ABSTRACT
Metastatic diffusion of Neuroblastoma (NB) cells in the bone marrow (BM) represents the most negative prognostic factors for NB patients. Multiple immune escape mechanisms are postulated as responsible. Our working hypothesis is that adenosine (ADO), an immunosuppressive molecule along with the ectoenzymatic pathways (CD39-CD73 and CD38-CD203a/PC-1-CD73) controlling its production, are involved in the dynamics of NB cells in the BM. The results indicate that ectonucleotidases are expressed by i) NB cell lines, ii) metastatic NB cells isolated from NB patients’ BM, iii) microvesicles (MV) derived from both NB cell types and iv) resident BM cell populations. BM infiltration by NB cells increased CD203a/PC-1 and CD73 expression on lymphoid and myeloid cells, respectively. Expressions of ectoenzymes and GD2 (NB-associated marker) were higher on MV from NB patients’ BM than in controls. Moreover, CD203a/PC-1 expression on BM-derived MV provide a basis for distinguishing NB patients with high or low BM infiltration. ADO production and consumption of related by-products were significantly higher when assessed on NB patients’ MV than those from controls. MV isolated from NB patients’ BM significantly downregulated in vitro T cell proliferation. Lastly, NB patients with worse prognosis are identified by a high percentage of CD38+ or CD73+ MV in the BM. In conclusion, ectonucleotidases are present and functional on NB cells, as well as in NB-infiltrated BM and in MV derived from BM. It is reasonable that MV are involved in BM infiltration by NB cells. Therefore, targeting these molecules may widen the therapeutic armamentarium for metastatic NB patients.
KEYWORDS: Neuroblastoma, metastasis, bone marrow, adenosine, microvesicles
Introduction
Neuroblastoma (NB), the most common extra-cranial solid pediatric tumor, is characterized by a peculiar biology and by variable prognosis. Indeed, clinical features of NB vary from spontaneous regression to aggressive metastatic disease.1 NB patients display different genetic alteration,2 including the amplification of the MYCN gene, an event that predicts a worse prognosis.3 Another adverse prognostic factor is the presence of metastasis at diagnosis [stage4]. High-risk NB patients are grouped in stage M, which displays less than 10% survival in case of no response to standard therapies or following relapse.5,6 Further, stage M patients are characterized by bone marrow (BM) infiltration by NB cells. Multiple evidences suggest that metastatic NB cells are different from primary tumor cell,7–10 and that both neoplastic and BM-resident cells are influenced by bi-directional signaling in the BM microenvironment.11
Metastatic NB cells in the BM exploit different mechanisms to escape the control of the immune system. The most known are downregulation of HLA molecules along with the expression and/or release of inhibitory molecules (i.e., HLA-G, MICA, B7H3 and calprotectin among the others.9,12,13 However, one of the strategies adopted by different human tumors [i.e. breast cancer,14 melanoma,15,16 prostate cancer,17 and gastric carcinom18] to impair the anti-tumor immune response relays on the local production of the immunosuppressive adenosine (ADO). Extracellular ADO is generated by a set of adenosinergic ectoenzymes, ruling the classical (the first to be identified) and alternative pathways. The first one relies on the metabolism of adenosine 5ʹ-triphosphate (ATP), metabolized by CD39, an ecto-nucleoside-triphosphate-diphosphohydrolase. ATP is converted to adenosine 5ʹ-diphospate (ADP), and the latter molecule into adenosine 5ʹ-monophospate (AMP).19 The alternative pathway starts from the metabolism of nicotinamide adenine dinucleotide (NAD+) operated by CD38, an ectoenzyme with ADP-ribosyl-cyclase/cyclic ADP ribose-hydrolase enzymatic activities, that converts NAD+ to adenosine diphosphate ribose (ADPR).20 The latter molecule may be converted to AMP in the presence of CD203a(PC-1) (an ectonucleotide-pyrophosphatase-phosphodiesterase-1). The same enzyme can also convert ATP to AMP. The two pathways converge to the action of CD73, a 5ʹ-nucleotidase, which converts AMP to AD.21,22
At the moment, expression and function of adenosinergic ectoenzymes on metastatic NB cells in the BM is not known. Hematopoietic cells, in fact, are sensitive to the action of AD,23 whose action is more efficient in closed systems (e.g. BM), considering its extremely limited half-life in vivo.
We recently proposed that the actions of ADO may include particle signals. This was demonstrated in the context of the BM niche of the multiple myeloma, where important signals are orchestrated by means of extracellular vesicles (EV).24 EV include exosomes, microvesicles (MV) and apoptotic bodies.25 Exosomes are smaller than MV (range 40–120 nm versus 50–1000) and are generated through an endocytic pathway. MV are directly released from the plasma membrane as a physiological step of the membrane dynamics: such release increases during physiological activation or neoplastic transformation.26,27 MV express on their surface specific markers [integrins, selectins, and phospatidylserine (PS)] and play a role in inter-cellular communications. They may be captured by distinct target cells through spontaneous or receptor-mediated endocytosis, or by direct fusion with the plasma membrane.28 EV released by tumor cells are reported to be involved in disease progression,29–31 through the inhibition of anti-tumor immune response.25,27
Recent studies on exosomes obtained from NB demonstrated that they can transfer oncogenic microRNAs to normal cells, contributing to metastasis and resistance to chemotherapy.32–35 In contrast, little is known about MV released by NB cells and their potential role in NB progression. A recent characterization of extracellular MV and exosomes released by mouse NB N2a cells demonstrated that they express multiple markers of the parental cell line, among which neural cell body protein UCH-L1, αII-spectrin, astroglial markers GFAP, BIII-tubulin, synaptophysin, and exosome marker Ali.36
The present study provides the results obtained by investigating the expression and function of adenosinergic ectoenzymes on metastatic NB cells present in the BM of NB patients, as compared with normal hematopoietic cells from patients and controls. A key question was whether BM infiltration by NB cells may influence the expression and function of these molecules within this microenvironment. The sequence of the study included the same analysis on MV released in the BM microenvironment of NB patients and controls. The last issue analyzed was the translational relevance of this study, by investigating possible correlations between the presence of adenosinergic ectoenzymes on MV isolated from BM at diagnosis and the prognosis of NB patients.
