ABSTRACT
Human pancreatic ductal adenocarcinoma (PDAC) exhibits marginal responses to anti-PD-1/PD-L1 immunotherapy and its mechanism remains poorly understood. We have investigated the effect of anti-PD-L1 and c-Myc inhibition in PDAC. Using 87 patients with PDAC from our hospital database we found a significant correlation between the expression of PD-L1 and c-Myc. Moreover, the expression of both PD-L1 and c-Myc was associated with poor overall survival. In addition, we confirmed this finding with the PDAC patients in the TCGA database. Using several PDAC cell lines we demonstrated a significant correlation between the expression of PD-L1 and c-Myc. We also found that expression of PD-L1 correlated with high-grade histology. JQ1, an inhibitor of c-Myc inhibited PD-L1 expression and tumor growth. Using xenograft models, we demonstrated that the combination of JQ1 and anti-PD-L1 antibody exerted synergistic inhibition of PDAC growth. Our data demonstrated that the expression of PD-L1 and c-Myc may be helpful prognostic biomarkers, and their inhibition may potentially serve as an effective treatment for PDAC.
KEYWORDS: C-Myc, PD-L1, JQ1, immunotherapy, pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the seventh leading cause of cancer-related deaths worldwide1 and the fourth leading cause of cancer-related deaths in the Western countries.2,3 There are many factors that are associated with the poor survival and treatment challenges of PDAC, including the lack of early detection, high risk of relapse after curative surgery, and poor response to chemotherapy, radiation, molecular targeted therapy, and immunotherapy.4
While immunotherapy using anti-PD-1/PD-L1 antibodies has been shown to be effective for many types of malignancies,5–9 its activity has been limited in PDAC, except in rare cases where the tumors harbor DNA mismatched repair gene deficiencies.9,10 Several factors may be related to the poor response of PDAC to immune checkpoint inhibitors. First, PD-L1 expression in tumors is an important indicator of checkpoint immunotherapy efficacy;11,12 however, PD-L1 expression in PDAC varies from 16.7% to 90%.13–16 Second, the high tumor burden causes immunosuppression in patients with pancreatic cancer.17 Third, almost all pancreatic cancers have non-immunogenic phenotypes.17,18 Among these factors the most important one is likely related to the immunosuppressive microenvironment of PDAC.
Mechanistically, though KRAS is the most commonly mutated oncogene in PDAC. However, c-Myc, a master transcription amplifier, is also commonly overexpressed and aberrantly activated in this disease.19 Targeting c-Myc for the treatment of PDAC has been an intense focus of the cancer research community.20 Activation of c-Myc has been shown to stimulate the expression of PD-L1 in some cancer cells causing immune evasion.21,22
JQ1 is an inhibitor of bromodomain containing four (BRD4), which is a co-activator of c-Myc transcription.23,24 JQ1 has been shown to inhibit c-Myc expression and disrupt its role as a master transcriptional amplifier in cancer cells.25 We investigated if PD-L1 expression is regulated by c-Myc in PDAC and if targeting c-Myc may be an effective approach to enhance the efficacy of anti-PD-L1 immunotherapy in PDAC.
Results
Patient characteristics
The clinicopathological characteristics of 87 patients with PDAC are presented in Table 1. The median age was 61 years (range 35–82), 43.7% were females, and most (78%) had TNM II (39 cases) and III (29 cases) stage. Fifty-seven (65.5%) lesions were located in the pancreatic head. The median tumor diameter was 4 cm. These resections were: 49 (56.3%) pancreaticoduodenectomies, 29 (33.3%) distal pancreatectomies, and 9 (10.4%) total pancreatectomies. The R0 resection rate was 35.6%. No neoadjuvant therapy was used in any patient of this cohort.
Table 1.
Clinicopathologic features.
| Expression of PD-L1 (n) |
||||
|---|---|---|---|---|
| n | High | Low | P-Value | |
| Gender | ||||
| Male | 49 (56.3%) | 16 | 33 | 0.711 |
| Female | 38 (43.7%) | 11 | 27 | |
| Age (years) | ||||
| ≥65 | 37 (42.5%) | 13 | 24 | 0.477 |
| <65 | 50 (57.5%) | 14 | 36 | |
| Tumor site | ||||
| Head | 57 (65.5%) | 14 | 43 | 0.072 |
| Other | 30 (34.5%) | 13 | 17 | |
| Diameter (cm) | ||||
| <4 | 39 (44.8%) | 13 | 26 | 0.676 |
| ≥4 | 48 (55.2%) | 14 | 34 | |
| CA 19-9 (U/ml) | ||||
| <37 | 23 (26.4%) | 5 | 18 | 0.261 |
| ≥37 | 64 (73.6%) | 22 | 42 | |
| Procedure | ||||
| Pancreaticoduodenectomy (PD) | 49 (56.3%) | 13 | 36 | 0.302 |
| Distal pancreatectomy | 29 (33.3%) | 12 | 17 | |
| Total pancreatectomy | 9 (10.4%) | 2 | 7 | |
| pT-staging | ||||
| pT1 | 2 (2.3%) | 0 | 2 | 0.923 |
| pT2 | 36 (41.4%) | 12 | 24 | |
| pT3 | 37 (42.5%) | 11 | 26 | |
| pT4 | 12 (13.8%) | 4 | 8 | |
| pN-staging | ||||
| pN0 | 35 (40.2%) | 12 | 23 | 0.591 |
| pN+ | 52 (59.8%) | 15 | 37 | |
| pStage | ||||
| IA | 1 (1.2%) | 1 | 0 | 0.878 |
| IB | 13 (14.9%) | 3 | 10 | |
| IIA | 14 (16.1%) | 4 | 10 | |
| IIB | 25 (28.7%) | 8 | 17 | |
| III | 29 (33.3%) | 9 | 20 | |
| IV | 5 (5.8%) | 2 | 3 | |
| Grading | ||||
| G1 | 3 (3.4%) | 1 | 2 | 0.007 |
| G2 | 67 (77.0%) | 16 | 51 | |
| G3 | 16 (18.4%) | 9 | 7 | |
| G4 | 1 (1.2%) | 1 | 0 | |
| Resection margins | ||||
| R0 | 31 (35.6%) | 11 | 20 | 0.504 |
| R1 | 56 (64.4%) | 16 | 40 | |
| Vascular invasion | ||||
| Yes | 26 (29.9%) | 9 | 17 | 0.637 |
| No | 61 (70.1%) | 18 | 43 | |
| Postoperative chemotherapy | ||||
| Yes | 41 (47.1%) | 13 | 28 | 0.898 |
| No | 46 (52.9%) | 14 | 32 | |
Correlation between PD-L1 and c-Myc expression in human PDAC
To understand if the expression of PD-L1 correlates with the level of c-Myc in PDAC, we retrieved the tumor specimens of 87 patients with PDAC and stained them with anti-PD-L1 and anti-c-Myc antibodies. Human placenta and lung tumor tissues were used as positive controls (Figure 1a and b). We found that PD-L1 was expressed in 31.0% and c-Myc was expressed in 43.7% of the tumors. The expression levels of c-Myc and PD-L1 in PDAC tissues were positively correlated at the protein level (p = 0.001; Table 2). We next evaluated the correlation between PD-L1 expression and patient characteristics. We found that compared to PD-L1-negative tumors, the PD-L1-positive tumors were associated with high histological grade (p = 0.007; Table 1), but not with sex, age, tumor site, CA 19–9, pT-stage, pN-stage, resection margins, vascular invasion, and postoperative chemotherapy.
