theory in modern chemistry. Chem. Soc. Rev. 2016, 45, 6432–6448. 10.1039/C6CS00448B. [DOI] [PubMed] [Google Scholar]; h Sauvage J.-P. From chemical topology to molecular machines (Nobel lecture). Angew. Chem., Int. Ed. 2017, 56, 11080–11093. 10.1002/anie.201702992. [DOI] [PubMed] [Google Scholar]; i Pairault N.; Niemeyer J. Chiral mechanically interlocked molecules – Applications of rotaxanes, catenanes and molecular knots in stereoselective chemosensing and catalysis. Synlett 2018, 29, 689–698. 10.1055/s-0036-1591934. [DOI] [Google Scholar]
- a Dietrich-Buchecker C. O.; Sauvage J.-P. A synthetic molecular trefoil knot. Angew. Chem., Int. Ed. Engl. 1989, 28, 189–192. 10.1002/anie.198901891. [DOI] [Google Scholar]; b Ashton P. R.; Matthews O. A.; Menzer S.; Raymo F. M.; Spencer N.; Stoddart J. F.; Williams D. J. A template-directed synthesis of a molecular trefoil knot. Liebigs Ann. Chem. 1997, 1997, 2485–2494. 10.1002/jlac.199719971210. [DOI] [Google Scholar]; c Dietrich-Buchecker C. O.; Hwang N. G.; Sauvage J.-P. A trefoil knot coordinated to two lithium ions: synthesis and structure. New J. Chem. 1999, 23, 911–914. 10.1039/a904476k. [DOI] [Google Scholar]; d Safarowsky O.; Nieger M.; Fröhlich R.; Vögtle F. A molecular knot with twelve amide groups—one-step synthesis, crystal structure, chirality. Angew. Chem., Int. Ed. 2000, 39, 1616–1618. . [DOI] [PubMed] [Google Scholar]; e Feigel M.; Ladberg R.; Engels S.; Herbst-Irmer R.; Fröhlich R. A trefoil knot made of amino acids and steroids. Angew. Chem., Int. Ed. 2006, 45, 5698–5702. 10.1002/anie.200601111. [DOI] [PubMed] [Google Scholar]; f Guo J.; Mayers P. C.; Breault G. A.; Hunter C. A. Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template. Nat. Chem. 2010, 2, 218–220. 10.1038/nchem.544. [DOI] [PubMed] [Google Scholar]; g Barran P. E.; Cole H. L.; Goldup S. M.; Leigh D. A.; McGonigal P. R.; Symes M. D.; Wu J. Y.; Zengerle M. Active metal template synthesis of a molecular trefoil knot. Angew. Chem., Int. Ed. 2011, 50, 12280–12284. 10.1002/anie.201105012. [DOI] [PubMed] [Google Scholar]; h Ponnuswamy N.; Cougnon F. B. L.; Clough J. M.; Pantoş G. D.; Sanders J. K. M. Discovery of an organic trefoil knot. Science 2012, 338, 783–785. 10.1126/science.1227032. [DOI] [PubMed] [Google Scholar]; i Prakasam T.; Lusi M.; Elhabiri M.; Platas-Iglesias C.; Olsen J.-C.; Asfari Z.; Cianférani-Sanglier S.; Debaene F.; Charbonnière L. J.; Trabolsi A. Simultaneous self assembly of a [2]catenane, a trefoil knot, and a solomon link from a simple pair of ligands. Angew. Chem. 2013, 125, 10140–10144. 10.1002/ange.201302425. [DOI] [PubMed] [Google Scholar]; j Ayme J.-F.; Gil-Ramírez G.; Leigh D. A.; Lemonnier J.-F.; Markevicius A.; Muryn C. A.; Zhang G. Lanthanide template synthesis of a molecular trefoil knot. J. Am. Chem. Soc. 2014, 136, 13142–13145. 10.1021/ja506886p. [DOI] [PubMed] [Google Scholar]; k Prakasam T.; Bilbeisi R. A.; Lusi M.; Olsen J.-C.; Platas-Iglesias C.; Trabolsi A. Postsynthetic modification of cadmium-based knots and links. Chem. Commun. 2016, 52, 7398–7401. 10.1039/C6CC02423H. [DOI] [PubMed] [Google Scholar]; l Zhang L.; August D. P.; Zhong J.; Whitehead G. F. S.; Vitorica-Yrezabal I. J.; Leigh D. A. Molecular trefoil knot from a trimeric circular helicate. J. Am. Chem. Soc. 2018, 140, 4982–4985. 10.1021/jacs.8b00738. [DOI] [PubMed] [Google Scholar]; m Cougnon F. B. L.; Caprice K.; Pupier M.; Bauzá A.; Frontera A. A strategy to synthesize molecular knots and links using the hydrophobic effect. J. Am. Chem. Soc. 2018, 140, 12442–12450. 10.1021/jacs.8b05220. [DOI] [PubMed] [Google Scholar]