Results
Expression of adenosinergic ectoenzymes on NB cells
First, we analyzed the expression of adenosinergic ectoenzymes on NB cells isolated from NB patients with infiltrated BM as qualitatively and quantitatively compared to NB cell lines. The results obtained are summarized in Table 1A.
Table 1.
Numerical data.
| A. Expression of ectoenymes on NB cells and on MV derived from NB cells (MRFI, median ± SE) | ||||||||||
| CD38 | CD39 | CD203a/PC-1 | CD73 | CD26 | ||||||
| Patients’ NB cells | 1 ± 0.03 | 1.5 ± 0.4 | 10.1 ± 2.4 | 28.1 ± 5.3 | 1 ± 0.2 | |||||
| Patients’ NB cellsco-cultured with BM cells | 3.8 ± 0.9 | **p = 0.003 | 3.8 ± 0.2 | *p = 0.01 | 3.6 ± 2.1 | *p < 0.05 | 4 ± 1 | **p = 0.001 | 1 ± 0.1 | ns |
| NB cell lines | 2.3 ± 0.7 | ***p = 0.0007 | 4.1 ± 0.3 | **p = 0.004 | 2.8 ± 15.6 | **p = 0.008 | 4.2 ± 0.9 | **p = 0.002 | 1 ± 0.3 | ns |
| MV from patients’ NB cells | 1 ± 0.03 | **p = 0.003 | 7.5 ± 1.4 | *p = 0.04 | 15.7 ± 4 | *p = 0.04 | 12.8 ± 5.3 | *p = 0.04 | 1 ± 0.2 | ns |
| MV from NB cell lines | 1.29 ± 0.3 | 2.7 ± 0.7 | 4.4 ± 1.3 | 4.6 ± 1.1 | 1 ± 0.3 | |||||
| B. Ectoenzymes expression on BM cells from NB patients (MRFI, median ± SE) | ||||||||||
| CD38 | CD39 | CD203a/PC-1 | CD73 | |||||||
| Lymphocytes | 70.7 ± 40.2 | ***p < 0.0001 | 25.7 ± 7.2 | 2.3 ± 7.7 | ***p = 0.0008 | 13.6 ± 4.3 | *** p = 0.0005 | |||
| Monocytes | 44.4 ± 27.4 | ***p < 0.0001 | 36 ± 11.2 | 3.7 ± 9.9 | ***p < 0.0001 | 4.8 ± 6.5 | ||||
| Granulocytes | 24.4 ± 5.1 | **p = 0.002 | 19.7 ± 23.5 | 3.9 ± 62.1 | ***p = 0.0003 | 3.3 ± 1.5 | ||||
| NB cells | 7.6 ± 11.5 | 4.5 ± 3 | 5.7 ± 1.1 | 4.6 ± 0.8 | ||||||
| C. Ectoenzymes expression on BM cells from NB patients with high or low BM infiltration (MRFI, median ± SE) | ||||||||||
| CD38 | CD39 | CD203a/PC-1 | CD73 | |||||||
| Lymphocytes | High | 69 ± 29.7 | ns | 26.4 ± 12.3 | ns | 2.8 ± 14.4 | *p = 0.03 | 15.6 ± 7.5 | ns | |
| Low | 70.7 ± 75.8 | 25.5 ± 7.3 | 2.3 ± 0.8 | 11.3 ± 4.1 | ||||||
| Monocytes | High | 64 ± 18.9 | ns | 32.1 ± 21.9 | ns | 3.7 ± 18 | ns | 5.4 ± 12.6 | **p = 0.009 | |
| Low | 41.6 ± 51.7 | 39.4 ± 5.8 | 3.7 ± 3.3 | 2.7 ± 2.3 | ||||||
| Granulocytes | High | 23.6 ± 10 | ns | 19.7 ± 5 | ns | 3.9 ± 15.4 | ns | 4.4 ± 1.8 | *p = 0.05 | |
| Low | 25.3 ± 3.1 | 19.8 ± 46.9 | 11.5 ± 124.2 | 2.8 ± 2.6 | ||||||
| NB cells | High | 13.1 ± 19.6 | ns | 3.9 ± 5.1 | ns | 5.8 ± 1.8 | ns | 3.6 ± 1.5 | ns | |
| D. Ectoenzymes expression on BM MNC cells from NB patients cultured in the presence or absence of NB cells (MRFI, median ± SE) | ||||||||||
| CD38 | CD203a/PC-1 | CD39 | CD73 | |||||||
| ctr | +NB cells | ctr | +NB cells | ctr | +NB cells | ctr | +NB cells | |||
| Lymphocytes | 49.1 ± 19.2 | 21.4 ± 3.9 | 13.5 ± 3.3 | 15.6 ± 3.8 | 9.5 ± 1.3 | 7.1 ± 0.6 | 6.2 ± 0.6 | 4 ± 0.2 | ||
| ns | ns | ns | ns | |||||||
| Monocytes | 19.1 ± 1.7 | 14.5 ± 1.1 | 6.1 ± 0.4 | 5.7 ± 0.3 | 7.6 ± 0.5 | 7 ± 0.5 | 3.8 ± 0.2 | 4.4 ± 0.3 | ||
| ns | ns | ns | ns | |||||||
| Granulocytes | 2.8 ± 0.3 | 2.7 ± 0.2 | 1.6 ± 0.2 | 1.9 ± 0.2 | 3.7 ± 0.5 | 8 ± 1.6 | 1.9 ± 0.3 | 2.3 ± 0.3 | ||
| Ns | * p = 0.01 | *p = 0.01 | *p = 0.01 | |||||||
CD38 and CD39 expressions were very low on metastatic NB cells, whereas they were highly expressed in NB cell lines (Figure 1, panel a). Conversely, CD203a/PC-1 and CD73 expressions were higher in NB cells from patients than in NB cell lines. CD26 was almost constantly absent in both NB cell types.
Figure 1.