Figure 1.
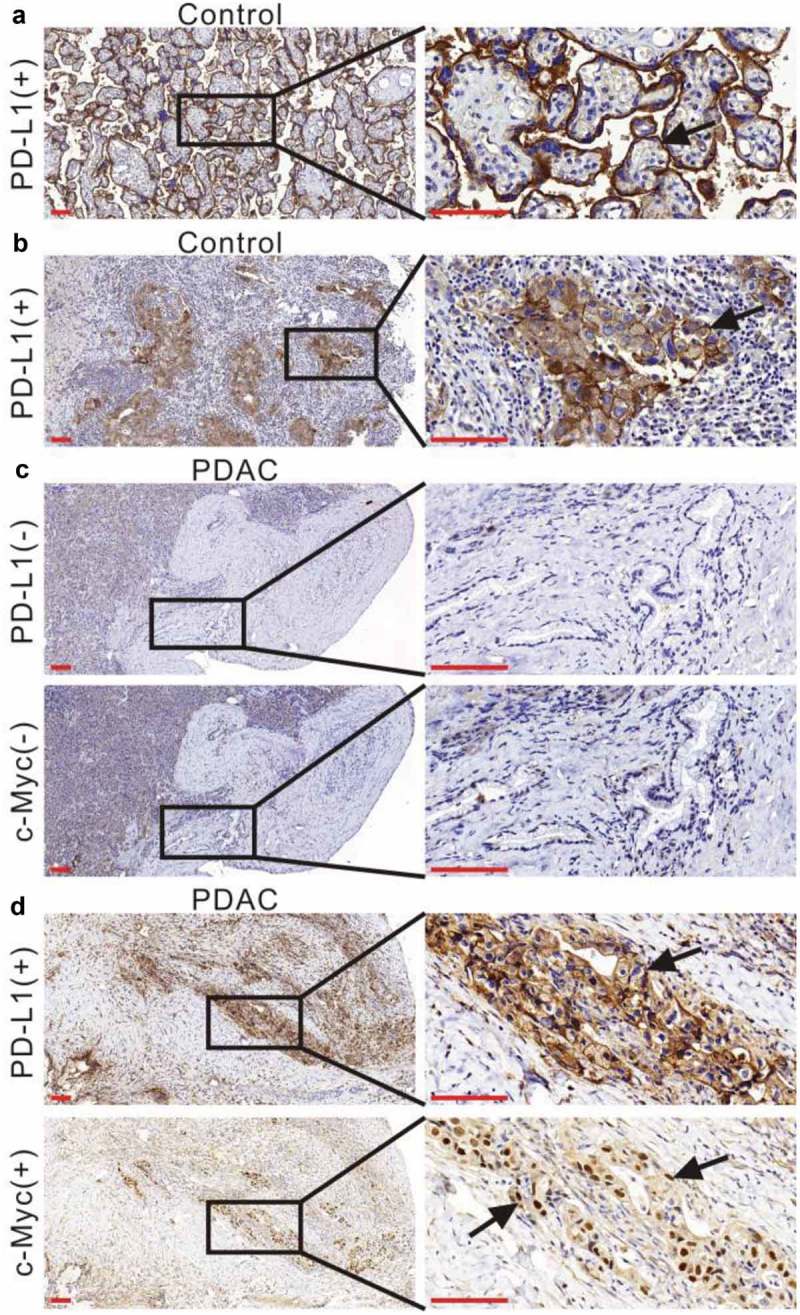
Immunostaining of PD-L1 and c-Myc in human PDAC. (a) Human placental tissue was stained with an anti-PD-L1 antibody as a positive control. Low magnification (100×) and high magnification (400×) images were obtained. The black arrow indicates positive PD-L1 staining on the cell membrane. (b) Human non-small cell lung cancer tissue was stained with an anti-PD-L1 antibody as a positive control. The left panel shows positive staining at low magnification (100×) and the right panel shows high magnification (400×). The black arrow indicates positive PD-L1 staining on the cell membrane. (c) Negative staining with an anti-PD-L1 antibody or anti-c-Myc antibody in a human PDAC tissue sample at low (100×) and high magnification (400×). (d) Positive staining with an anti-PD-L1 antibody or anti-c-Myc antibody in a human PDAC tissue sample at low (100×) and high magnification (400×). The black arrows indicate PD-L1-positive or c-Myc-positive tumor cells. Scale bar = 50 µm (red line at the bottom left).
Table 2.