- Adams C. C.The Knot Book: An Elementary Introduction to the Mathematical Theory of Knots; American Mathematical Society: Providence, RI, 2004. [Google Scholar]
- a Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. A synthetic molecular pentafoil knot. Nat. Chem. 2012, 4, 15–20. 10.1038/nchem.1193. [DOI] [PubMed] [Google Scholar]; b Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. Pentameric circular iron(II) double helicates and a molecular pentafoil knot. J. Am. Chem. Soc. 2012, 134, 9488–9497. 10.1021/ja303355v. [DOI] [PubMed] [Google Scholar]; c Ponnuswamy N.; Cougnon F. B. L.; Pantoş G. D.; Sanders J. K. M. Homochiral and meso figure eight knots and a Solomon link. J. Am. Chem. Soc. 2014, 136, 8243–8251. 10.1021/ja4125884. [DOI] [PubMed] [Google Scholar]; d Marcos V.; Stephens A. J.; Jaramillo-Garcia J.; Nussbaumer A. L.; Woltering S. L.; Valero A.; Lemonnier J.-F.; Vitorica-Yrezabal I. J.; Leigh D. A. Allosteric initiation and regulation of catalysis with a molecular knot. Science 2016, 352, 1555–1559. 10.1126/science.aaf3673. [DOI] [PubMed] [Google Scholar]; e Danon J. J.; Krüger A.; Leigh D. A.; Lemonnier J.-F.; Stephens A. J.; Vitorica-Yrezabal I. J.; Woltering S. L. Braiding a molecular knot with eight crossings. Science 2017, 355, 159–162. 10.1126/science.aal1619. [DOI] [PubMed] [Google Scholar]; g Cougnon F. B. L. Tight embrace in a molecular knot with eight crossings. Angew. Chem., Int. Ed. 2017, 56, 4918–4919. 10.1002/anie.201701308. [DOI] [PubMed] [Google Scholar]; h Kim D. H.; Singh N.; Oh J.; Kim E.-H.; Jung J.; Kim H.; Chi K.-W. Coordination-driven self-assembly of a molecular knot comprising sixteen crossings. Angew. Chem., Int. Ed. 2018, 57, 5669–5673. 10.1002/anie.201800638. [DOI] [PubMed] [Google Scholar]; i Leigh D. A.; Lemonnier J.-F.; Woltering S. L. Comment on “Coordination-driven self-assembly of a molecular knot comprising sixteen crossings. Angew. Chem., Int. Ed. 2018, 57, 12212–12214. 10.1002/anie.201804904. [DOI] [PubMed] [Google Scholar]; j Zhang L.; Lemonnier J.-F.; Acocella A.; Calvaresi M.; Zerbetto F.; Leigh D. A. Effects of knot tightness at the molecular level. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 2452. 10.1073/pnas.1815570116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Carina R. F.; Dietrich-Buchecker C. O.; Sauvage J.-P. Molecular composite knots. J. Am. Chem. Soc. 1996, 118, 9110–9116. 10.1021/ja961459p. [DOI] [Google Scholar]; b Danon J. J.; Leigh D. A.; Pisano S.; Valero A.; Vitorica-Yrezabal I. J. A Six-Crossing Doubly Interlocked [2]Catenane with Twisted Rings, and a Molecular Granny Knot. Angew. Chem., Int. Ed. 2018, 57, 13833–13837. 10.1002/anie.201807135. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang L.; Stephens A. J.; Nussbaumer A. L.; Lemonnier J.-F.; Jurček P.; Vitorica-Yrezabal I. J.; Leigh D. A. Stereoselective synthesis of a composite knot with nine crossings. Nat. Chem. 2018, 10, 1083–1088. 10.1038/s41557-018-0124-6. [DOI] [PubMed] [Google Scholar]
- Marenda M.; Orlandini E.; Micheletti C. Discovering privileged topologies of molecular knots with self-assembling models. Nat. Commun. 2018, 9, 3051. 10.1038/s41467-018-05413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The interwoven grid strategy used for knots was originally used in the synthesis of a Solomon link, see:Beves J. E.; Danon J. J.; Leigh D. A.; Lemonnier J.-F.; Vitorica-Yrezabal I. J. A Solomon link through an interwoven molecular grid. Angew. Chem., Int. Ed. 2015, 54, 7555–7559. 10.1002/anie.201502095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hanessian S.Total Synthesis of Natural Products: The ‘Chiron Approach’; Pergamon: Oxford, 1984. [Google Scholar]; b Hanessian S. The Enterprise of Synthesis: From Concept to Practice. J. Org. Chem. 2012, 77, 6657–6688. 10.1021/jo300902m. [DOI] [PubMed] [Google Scholar]