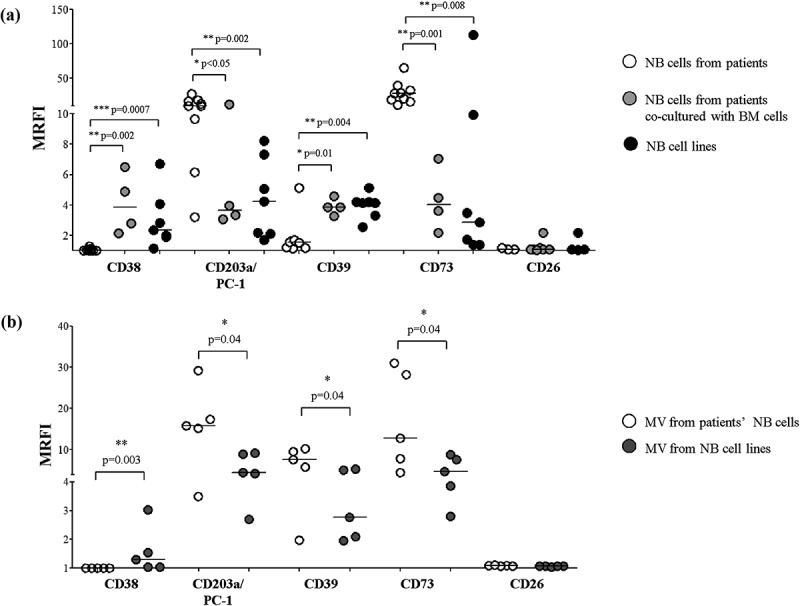
Expression of adenosinergic ectoenzymes on NB cells.
Panel a: The expression of CD38, CD39, CD203a/PC-1, CD73, and CD26 has been evaluated by flow cytometry on NB cell lines (n = 7, black dots) and metastatic NB cells isolated from patients’ BM samples, either freshly isolated (n = 8, white dots) or co-cultured with autologous BM MNC cells (n = 4, gray dots). The same analysis was performed on MV isolated from NB cell lines (n = 5, gray dots) and patients’ metastatic NB cells (n = 5, white dots) (Panel b). Results are expressed as mean relative of fluorescence intensity (MRFI). Horizontal bars indicated medians. p values are indicated where differences are statistically significant (Mann-Whitney test).
The first conclusion is that the expressions of adenosinergic ectoenzymes in NB cells from patients are definitely different from that of stabilized NB cell lines. A reasonable explanation is that the latter cells lost some of their original features during a long stay (years) in culture, but prolonged culture (weeks) of BM-derived freshly isolated NB cells did not affect their phenotype (data not shown). Another possibility was that the observed diversity may be due to interactions between NB cells and cellular and soluble components of the BM environment. To test this hypothesis, we cultured freshly isolated NB cells in the presence (or absence) of BM-resident mononuclear cells (MNC). As shown in Figure 1 (panel a), the phenotype of BM-derived NB cells after co-culture with BM MNC cells is similar the phenotype of NB cell lines: indeed, CD38 and CD39 were significantly upregulated, whereas CD203a/PC-1 and CD73 were significantly downregulated. These results confirmed that the interaction of NB cells with BM MNC cells may alter the expression of adenosinergic ectoenymes on neoplastic cells.
Phenotype of MV isolated from NB cells
Tumor and normal cells growing in a closed environment (e.g., BM) may use microvesicles (MV) to communicate inside and outside the space context. Thus, the expression of adenosinergic ectoenzymes (completed by CD26) was analyzed on MV isolated from NB cells infiltrating the BM and from NB cell lines (Figure 1, panel b). The purity of MV preparation was confirmed by the presence of PS, tested as specific marker of MV, as previously reported24 (data not shown). The phenotype of the MV turned out to be identical to that displayed by parental cells. Indeed, MV isolated from patients’ NB cells expressed high levels of CD203a/PC-1 and CD73, whereas MV from NB cell lines were characterized by high levels of CD38. However, a significant difference between cells and the corresponding MV concerned CD39 expression, that was higher in MV from patients’ NB cells than in those from NB cell lines. Table 1A summarizes these findings.
Expression of adenosinergic ectoenzymes on cell populations in the BM
In order to identify the potential causes for the differences in NB phenotype, observed in metastatic cells, we defined the full picture of the ectoenzymes present on the infiltrating NB cells and on the main cell populations, lymphocytes, monocytes, and granulocytes, present in the BM. Samples were obtained from 17 NB patients, and data are summarized in Table 1B. CD38 expression was very high in lymphocytes and monocytes, and low in granulocytes, however, CD38 expression was significantly higher in all MNC populations than in NB cells. Conversely, CD203a/PC-1 expression was low to intermediate on lymphocytes, monocytes, granulocytes, and NB cells, and no significant differences were detected between MNC and NB cells (Figure 2, panel a and b, respectively). CD39 expression was high in lymphocytes, monocytes, and granulocytes, whereas NB cells display a significantly lower expression than MNC (Figure 2, panel c). Lastly, CD73 expression was almost identical in NB cells and MNC: an exception is represented by lymphocytes, characterized by an expression of CD73 significantly higher than NB cells (Figure 2, panel d). Taken together, these observations suggest that the resident BM leukocytes express levels of ectoenzymes higher than that of infiltrating NB cells; therefore MV isolated from BM samples may derive either from MNC or NB cells.
Figure 2.
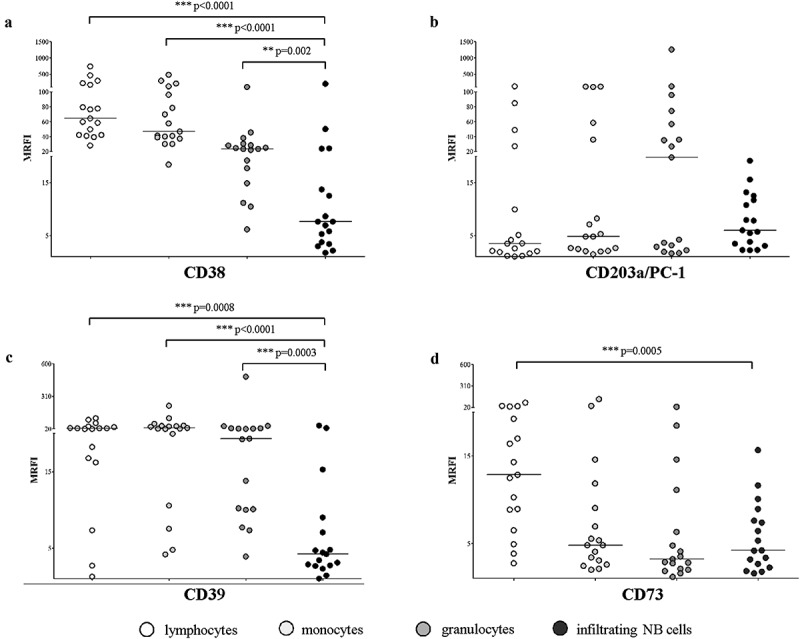
Expression of adenosinergic ectoenzymes on BM-resident cell populations.