Correlation between the expression of MYC and PD-L1 in PDAC.
| MYC |
Spearman’s correlation |
||||
|---|---|---|---|---|---|
| PD-L1 | Positive | Negative | Total | r | P-values |
| Positive | 19 | 8 | 27 | 0.361 | 0.001 |
| Negative | 19 | 41 | 60 | ||
| Total | 38 | 49 | 87 | ||
Tumor expression of PD-L1 and c-Myc correlated with poor outcome in patients with PDAC
Median follow-up and overall survival (OS) was 9.6 and 11.5 months, respectively, for the entire cohort. Univariate analysis showed that the variables associated with OS included tumor site [hazard ratio (HR) = 0.558; p = 0.044], diameter (HR = 1.705; p = 0.049), TNM stage (HR = 3.001, 2.438, 1.611; p = 0.026, 0.001, 0.037), grade (HR = 1.981; p = 0.012), resection margins (HR = 1.986; p = 0.019; Table 3). Patients with positive c-Myc expression correlated with worse OS (HR = 2.123; p = 0.006; Table 3; Figure 2a), compared to those with negative c-Myc expression. However, PD-L1 expression (p = 0.127; Figure 2b), sex (p = 0.118), age (p = 0.656), CA19-9 (p = 0.817), vascular invasion (p = 0.911), and postoperative chemotherapy (p = 0.051) are not significantly associated with OS. We further analyzed the correlation of PD-L1/c-Myc expression with clinical outcomes by dividing 87 PDAC patients into four PD-L1/c-Myc expression groups (expression of none, either, or both). Using univariate analysis, we found that PD-L1 and c-Myc double-positive tumors were associated with worse OS (HR = 4.0878, p < 0.001; Table 3; Figure 2c) compared to the other groups (PD-L1-positive and c-Myc-negative, PD-L1-negative and c-Myc-positive, and PD-L1 and c-Myc-double negative groups). Multivariate analysis was performed to determine if PD-L1/c-Myc expression remain independent predictors of OS (Table 3). Because PD-L1/c-Myc double positive group included some of the c-Myc positive patients, only variables of PD-L1/c-Myc expression, tumor site, diameter, TNM stage, grade, and resection margins were used in the multivariate analysis. We found that PD-L1/c-Myc expression (HR = 5.87; p < 0.001), grade (HR = 1.715; p = 0.046), and TNM stage (HR = 2.19, 2.388, 3.628; p = 0.194, 0.005, 0.005) were independent factors for prognosis (Table 2).
Table 3.
Univariate and multivariate Cox proportional analysis for overall survival.
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Variable | n | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Gender | |||||||
| Male | 49 | 1 | |||||
| Female | 38 | 0.639 | 0.364–1.121 | 0.118 | |||
| Age (years) | |||||||
| ≥65 | 37 | 1 | |||||
| <65 | 50 | 0.994 | 0.976–1.022 | 0.656 | |||
| Tumor site | |||||||
| Head | 57 | 1 | 1 | ||||
| Other | 30 | 0.558 | 0.316–0.984 | 0.044 | 0.503 | 0.198–1.278 | 0.149 |
| Diameter (cm) | |||||||
| <4 | 39 | 1 | 1 | ||||
| ≥4 | 48 | 1.705 | 1.000–2.910 | 0.049 | 1.751 | 0.948–3.233 | 0.073 |
| CA 19-9 (U/ml) | |||||||
| <37 | 23 | 1 | |||||
| ≥37 | 64 | 0.933 | 0.520–1.675 | 0.817 | |||
| TNM Stage | |||||||
| I | 14 | 1 | 1 | ||||
| II | 39 | 3.001 | 1.143–7.882 | 0.026 | 2.139 | 0.679–6.743 | 0.194 |
| III | 29 | 2.438 | 1.462–4.066 | 0.001 | 2.388 | 1.306–4.366 | 0.005 |
| IV | 5 | 1.611 | 1.030–2.521 | 0.037 | 3.628 | 1.462–9.003 | 0.005 |
| Grading | |||||||
| G1a | 3 | – | – | – | – | – | – |
| G2 | 67 | 1 | 1 | ||||
| G3 | 16 | 1.981 | 1.165–3.270 | 0.012 | 1.715 | 1.009–2.916 | 0.046 |
| G4a | 1 | – | – | – | – | – | – |
| Resection margins | |||||||
| R0 | 31 | 1 | 1 | ||||
| R1 | 56 | 1.986 | 1.119–3.526 | 0.019 | 1.338 | 0.725–2.470 | 0.352 |
| Vascular invasion | |||||||
| No | 61 | 1 | |||||
| Yes | 26 | 1.033 | 0.582–1.833 | 0.911 | |||
| Postoperative chemotherapy | |||||||
| Yes | 41 | 1 | |||||
| No | 46 | 1.713 | 0.998–2.940 | 0.051 | |||
| PD-L1 expression | |||||||
| Negative | 60 | 1 | |||||
| Positive | 27 | 1.447 | 0.805–2.598 | 0.127 | |||
| c-Myc expression | |||||||
| Negative | 49 | 1 | |||||
| Positive | 38 | 2.123 | 1.243–3.627 | 0.006 | |||
| PD-L1 and c-Myc expression | |||||||
| Other groups | 68 | 1 | 1 | ||||
| Double positive group | 19 | 4.078 | 2.146–7.749 | <0.001 | 5.87 | 2.696–12.777 | <0.001 |
| Stage I cohort | |||||||
| Other groups | 12 | ||||||
| Double positive groupa | 2 | – | – | – | – | – | – |
| Stage II cohort | |||||||
| Other groups | 33 | 1 | 1 | ||||
| Double positive group | 8 | 4.059 | 1.453–11.340 | 0.008 | 5.797 | 1.913–17.56 | 0.002 |
| Stage III cohort | |||||||
| Other groups | 21 | 1 | |||||
| Double positive group | 8 | 2.101 | 1.418–3.113 | 0.013 | 2.986 | 1.110–8.031 | 0.009 |
| Stage IV cohort | |||||||
| Other groups | 4 | ||||||
| Double positive groupa | 1 | – | – | – | – | – | – |
Abbreviations: HR, hazard ratio; CI, confidence interval;
Other groups: MYC and PD-L1 double negative group, MYC positive and PD-L1 negative group, and MYC
negative and PD-L1 positive group
Double positive group: MYC and PD-L1 double positive group
aBecause small number of cases in these groups, the survival analysis was meaningless.
Figure 2.

Kaplan–Meyer plot of OS of 87 PDAC patients according to tumor expression of c-Myc and PD-L1. (a) Kaplan–Meyer plot of OS in 87 PDAC patients according to tumor expression of c-Myc. (b) Kaplan–Meyer plot of OS in 87 PDAC patients with positive or negative tumor PD-L1 expression. (c–e) The red curve indicates positive staining for both c-Myc and PD-L1 (double positive), the gold curve indicates c-Myc-negative but PD-L1-positive staining, green shows c-Myc-positive and PD-L1-negative, and blue indicates double negative. The p-values were calculated by performing a Log-rank analysis.