- Adams H.; Ashworth E.; Breault G. A.; Guo J.; Hunter C. A.; Mayers P. C. Knot tied around an octahedral metal centre. Nature 2001, 411, 763. 10.1038/35081143. [DOI] [PubMed] [Google Scholar]
- a Gil-Ramírez G.; Hoekman S.; Kitching M. O.; Leigh D. A.; Vitorica-Yrezabal I. J.; Zhang G. Tying a molecular overhand knot of single handedness and asymmetric catalysis with the corresponding pseudo-D3-symmetric trefoil knot. J. Am. Chem. Soc. 2016, 138, 13159–3162. 10.1021/jacs.6b08421. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Leigh D. A.; Pirvu L.; Schaufelberger F.; Tetlow D. J.; Zhang L. Securing a supramolecular architecture by tying a stopper knot. Angew. Chem., Int. Ed. 2018, 57, 10484–10488. 10.1002/anie.201803871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zhang G.; Gil-Ramírez G.; Markevicius A.; Browne C.; Vitorica-Yrezabal I. J.; Leigh D. A. Lanthanide template synthesis of trefoil knots of single handedness. J. Am. Chem. Soc. 2015, 137, 10437–10442. 10.1021/jacs.5b07069. [DOI] [PubMed] [Google Scholar]; See also:; b Kotova O.; Kitchen J. A.; Lincheneau C.; Peacock R. D.; Gunnlaugsson T. Probing the effects of ligand isomerism in chiral luminescent lanthanide supramolecular self-assemblies: a Europium “Trinity sliotar” study. Chem. - Eur. J. 2013, 19, 16181–16186. 10.1002/chem.201303660. [DOI] [PubMed] [Google Scholar]; c Kitchen J. A. Lanthanide-based self-assemblies of 2,6-pyridyldicarboxamide ligands: Recent advances and applications as next-generation luminescent and magnetic materials. Coord. Chem. Rev. 2017, 340, 232–246. 10.1016/j.ccr.2017.01.012. [DOI] [Google Scholar]; d Barry D. E.; Caffrey D. F.; Gunnlaugsson T. Lanthanide-directed synthesis of luminescent self-assembly supramolecular structures and mechanically bonded systems from acyclic coordinating organic ligands. Chem. Soc. Rev. 2016, 45, 3244–3274. 10.1039/C6CS00116E. [DOI] [PubMed] [Google Scholar]
- In preliminary studies, we were unable to find spacers of a length and conformation that tolerated the one-step combination of entanglement synthons to form composite knots (e.g., dimerization of an entangled bis-alkene to form a granny knot or combination of an entangled bis-azide and bis-alkyne of different handedness to form a square knot).
- Molecular modeling indicates that a double overhand knot such as (Λ,Δ)-L3•[Lu]2 adopts a conformation that places the reactive endgroups a relatively long distance away from each other, hence the use of relatively short spacers to the azide and alkyne for the CuAAC step that couples the synthons and relatively long and flexible chains for the subsequent RCM ring closure (see Figure S11).
- Garber S. B.; Kingsbury J. S.; Gray B. L.; Hoveyda A. H. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. 10.1021/ja001179g. [DOI] [Google Scholar]
- De Gennes P. G. Reptation of a polymer chain in the presence of fixed obstacles. J. Chem. Phys. 1971, 55, 572–579. 10.1063/1.1675789. [DOI] [Google Scholar]
- There is a small difference in intensity of the CD responses recorded for the two isolated granny knots when the spectra are normalized for UV-absorbance (Figure 5). This likely results from a trace impurity in (Δ,Δ)-1. No epimerization of asymmetric centers occurs during any of the synthetic steps (the resulting diastereomers are detectable by 1H NMR10a,11a), and folding to an entanglement of opposite handedness is not possible on steric grounds for any of the lanthanide-coordinated overhand knot intermediates.10a,11a
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