The expression of CD38 (panel a), CD203a/PC-1 (panel b), CD39 (panel c) and CD73 (panel d) has been evaluated by flow cytometry on BM-resident cells in BM samples from 17 NB patients, gating on lymphocytes (white dots), monocytes (light gray dots), granulocytes (gray dots) and infiltrating NB cells (dark gray dots). Results are expressed as MRFI. Horizontal bars indicated medians. p values are indicated where differences (normal cells vs NB cells) are statistically significant (Mann-Whitney test)
Next, we asked whether the amount of NB infiltration in the BM may influence the expression of adenosinergic ectoenzymes by the BM-resident cells. Thus, we compared BM samples with high or low infiltration NB cells’ infiltration. The cut-off value for NB infiltration (10%) was calculated by ROC curves analysis, and results are summarized in Table 1C. As shown in Figure 3 (panel a) CD38 expressed by lymphocytes, monocytes, granulocytes, and NB cells was not significantly influenced by the degree of infiltration. CD203a/PC-1 expression on lymphocytes was significantly higher in highly infiltrated BM, whereas CD203a/PC-1 expression in monocytes, granulocytes, and NB cells was independent from BM infiltration (Figure 3, panel b). Similarly, CD39 expression on lymphocytes, monocytes, granulocytes, and NB cells was unaffected by the degree of BM infiltration (Figure 3, panel c). Lastly, CD73 expression was increased on monocytes and granulocytes from highly infiltrated BM samples, whereas its expression on lymphocytes and NB cells was independent from the degree of infiltration (Figure 3, panel d).
Figure 3.
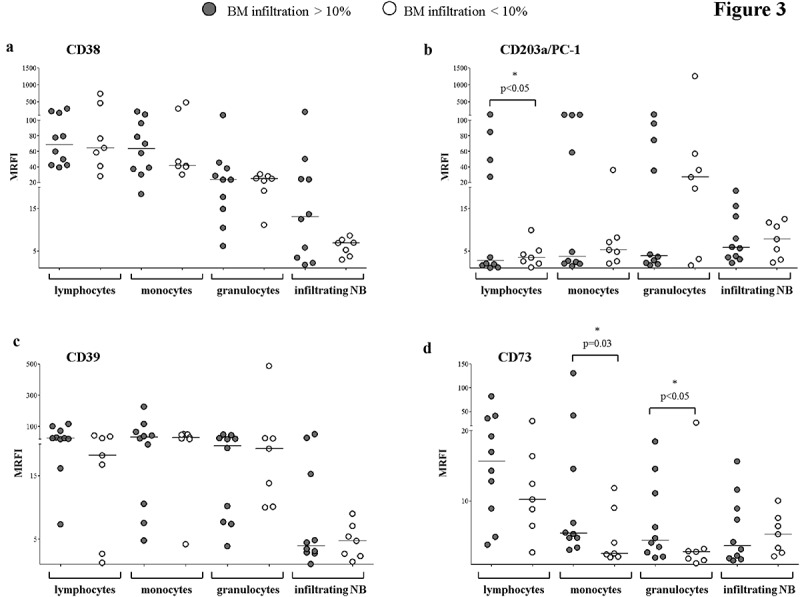
Expression of adenosinergic ectoenzymes on BM-resident cell populations in BM samples with high or low NB infiltration.
The expression of CD38 (panel a), CD203a/PC-1 (panel b), CD39 (panel c) and CD73 (panel d) was evaluated by flow cytometry on BM-resident cells, gating on lymphocytes, monocytes, granulocytes and infiltrating NB cells. BM samples with high (n = 10, gray dots) or low (n = 7, white dots) NB infiltration have been compared. Results are expressed as MRFI. Horizontal bars indicated medians. p values are indicated where differences are statistically significant (t-test for lymphocytes and NB cells, Mann-Whitney test for the other cell populations)
Adenosinergic ectoenzymes are up-regulated on myeloid cell populations upon co-colture with metastatic NB cells
Based on these observations, we asked whether the increased expression of adenosinergic ectoenzymes on MNC from NB-infiltrated BM samples could be related to a direct interaction between resident BM cells and NB cells and/or to the release of soluble factors by NB cells.
To address this issue, BM MNC cells were cultured alone or in the presence of metastatic NB cells (at 1:1 MNC:NB ratio) for 48 h. Then, the expression of adenosinergic ectoenzymes was analyzed on CD45+ cells, gating on lymphocytes, monocytes, and granulocytes on the basis of physical parameters. Results are summarized in Table 1D. As shown in Figure 4, the expression of CD38, CD203a/PC-1, CD39 and CD73 was not significantly modulated by metastatic NB cells on lymphocytes (panel A) and monocytes (panel B). In contrast, the expression of CD203a/PC-1, CD39, and CD73 (but not of CD38) was significantly upregulated on granulocytes upon co-colture with NB cells (panel C), thus suggesting a selective action of this latter cell population.
Figure 4.
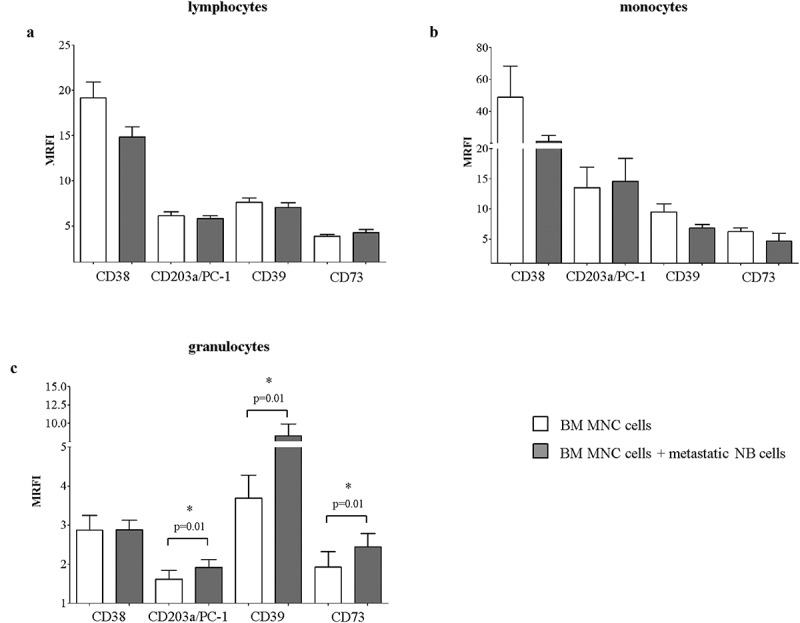
Expression of adenosinergic ectoenzymes on BM MNC upon co-culture with metastatic NB cells.