Prognostic value of PD-L1/c-Myc expression stratified by tumor TNM stage
To further determine the prognostic value of PD-L1/c-Myc expression, patients were divided into four subgroups based on tumor TNM stage (Table 3). When stratified by TNM stage, we found that in the stages II and III cohorts, patients with PD-L1/c-Myc double positive expression had worse OS compared with the other groups, both by the univariate analysis (HR = 4.059, p = 0.008; HR = 2.101, p = 0.013; Figure 2d, e) and multivariate analysis (HR = 5.797, p = 0.002; HR = 2.986, p = 0.009). In addition, because only two and one patients in Stage I and stage IV cohort had PD-L1/c-Myc double positive expression, the survival analysis was not performed.
c-Myc regulates PD-L1 expression in PDAC
Some recent studies showed that c-Myc regulated PD-L1 expression in certain malignancies.22,26,27 We investigated the expression of PD-L1 and c-Myc in five human PDAC cell lines and one murine PDAC cell line using Western blotting to determine their correlation. c-Myc was expressed at various levels in all six cell lines (Figure 3a); two cell lines showed distinct PD-L1 expression, one showed faint expression, and the others showed no expression (Figure 3a). The expression of PD-L1 was further confirmed with immunofluorescence (Figure 3b) in three human PDAC cell lines (SW1990, BxPC-3, and CFPAC-1). Using shRNA, we found that knockdown of c-Myc significantly reduced PD-L1 expression in SW1990 and BxPC-3 cells (Figure 3c–g). In addition, overexpression of c-Myc increased the expression of PD-L1 in a dose-dependent manner (Figure 3h–j).
Figure 3.
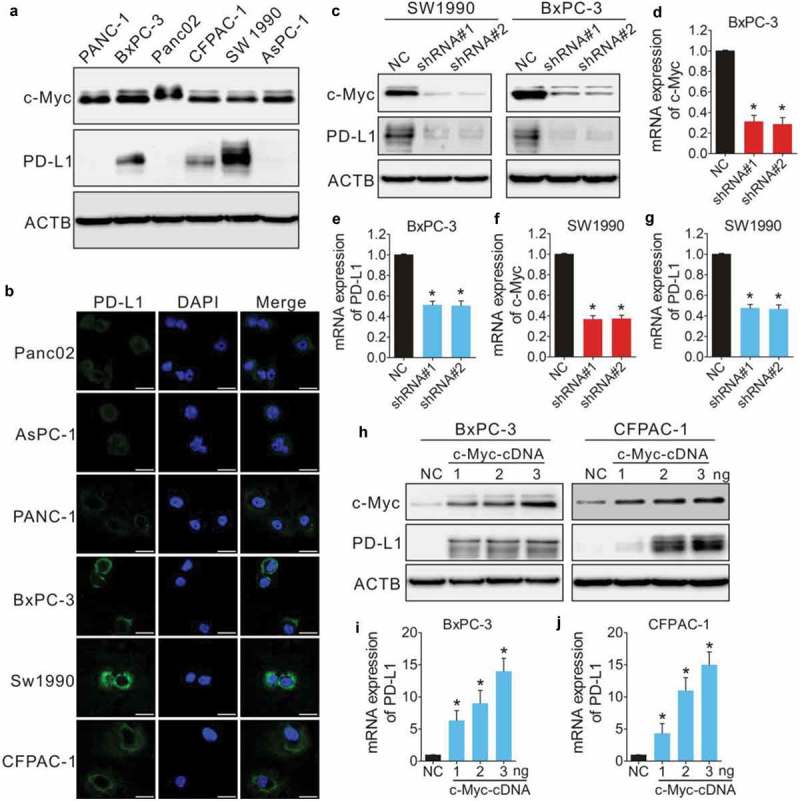
Expression of PD-L1 is regulated by c-Myc in PDAC. (a) Immunoblotting of c-Myc and PD-L1 in PDAC cell lines. ACTB (β-actin) was used as a normalization control. (b) Immunofluorescence analysis of PD-L1 expression in PDAC cells. Scale bars, 20 µm (white line at the bottom right). SW1990 and BxPC-3 cells were transfected with negative control shRNA (NC) or shRNA-c-Myc (shRNA#1 or shRNA#2) and c-Myc and PD-L1 expression were evaluated by immunoblotting (c) and qPCR (d–g). BxPC-3 and CFPAC-1 cells were transfected with an empty lentiviral vector as a negative control (NC), or a vector harboring c-Myc cDNA at different concentrations (1, 2, 3 ng) and the expression of c-Myc and PD-L1 was evaluated by immunoblotting (h) and qPCR (I and J). *p < 0.01 versus control.
JQ1 is a BRD4 inhibitor that inhibits c-Myc activity.28 As shown in Figure 4a–c, JQ1 treatment at 1.6, 3.2, and 4.8 nM decreased the expression of PD-L1 in SW1990 and BxPC-3 cell lines. Based on a xenograft model generated by injecting PDAC cells subcutaneously into athymic nude mice, we also found that inhibition of c-Myc by JQ1 resulted in a significant reduction in PD-L1 protein expression (Figure 4d–g), indicating that the c-Myc oncogene regulates PD-L1 expression in PDAC cells both in vitro and in vivo.
Figure 4.

Pharmacological inhibition of c-Myc by JQ1 suppresses PD-L1 expression. Immunoblotting (a) and qPCR (b and c) were performed to evaluate the expression of PD-L1 in SW1990 and BxPC-3 cells following JQ1 treatment for 48 hours at different concentrations (1.6, 3.2, and 4.8 nM). The data represent the mean ± SD of three independent experiments. *p < 0.01 when compared to the control group; #p < 0.01 when compared to the JQ1-1.6 nM group. (d–g) BxPC-3 and SW1990 xenografts from nude mice were treated intraperitoneally with control solvent or JQ1 at 25 mg/kg body weight twice daily. Each group contained five animals. Tumor weight was then measured (d and f). Representative examples of c-Myc and PD-L1 staining using BxPC-3 (e) or SW1990 (g) tumors grown in nude mice and treated with control solvent or JQ1. c-Myc+ and PD-L1+ tumor cells are shown in brown. Scale bars, 50 µm (red line at the bottom left).