The expression of CD38, CD203a/PC-1, CD39, and CD73 was evaluated by flow cytometry on BM MNC cultured in the presence (gray bars) or absence (white bars) of metastatic NB cells, at 1:1 MNC:NB ratio. The expression of each molecule was analyzed in CD45+ cells, gating on lymphocytes (panel a), monocytes (panel b) and granulocytes (panel c). Results are expressed as MRFI. p values are indicated where differences are statistically significant (Mann-Whitney test)
Expression of adenosinergic ectoenzymes in microvesicles from BM plasma samples
To differentiate the cell origin of MV, we analyzed the expression of GD2, a surface disialoganglioside highly expressed by NB cells. Figure 5 (panel a) shows that the expression of GD2 on MV isolated from NB patients is significantly higher than in MV from controls, confirming that MV – at least in part – originate from the NB cells infiltrating the BM.
Figure 5.
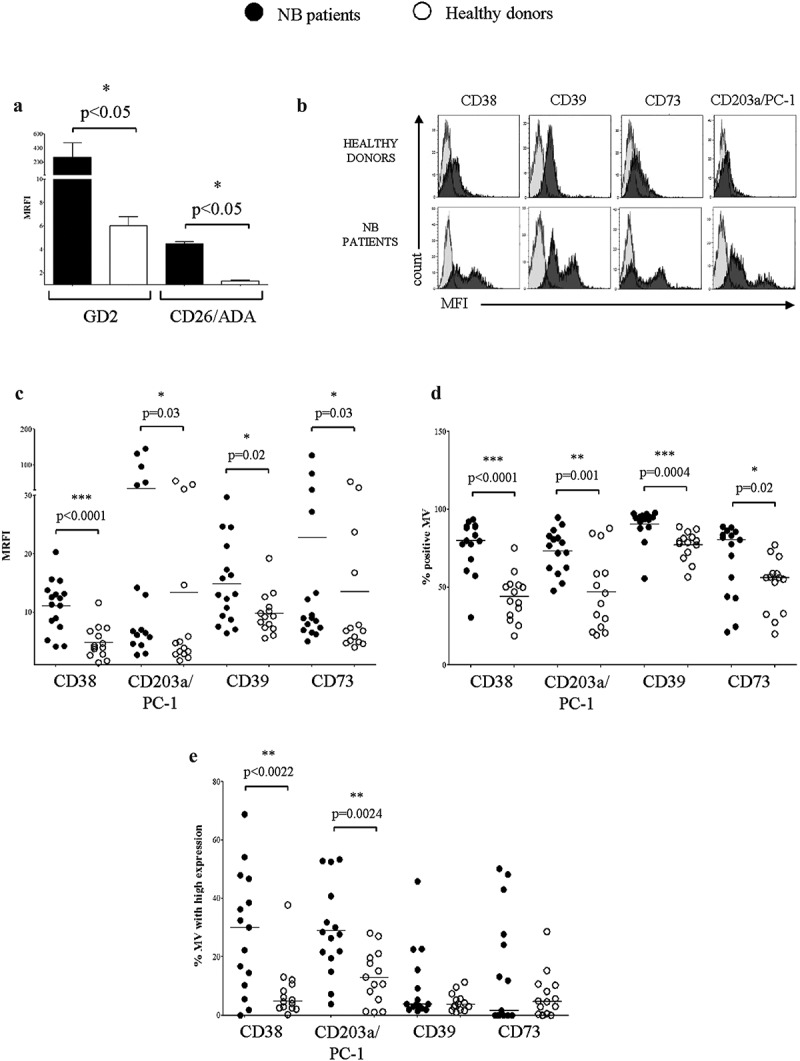
Expression of adenosinergic ectoenzymes on MV isolated from BM plasma samples.
Panel a. The expression of GD2 and CD26/ADA has been evaluated by flow cytometry on MV isolated from BM plasma samples obtained from NB patients (n = 4, black bars) or healthy controls (n = 4, white bars). Results are expressed as MRFI (median ± SE). p values are indicated where differences are statistically significant (Mann-Whitney test). The expression of CD38, CD203a/PC-1, CD39 and CD73 has been evaluated by flow cytometry on MV isolated from BM plasma samples obtained from NB patients and controls. Histograms in panel b shows a representative experiment. Grey profiles indicate staining with irrelevant isotype-matched mAbs, whereas black profiles indicate staining with specific mAbs. Pooled results obtained from NB patients (n = 16, black dots) and controls (n = 14, white dots) are expressed as MRFI (panel c), percentage of positive MV (panel d) or percentage of MV with high expression (panel e). Horizontal bars indicated medians. p values are indicated where differences are statistically significant (t-test for CD38 and CD203a/PC-1, Mann-Whitney test for the other ectoenzymes)
To have a dynamic representation of ADO metabolism, we then analyzed the expression of CD26, a surface molecule harboring adenosine deaminase (ADA), on MV. As shown in Figure 5 (panel a), the expression of CD26 on MV isolated from NB patients is higher than in controls. Quantitative differences between MV isolated from NB patients as compared to controls are summarized in Table 2A.
Table 2.
Numerical data.
| A. Expression of ectoenymes on MV derived from BM plasma samples of NB patients and controls (median ± SE) | |||||||||||||
| GD2 | CD26 | CD38 | CD203a/PC-1 | CD39 | CD73 | ||||||||
| % of positive MV | NB patients | 77.05 ± 16.95 | ***p < 0.0001 | 73.34 ± 14 | **p = 0.001 | 90.57 ± 10.79 | ***p = 0.0004 | 67.71 ± 23.61 | *p = 0.02 | ||||
| controls | 43.44 ± 14.83 | 46.95 ± 44.63 | 77.12 ± 9.1 | 51.99 ± 17.33 | |||||||||
| % of MV with high expression | NB patients | 28.41 ± 20.58 | **p = 0.0022 | 28.86 ± 15.53 | **p = 0.0024 | 3.8 ± 3.1 | ns | 1.6 ± 4.9 | ns | ||||
| controls | 8 ± 9.44 | 12.85 ± 9.1 | 3.6 ± 0.8 | 4.7 ± 2 | |||||||||
| MRFI | NB patients | 88.1 ± 20.4 | *p = 0.05 | 4.6 ± 0.15 | *p = 0.05 | 11 ± 4.4 | ***p < 0.0001 | 33.9 ± 48 | *p = 0.03 | 14.8 ± 7 | *p = 0.02 | 22.7 ± 33.1 | *p = 0.03 |
| controls | 6 ± 0.78 | 1.2 ± 0.12 | 4.8 ± 2.7 | 13.3 ± 18.2 | 9.7 ± 3.5 | 13.5 ± 15 | |||||||
| B. Expression of ectoenymes on MV derived from BM plasma samples of NB patients with high or low BM infiltration (median ± SE) | |||||||||||||
| CD38 | CD203a/PC-1 | CD39 | CD73 | ||||||||||
| % of positive MV | High | 79.9 ± 11 | ns | 74.3 ± 14.5 | ns | 93.9 ± 2.4 | ns | 76 ± 15.6 | ns | ||||
| Low | 74.1 ± 24.3 | 71.7 ± 14.2 | 85.5 ± 16.2 | 55.2 ± 29.3 | |||||||||
| % of MV with high expression | High | 28.2 ± 17.2 | ns | 34.2 ± 12 | *p = 0.03 | 10 ± 14.9 | ns | 18.5 ± 22.8 | ns | ||||
| Low | 28.6 ± 26.6 | 20.7 ± 17.7 | 9.2 ± 8.3 | 8.8 ± 11.1 | |||||||||
| MRFI | High | 12.5 ± 4.4 | ns | 33.9 ± 49.2 | ns | 16.1 ± 6.1 | ns | 17.1 ± 21.7 | ns | ||||
The expression of adenosinergic ectoenzymes belonging to conventional (CD39, CD73) and alternative (CD38/CD203a-PC-1/CD73) adenosinergic pathways was then analyzed on MV isolated from BM plasma samples from both NB patients and controls. Data are summarized in Table 3A and Figure 5 (panel b) shows the results of a representative experiment.
Table 3.
| A) Functional characterization of adenosinergic ectoenzymes expressed on MV (* = substrate, results expressed as nmol/30ʹ) | |||||||||
| ATP | ADPR | NIC | ADP | AMP | ADO | IMP | INO | ||
| CD73 | NB | - | - | - | - | 18.08* | 63.9 | - | 2.95 |
| Controls | - | - | - | 79.64* | 0.96 | - | 0 | ||
| CD203a/PC-1 | NB | - | 89.13* | - | - | 0.45 | 0.22 | 8.4 | 18.17 |
| Controls | - | 79.10* | - | - | 2.08 | 0.03 | 4 | 1.66 | |
| CD39 | NB | 13.85* | - | - | 47.01 | 38.83 | 1.34 | 4.4 | 3.55 |
| Controls | 71.20* | - | - | 12.85 | 7.28 | 0 | 4.95 | 0 | |
| CD38 | NB | - | 54.24 | 10.33 | - | 0.42 | 0.16 | 13.22 | 3.36 |
| Controls | - | 29.15 | 5.33 | - | 1.86 | 0.08 | 0 | 0 | |
| B) Inhibition of T cell proliferation by BM-derived MV | |||||||||
| % of proliferatingT cells | |||||||||
| T cells + anti-CD3/CD28 beads | 95.7 ± 0.1 | ||||||||
| T cells+ anti-CD3/CD28 beads+ BM-derived MV | 69.6 ± 2.2 | ||||||||
p=0.01
MV obtained from NB patients featured a higher expression of CD38, CD203a/PC-1, CD39 and CD73 (Figure 5, panel c). Moreover, the percentage of MV expressing CD38, CD203a/PC-1, CD39 and CD73 (panel D) was higher than on control MV. Quantitative dissection of the percentage of MV expressing high levels of each ectoenzyme (see Materials and Methods) showed that samples isolated from NB patients were characterized by a percentage of CD38hi and CD203a/PC-1hi MV higher than controls. On the contrary, no differences were observed for CD39hi and CD73hi MV between NB patients and controls (Figure 5, panel e).
Collectively, these findings indicate that MV from NB patients are equipped with a complete set of ADO-producing ectoenzymes and the machinery able to degrade ADO, likely more efficient than in control MV.
Expression of adenosinergic ectoenzymes by MV is only modestly influenced by BM infiltration
We next asked whether the degree of BM infiltration by NB cells would influence the expression of adenosinergic ectoenymes on MV isolated from BM plasma. To answer this issue, we compared MV isolated from BM samples characterized by high or low NB infiltration (cutoff value 10%). Results are summarized in Table 2B.
As shown in Figure 6, MV isolated from BM samples of NB patients with high or low infiltration displayed almost overlapping expression levels of each ectoenzyme (panel A). Similarly, the percentage of MV expressing CD38, CD203a/PC-1, CD39, and CD73 was almost identical in the two groups (panel B). Also, the percentage of MV expressing high levels of CD38hi, CD39hi, and CD73hi was similar in the two groups (Figure 6, panel c). On the contrary, the percentage of MV expressing high levels of CD203a/PC-1 was significantly increased in BM samples with high NB infiltration (Figure 6, panel c), in accordance with the observed up-regulation of surface CD203a/PC-1 expression in the BM environment.
Figure 6.