We then retrieved 177 PDAC cases from the TCGA database and investigated the correlation between the level of CD274 (PD-L1) expression and c-Myc. Consistent with our data, CD274 expression was positively correlated with c-Myc expression (Figure 5a). To better understand the relationship between CD274 and c-Myc, we investigated the correlation between the mRNA expression level of PD-L1 and c-Myc-target genes,29 We showed that PD-L1 expression correlated well with the expression of several c-Myc target genes including ST3GAL3, BRCA1, SOCS3 and CCNG2 (Figure 5b–e). We further examined MYCN expression in PDAC and found an inverse correlation between c-Myc and MYCN (Figure 5f) as well as between CD274 and MYCN (Figure 5g), suggesting that MYCN may also regulate PD-L1 expression in PDAC. In addition, the majority of the 177 PDAC patients from the TCGA database (96%) had stage I or II disease (Table S1). There were no significant differences in the c-Myc and PD-L1 expression between stage I and stage II patients (29.39 ± 16.52 vs 25.92 ± 15.8, p = 0.373; 1.42 ± 1.06 vs 1.22 ± 0.89, p = 0.398). Kaplan-Meier analysis showed that low c-Myc expression was associated with significantly longer OS compared to the cases with high c-Myc expression (Figure 5h, cut-off value: median). However, CD274 expression (high or low; cut-off value: median) did not correlate with OS (Figure 5i).
Figure 5.
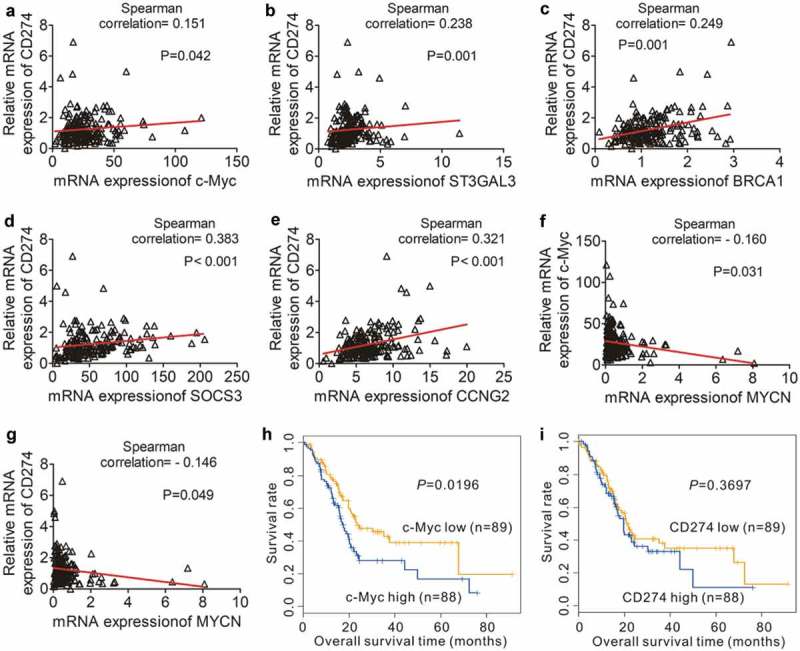
Correlation PD-L1 expression and the expression of c-Myc target genes in PDAC. (a) Positive correlation between the expression of c-Myc and the expression of CD274 in 177 individual human PDAC specimens. (b–e) Positive correlation between the expression of c-Myc-target genes [ST3GAL3 (b), BRCA1 (c), SOCS3 (d) and CCNG2 (e)] and CD274 expression in 177 human PDAC samples. (f–g) Inverse correlation between MYCN expression and c-Myc/CD274 expression in 177 human PDAC samples. (h) OS in 177 PDAC patients with high (blue line) or low (yellow line) c-Myc-expressing tumors. (i) OS in 177 PDAC patients with high (blue line) or low (yellow line) CD274-expressing tumors. P-values were calculated by performing a Log-rank test.
Pharmacological suppression of c-Myc and anti-PD-L1 enhances the efficacy of immunotherapy
We next asked if JQ1 enhance the efficacy of anti-PD-L1 therapy. We transplanted Panc02 and MPC-83 cells to C57BL/6 and Kunming (KM) mice. Panc02 cells do not express PD-L1 in vitro, but the xenograft tumor cells express both MYC and PD-L1 (Figure 6a and b). The MPC-83 cells express c-Myc and PD-L1 both in vitro and in vivo (Figure 6a and c). The tumor-bearing mice were treated with JQ1 and an anti-PD-L1 monoclonal antibody (mAb), alone or in combination. As shown in Figure 7a and b, JQ1 and anti-PD-L1 mAb therapy alone resulted in reduced tumor growth compared to that of untreated animals. When the anti-PD-L1 mAb was combined with JQ1, the inhibition of tumor growth was synergistic compared to either anti-PD-L1 or JQ1 therapy alone, as assessed by tumor volume (p = 0.02; p = 0.011) and weight (p = 0.004; 0.008). JQ1 and the anti-PD-L1 mAb also reduced tumor cell proliferation and increased tumor cell apoptosis (Figure 7c and d; Figure S1). Moreover, JQ1 amplified the inhibitory effect of anti-PD-L1 on tumor cell proliferation (p < 0.001; p < 0.001) while enhanced its induction of tumor cell apoptosis (p < 0.001; p < 0.001). These data suggest that JQ1 and anti-PD-L1 mAb therapy may be an effective combination therapy for PDAC.
Figure 6.
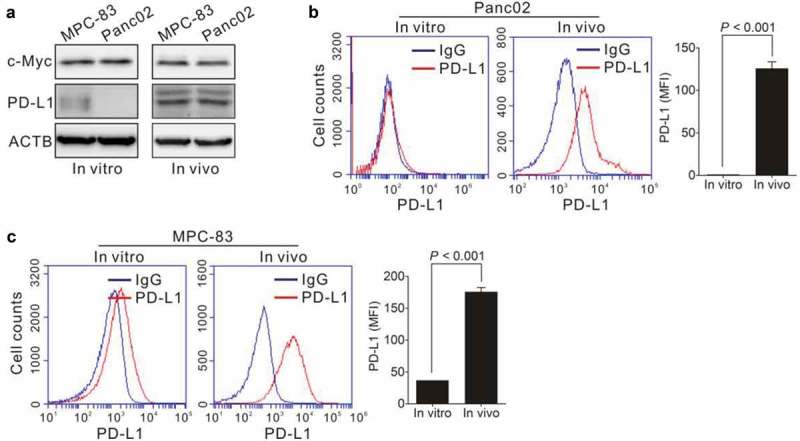
Expression of c-Myc and PD-L1 in PDAC in vitro and in vivo. (a) Protein levels of c-Myc and PD-L1 plot were analyzed by immunoblotting. (b–c) Panc02 cells or MPC-83 cells were subcutaneously transplanted into C57BL/6 mice or KM mice to establish pancreatic tumors. PD-L1 expression level on cultured Panc02 cells (in vitro) and Panc02 xenograft (in vivo) as shown in (b). PD-L1 expression level on cultured MPC-83 cells (in vitro) and MPC-83 xenograft (in vivo) as shown in (c). The expression of PD-L1 (mean fluorescent intensity, MFI) was analyzed by Student’s t-test (right panel). Data represent the mean ± SD.
Figure 7.

Effect of JQ1 and anti-PD-L1 antibody on the growth of PDAC. (a) Panc02 cells were transplanted to C57BL/6 mice. When the tumor reached 100 mm3, tumor-bearing mice were divided into four groups and treated with JQ1 (25 mg/kg body weight) and anti-PD-L1 antibody (200 μg/mouse), either alone or in combination for 14 days. The tumors removed from each group are shown in the left panel; tumor volume (middle panel) and weight (right panel) were compared to those of the untreated control. P-values were calculated based on a Student’s t-test. (b) MPC-83 cells were transplanted to KM mice. When the tumor reached 100 mm3, tumor-bearing mice were treated as in (a). The tumors removed from each group are shown in the left panel; tumor volume (middle panel) and weight (right panel) were compared to those of the untreated control. P-values were calculated based on a Student’s t-test. (c–d) Four different groups of Panc02 xenograft tumor tissues were stained for Ki-67 (c) and TUNEL (d), and representative images are shown: (a) control, (b) JQ1, (c) anti-PD-L1 monoclonal antibody (mAb), (d) JQ1 + anti-PD-L1 mAb. Scale bars, 50 µm (red line at the bottom left). Ki-67-positive (brown) or TUNEL-positive cells (green) were analyzed by performing a Student’s t-test. Data represent the mean ± SD.
Discussion
Human PDAC is essentially a “cold” tumor and responds poorly to immune checkpoint inhibitors. The expression of PD-L1 may be a factor related to its very poor prognosis and resistance to therapy.13,30 We have shown that expression of both c-Myc and PD-L1 was associated with worse outcomes in patients with PDAC compared to either markers alone, indicating that both c-Myc and PD-L1 play are in part responsible for the aggressive biology of PDAC and its poor response to immune checkpoint inhibitors.
Frequently overexpressed in PDAC, c-Myc has been also found to be highly associated with poor patient outcomes.31,32 It regulates lactate dehydrogenase A (LDHA), promotes the production of lactate, and contributes to the acidification of the tumor microenvironment which likely limits the efficacy of anti-PD-L1 therapy.33–35 We have confirmed that c-Myc regulates the expression of PD-L1 in PDAC as well. And its silencing using shRNA or inhibition via JQ1 suppressed PD-L1 expression in vitro and xenograft models. Our results thus suggest that c-Myc inhibitors might represent potential anti-tumor immunotherapeutic agents for pancreatic cancer. Since c-Myc was shown to directly bind the promoter of PD-L1 to regulate its expression,22 more studies have explored the relationship between these two proteins in human tumor cells.26,28 Our data using our own patient cohort as well as the TCGA database demonstrated that c-Myc expression correlated with PD-L1 expression. We also found that expression of MYCN was correlated with PD-L1 expression based on the TCGA cohort. In addition, c-Myc/PD-L1-double positivity significantly correlated with poor outcomes in PDAC patients. Furthermore, multivariate analyses demonstrated that c-Myc/PD-L1-double positivity was an independent predictor of poor prognosis for this disease.
Combination therapy strategies are being actively investigated for overcoming the resistance and turning “non-immunogenic” (cold) neoplasms into “immunogenic” (hot) neoplasms.17,35,36 Several groups have studied various approaches including combining anti-PD-1/PD-L1 therapy with other treatment methods such as surgery, chemotherapy, radiotherapy, molecular targeted therapy, or other immunotherapies.17 JQ1 is an inhibitor of the epigenetic modifier protein BRD4,37 which is a transcriptional regulator that controls the expression of c-Myc.20,38 JQ1, an inhibitor of c-Myc, has been shown to exert a potent inhibitory effect on PDAC cells.28 JQ1 is currently being tested in several clinical trials.39 Our study showed that either JQ1 or anti-PD-L1 mAb alone significantly inhibited tumor growth. However, the combination of both agents resulted in a synergistic effect.
In summary, our data demonstrate that PD-L1 expression correlated with c-Myc expression in PDAC and may serve as prognostic predictors clinically. In addition, the combined inhibition of both molecules may be an effective therapeutic approach for PDAC and worth further investigations.
Materials and methods
Patients and tissue samples
Human pancreatic cancer samples were collected from patients undergoing surgery at Fujian Medical University Union Hospital, Fuzhou, China, from November 2010 to April 2017. All patients received surgery and had histologically confirmed PDAC. Patients with neoadjuvant treatment, inflammatory diseases or active infection were excluded. A total of 87 patients who had been diagnosed with PDAC were enrolled in the study. Data collected and evaluated included sex, age, tumor site, tumor diameter, CA19-9, TNM stage, grade, resection margins, vascular invasion, and postoperative chemotherapy. The stage of each patient was assessed based on the American Joint Committee on Cancer version 7 (AJCC 7). R0 resections were assumed if all tumor-free margins were at least 1 mm wide. Informed consent was obtained before sample collection. The study was approved by the Committee for the Ethical Review of Research, Fujian Medical University Union Hospital (No. 2016-ZQN-34). Formalin-fixed paraffin-embedded samples were obtained for immunohistochemistry analysis.
Cell lines, mice, and reagents
The murine PDAC cell line Panc02 and MPC-83, syngeneic to C57BL/6 mice and Kunming (KM) mice, were obtained from Shanghai Aolu Biological Technology Co. Ltd (Shanghai, China). Human pancreatic cancer cell lines including PANC-1, BxPC-3, SW1990, CFPAC-1, and AsPC-1 were obtained from the Cell Bank, Chinese Academy of Sciences (Shanghai, China). All cell lines were genotyped for identification by the Cell Bank, Chinese Academy of Sciences and were tested to rule out mycoplasma contamination. Cells were treated with 1.6, 3.2, and 4.8 nM JQ1 (HY-13030, MedChemExpress, MCE) for 2 days before harvesting for protein and mRNA expression assays.