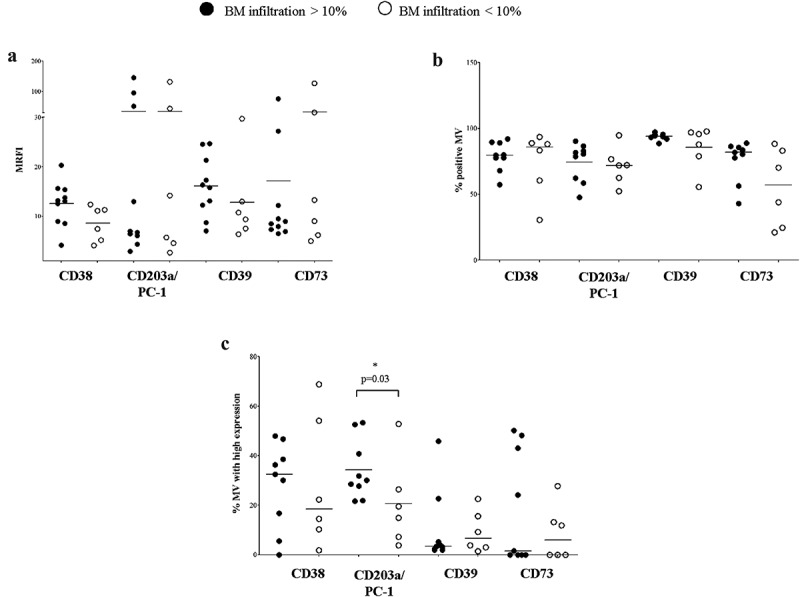
Expression of adenosinergic ectoenzymes on MV isolated from BM plasma samples with low or high NB infiltration.
The expression of CD38, CD203a/PC-1, CD39, and CD73 has been evaluated by flow cytometry on MV isolated from BM plasma samples obtained from NB patients with high (n = 10, black dots) or low (n = 6, white dots) NB infiltration. Results are expressed as MRFI (panel a), percentage of positive MV (panel b) or percentage of MV with high expression (panel c). Horizontal bars indicated medians. p values are indicated where differences are statistically significant (t-test for CD38 and PC-1, Mann-Whitney test for the other ectoenzymes)
MV obtained from NB patients are enzymatically more efficient in terms of ADO production than those from controls
To test whether the ectoenzymes present on MV isolated from BM samples were functional, we measured ADO production by MV after exposing them to different substrates. Precisely, MV obtained from 5 distinct NB patients and from 5 distinct controls were separately pooled and challenged with ATP, NAD+, ADPR, and AMP. Results obtained are summarized in Table 3A. As shown in Figure 7, panel a, AMP consumption was higher in MV isolated from NB patients than in those from controls. The production of ADO, inosine monophosphate (IMP) and inosine (INO) was consequently higher in the former than in the latter samples. These findings indicated that the enzymatic activity exerted by CD73 was increased in MV obtained from NB patients.
Figure 7.
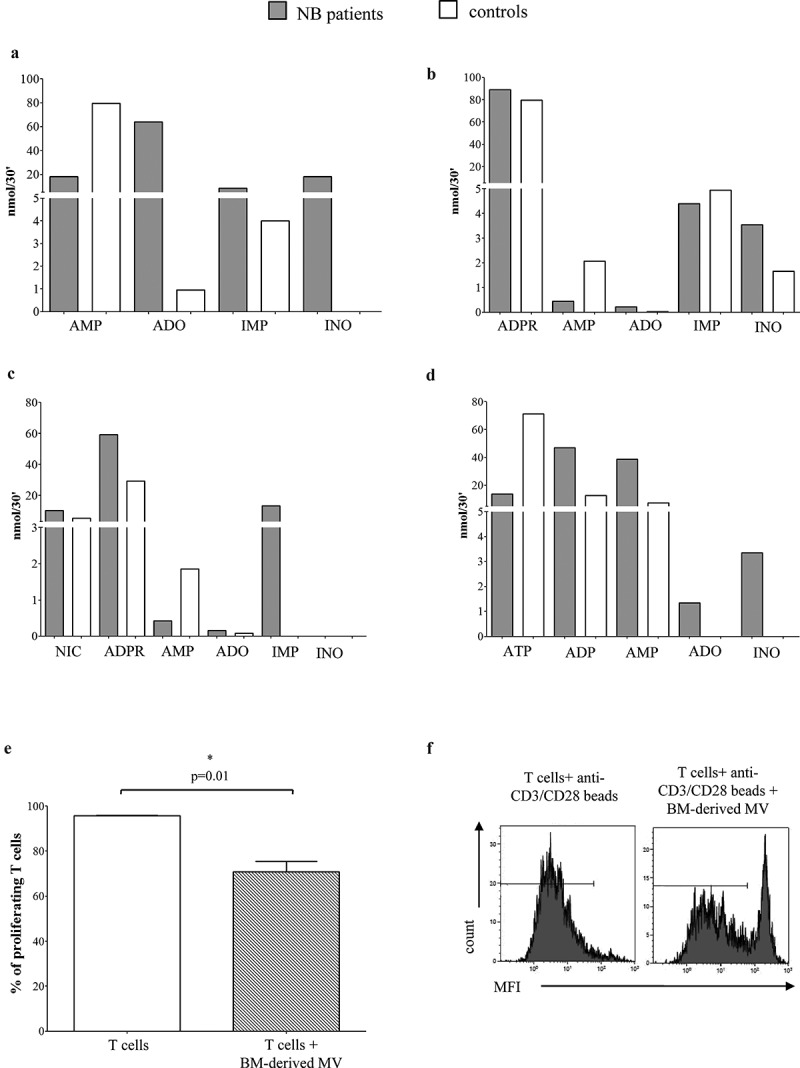
Characterization of the enzymatic and immunosuppressive function of adenosinergic ectoenzymes on MV.
The enzymatic function of CD73, CD203a/PC-1, CD38, and CD39 was evaluated on pooled MV from five NB patients (gray bars) and five controls (white bars), using as substrate adenosine 5ʹ-monophosphate (AMP, panel a), adenine diphosphate ribose (ADPR, panel b), nicotinamide adenine dinucleotide (NAD+, panel c) and adenosine 5ʹ-triphosphate (ATP, panel d). Results are expressed as nmol of substrate, adenosine (ADO) or the byproducts AMP, adenosine 5ʹ-monophosphate (ADP), ADPR, inosine (INO), inosine monophosphate (IMP) and nicotinamide (NIC). The immunosuppressive function of MV was evaluated by co-culturing T cells isolated from four healthy donors and stimulated with anti-CD3/CD28 beads in the presence (gray bar) or absence (white bar) of NB patients’ BM-derived MV. Histograms in panel E shows the median of four different experiments ± SEM. Histograms in panel F shows a representative experiment.