For in vivo studies, animal experiments were approved by the Ethics Committee for Animal Research of Fuzhou General Hospital of Chinese PLA Nanjing Military Command. Male athymic nude (BALB/c-nu) mice, 4–5 weeks of age, male C57BL/6 mice, 5 weeks of age, and male KM mice, 5 weeks of age, were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The xenograft model was established in accordance with our previously described protocol.40 SW1990 and BxPC-3 xenografts from nude mice were treated with control solvent (10% hydroxypropyl β-cyclodextrin in water) or JQ1 (five mice, respectively). JQ1 injections were performed intraperitoneally (i.p; 25 mg/kg, twice daily per mouse) for 14 days. Panc02 cells or MPC-83 cells were subcutaneously implanted into 32 C57BL/6 mice or 32 KM mice. When the tumor reached 100 mm3, tumor-bearing mice were randomly divided into four groups. Then, tumor-bearing mice were treated with control solvent, JQ1 (25 mg/kg, twice daily i.p.), an anti-PD-L1 (mAb; 200 μg/day i.p., Clone No. 10F.9G2, BioXcell), or JQ1 + anti-PD-L1 mAb. After 2 weeks of treatment, mice were sacrificed, and tumors were removed and weighed.
Oligonucleotide transfection
c-Myc short hairpin RNA (shRNA), cDNA encoding the whole sequence of c-Myc, and respective negative control vectors were obtained from Genepharma; sequences are shown in Supplementary Table 2. Oligonucleotide transfection was performed using the Lipofectamine 3000 reagent (Invitrogen) and transfection was performed according to the manufacturers’ recommendations.
Antibodies and immunoblotting
Anti-PD-L1 (E1L3N, #13684) was obtained from Cell Signaling Technology and the anti-c-Myc antibody (Y69) was from Abcam (ab32072). Western blotting for PD-L1 and c-Myc in pancreatic cancer cells was performed using methods described previously.40
Immunohistochemistry (IHC)
Immunohistochemical analysis was performed as previously described.41 PD-L1 was detected in the cytoplasm and on the cytomembranes of tumor cells, whereas c-Myc was detected in the nucleoplasm of tumor cells. Sections of human placenta and lung cancer tissue were used as positive controls (Figure 1a and b). PD-L1 status was defined as positive if ≥40% of tumor cells exhibited cytosolic staining of any intensity. c-Myc status was analyzed based on histopathological scoring, as described by Warner et al.,42 and status was defined as positive based on a score ≥4. All specimens were evaluated by two pathologists who were blinded to the patients’ clinical information.
Immunofluorescence
Immunofluorescence assays were performed to identify the location of PD-L1 in pancreatic cancer cells, as previously described.43
Quantitative PCR (qPCR)
c-Myc and PD-L1 mRNA was measured by qPCR using SYBR Premix Ex Taq (Takara, Dalian, China) and normalized to β-actin expression. The qPCR reactions were performed using an ABI Stepone plus Real-Time PCR System (Applied Biosystems). The primer sequences are listed in Supplementary Table 3.
Flow cytometry analysis
The PD-L1 expression on murine PDAC cells was determined by flow cytometry after staining with anti-mouse specific PD-L1 antibody conjugated with APC (17-5983-42, MIH1, eBioscience).
The cancer genome atlas (TCGA) data analysis
RNAseq and clinical data from 177 patient samples, defined by the TCGA pathologist as PDAC, were obtained from the TCGA Data Portal (https://tcga-data.nci.nih.gov). All data were downloaded from the November 22, 2017 standard dataset. For RNAseq data, expression levels were TPM-normalized and ENSG-ID transformed.
Statistical analysis
Quantitative data were expressed as the mean ± standard deviation (SD) and analyzed based on variance and Student’s t-tests. Chi-square tests were performed to compare PD-L1 expression and clinical features. Spearman’s rank correlation was evaluated to determine the correlation between PD-L1 and c-Myc expression at the protein level. OS was measured from the date of diagnosis to the day of death from any cause or the last censored follow-up. Survival analysis methods were similar to those previously described.13
Funding Statement
This study was supported by the Medical Talents Training Program of Health and Family Planning Commission of Fujian Province (2016-ZQN-34); The Medical Center of Minimally Invasive Technology of Fujian Province (No. 171, 2017 and No. 4, 2017); Joint Funds of Scientific and Technological Innovation Program of Fujian Province (2017Y9059);Joint Funds of Scientific and Technological Innovation Program of Fujian Province [2017Y9059];The Medical Center of Minimally Invasive Technology of Fujian Province [No. 171, 2017 and No. 4, 2017].
Acknowledgments
The authors thank Wei Zheng and Wewei Liu (Department of Pathology, Fujian Medical University, Fuzhou, China) for providing histological technology and analysis. The authors also thank He-ping Zheng, Yuan Dang, Fu-li Wen, and Lai-en Xue for their help with the animal studies at the Comparative Medicine Center of Fuzhou General Hospital.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contribution
YP, FL, and HH conceived the concept. HH and MP supervised the study. YP, QF, PX, JY, ZZ, and XL designed and performed the experiments. YP and MP wrote the manuscript. All authors approve the manuscript.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the Helsinki declaration. And all patients whose tissue samples were used in this research provided written informed consent, and the study protocol was approved by the Committee for the Ethical Review of Research, Fujian Medical University Union Hospital. Animal experiment protocols were approved by the Ethics Committee for Animal Research of Fuzhou General Hospital of Chinese PLA Nanjing Military Command.
Supplementary materials
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F.. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A.. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:104–117. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4.Collisson EA, Olive KP. Pancreatic cancer: progress and challenges in a rapidly moving field. Cancer Res. 2017;77:1060. doi: 10.1158/0008-5472.CAN-16-2452. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Brahmer J. Nivolumab in nonsquamous non-small-cell lung cancer. N Engl J Med. 2016;374:492–494. doi: 10.1056/NEJMc1514790. [DOI] [PubMed] [Google Scholar]
- 7.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Mcdermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. Lancet Oncol. 2016;17:917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 9.Zheng L. PD-L1 expression in pancreatic cancer. J Natl Cancer Inst. 2017;109(djw304). doi: 10.1093/jnci/djx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, SS Tykodi, Chow LQM, Hwu WJ, Topalian SL, Hwu P Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC. Safety and activity of Anti–PD-L1 antibody in patients with advanced cancer. J Urol. 2012;188:2148–2149. doi: 10.1016/j.juro.2012.08.169. [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 13.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059–1065. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- 15.Hutcheson J, Balaji U, Porembka MR, Wachsmann M, Mccue PA, Knudsen E, Witkiewicz AK. Immunological and metabolic features of pancreatic ductal adenocarcinoma define prognostic subtypes of disease. Clin Cancer Res. 2016;22:3606. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu C, Paschall AV, Shi H, Savage N, Waller JL, Sabbatini ME, Oberlies NH, Pearce C, Liu K. The MLL1-H3K4me3 axis-mediated PD-L1 expression and pancreatic cancer immune evasion. J Natl Cancer Inst. 2017;109:S587–S589. doi: 10.1093/jnci/djw283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, You L, Zheng L, Zhang T, Zhao Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Coaña PD Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med. 2015;21:482–491. doi: 10.1016/j.molmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Wirth M, Mahboobi S, Krämer OH, Schneider G. Concepts to target MYC in pancreatic cancer. Mol Cancer Ther. 2016. doi: 10.1158/1535-7163.MCT-16-0050. [DOI] [PubMed] [Google Scholar]
- 20.Wirth M, Schneider G. MYC: A stratification marker for pancreatic cancer therapy. Trends Cancer. 2016;2:1–3. doi: 10.1016/j.trecan.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Hessmann E, Schneider G, Ellenrieder V, Siveke JT. MYC in pancreatic cancer: novel mechanistic insights and their translation into therapeutic strategies. Oncogene. 2015;35:1609. doi: 10.1038/onc.2015.216. [DOI] [PubMed] [Google Scholar]
- 22.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol Cell. 2014;54:728–736. doi: 10.1016/j.molcel.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi J. Bromodomain and extraterminal domain inhibitors (BETi) for cancer therapy: chemical modulation of chromatin structure. Cold Spring Harb Perspect Biol. 2014;6(12):a018663. doi: 10.1101/cshperspect.a018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal R, Qi J, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim EY, Kim A, Kim SK, Chang YS. MYC expression correlates with PD-L1 expression in non-small cell lung cancer. Lung Cancer. 2017;110:63. doi: 10.1016/j.lungcan.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Melaiu O, Mina M, Chierici M, Boldrini R, Jurman G, Romania P et al PD-L1 is a therapeutic target of the bromodomain inhibitor JQ1 and, combined with HLA class I, a promising prognostic biomarker in neuroblastoma. Clin Cancer Res. 2017;23:4462–4472. doi: 10.1158/1078-0432.CCR-16-2601. [DOI] [PubMed] [Google Scholar]
- 28.Mazur PK, Herner A, Mello SS, Wirth M, Hausmann S, Sanchez-Rivera FJ, Lofgren SM, Kuschma T, Hahn SA, Vangala D, et al. Combined inhibition of BET family proteins and histone deacetylases as a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat Med. 2015;21:1163–1171. doi: 10.1038/nm.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LL, Lin HP, Zhou WJ, He CX, Zhang ZY, Cheng ZL, Song JB, Liu P, Chen XY, Xia YK, Chen XF, SNIP1 recruits TET2 to regulate c-MYC target genes and cellular DNA damage response. Cell Rep. 2018;25:1485–1500. doi: 10.1016/j.celrep.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaki S, Yanagimoto H, Tsuta K, Ryota H, Kon M. PD-L1 expression in pancreatic ductal adenocarcinoma is a poor prognostic factor in patients with high CD8+ tumor-infiltrating lymphocytes: highly sensitive detection using phosphor-integrated dot staining. Int J Clin Oncol. 2017;22:1–8. doi: 10.1007/s10147-016-1065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15:462–469. doi: 10.1038/modpathol.3880547. [DOI] [PubMed] [Google Scholar]
- 32.Witkiewicz AK, Mcmillan EA, Balaji U, Baek G, Lin WC, Mansour J, Mollaee M, Wagner K-U, Koduru P, Yopp A, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahlström T, Arsenian HM. Impact of MYC in regulation of tumor cell metabolism. BBA Gene Regul Mech. 2015;1849:563–569. doi: 10.1016/j.bbagrm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Hinzman CP, Aljehane L, Brown-Clay JD, Kallakury B, Sonahara F, Goel A, Trevino J, Banerjee PP. Aberrant expression of PDZ-binding kinase/T-LAK cell-originated protein kinase modulates the invasive ability of human pancreatic cancer cells via the stabilization of oncoprotein c-MYC. Carcinogenesis. 2018. doi: 10.1093/carcin/bgy114. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wolfgang CL, Zheng L. Precision immuno-oncology: prospects of individualized immunotherapy for pancreatic cancer. Cancers. 2018;10:39. doi: 10.3390/cancers10110400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smyth MJ, Ngiow SF, Ribas A, Teng MWL. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2015;13:143. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Fan P, Zhao J, Wu H, Jin X, Wu H. FBP1 loss contributes to BET inhibitors resistance by undermining c-Myc expression in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2018;37:224. doi: 10.1186/s13046-018-0888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Tong Y, Zhang X, Pan M, Chen S. Arsenic sulfide combined with JQ1, chemotherapy agents, or celecoxib inhibit gastric and colon cancer cell growth. Drug Des Devel Ther. 2015;9:5851–5862. doi: 10.2147/DDDT.S92943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrieu G, Belkina AC, Denis GV. Clinical trials for BET inhibitors run ahead of the science. Drug Discov Today Technol. 2016;19:45–50. doi: 10.1016/j.ddtec.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Y, Lu F, Xiong P, Pan M, Zhang Z, Lin X, Pan M, Huang H. WIPF1 antagonizes the tumor suppressive effect of miR-141/200c and is associated with poor survival in patients with PDAC. J Exp Clin Cancer Res. 2018;37:167. doi: 10.1186/s13046-018-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funamizu N, Hu C, Lacy C, Schetter A, Zhang G, He P, Gaedcke J, Ghadimi MB, Ried T, Yfantis HG, et al. Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2013;132:785–794. doi: 10.1002/ijc.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warner SL, Stephens BJ, Nwokenkwo S, Hostetter G, Sugeng A, Hidalgo M, Trent JM, Han H, Von Hoff DD. Validation of TPX2 as a potential therapeutic target in pancreatic cancer cells. Clin Cancer Res. 2009;15:6519–6528. doi: 10.1158/1078-0432.CCR-09-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Jia Y, Zhao S, Zhang X, Wang X, Han X, Wang Y, Ma M, Shi J, Liu L. BIN1 reverses PD-L1-mediated immune escape by inactivating the c-MYC and EGFR/MAPK signaling pathways in non-small cell lung cancer. Oncogene. 2017;36:6235–6243. doi: 10.1038/onc.2017.217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