The consumption of ADPR (Figure 7, panel b) and NAD+ (Figure 7, panel c) resulted similar in patients and controls, suggestive of similar CD203a/PC-1 and CD38 enzymatic activities. However, differences were observed in terms of performances. Indeed, MV from NB patients produced higher amounts of ADO and INO from ADPR, as well as higher amount of ADPR and IMP from NAD+ than those from controls. On the other side, MV from controls yielded higher amounts of AMP, suggesting an increased degradation of AMP in MV from NB patients, likely due to an increased CD73 enzymatic activity. Lastly, ATP consumption and production of by-products (ADP, AMP, ADO, and INO) was higher in MV from NB patients than in MV from controls (Figure 7, panel d), suggesting that the CD39 enzymatic activities are increased in MV derived from NB patients as compared to those from controls.
MV isolated from NB patients’ BM samples inhibited t cell proliferation
We next asked whether MV isolated from NB patients’ BM samples exert immunosuppressive functions. To answer this issue, we analyzed the proliferation of T cells isolated from normal donors in response to polyclonal stimuli, in the presence or absence of these MV preparations. Data are summarized in Table 3B. As shown in Figure 7, panel e, T cell proliferation was significantly reduced in the presence of BM-derived MV, thus suggesting that these MV may participate in the local immunosuppression in the BM microenvironment, thus favoring the metastatic spread of NB cells.
CD38 and CD73 expression on MV in the BM correlated with a worse prognosis for NB patients
A key question is whether adenosinergic ectoenzymes expressed by MV released in the BM of NB patients may influence their clinical outcome. To test this issue we first defined cut-off levels of expression for CD38, CD39, CD203a/PC-1, and CD73 by considering MRFI, percentage of positive MV and percentage of MV with high expression for each ectoenzyme. Then we analyzed event-free survival (EFS) and overall survival (OS) of 10 NB patients whose follow-up data were available, after stratifying them above or below the determined cut-off levels.
As shown in Figure 8, NB patients with a percentage of BM-derived MV expressing CD38 and CD73 above the cut-off level (panel A and C, respectively) displayed a worse EFS than patients with percentages of BM-derived MV below the cut-off. Conversely, the percentage of MV expressing CD39 or CD20a/PC-1 did not significantly influence the EFS of NB patients (panel B and D, respectively). In addition, no correlation was found between i) EFS and MRFI or percentage of MV expressing high levels of each ectoenzyme and ii) OS and ectoenzyme expression on MV. Taken together, these results support the hypothesis that MV expressing CD38 and CD73 released in the BM microenvironment represent an immune escape mechanism adopted by infiltrating NB cells, a step which eventually favors the relapse of NB.
Figure 8.
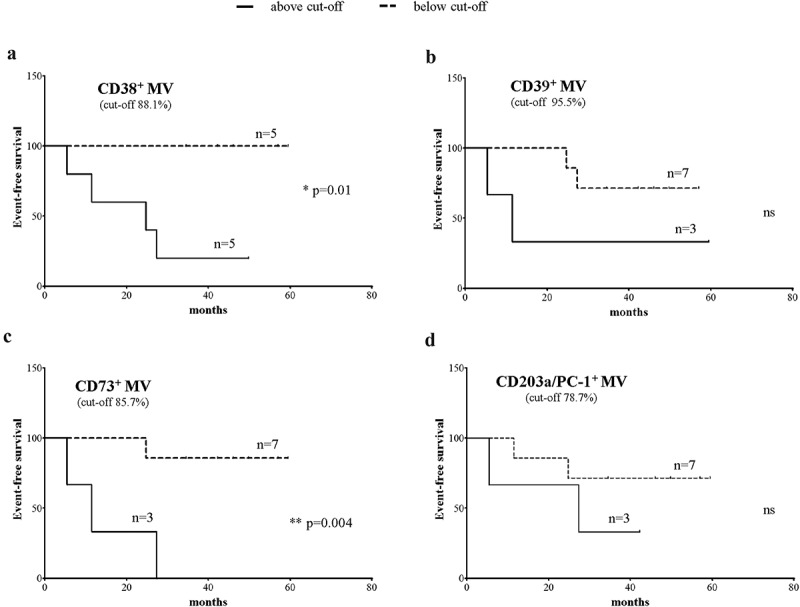
Correlation between expression of ectoenzymes on MV and prognosis of NB patients.
Kaplan-Meier analysis of event-free survival (EFS) of 10 NB patients with a percentage of MV expressing CD38 (panel a), CD39 (panel b), CD73 (panel c) and CD203a/PC-1 (panel d) above (black line) or below (dashed line) the cut-off level determined by ROC curves for each parameter. Y-axes indicate the percentage of relapse-free patients, whereas X-axes indicate the time to relapse (months). Curve comparison was performed using Log-rank (Mantel-Cox) test. p values are indicated where the difference is statistically significant.
Discussion
Bone marrow (BM) is the elective site for metastatic spread of malignant cells in NB patients, representing the most adverse prognostic factor.37 Reports indicate that NB infiltration may affect the phenotype and function of BM-resident cells.10,38,39 The results of the present study show that both metastatic NB cells and NB cell lines express adenosinergic ectoenzymes, thus NB cells may contribute themselves to ADO production in the BM microenvironment. Moreover, BM infiltration by metastatic NB cells is paralleled by an altered expression of ectoenzymes on resident BM cell populations. Precisely, BM samples with high NB infiltration showed the presence of lymphoid and myeloid cell subsets with an increased expression of CD203a/PC-1 and CD73, respectively. Reports from our group indicate that ADO production within the BM niche of patients with multiple myeloma may contribute to tumor progression.24,40,41 In line with this, it is reasonable to speculate that the combined adenosinergic activity of ectoenzymes located on infiltrating NB cells and on BM-resident cells lead to an increased ADO production inside BM microenvironment, that may lead to the generation of an impaired local anti-tumor immune response, with a consequent spreading of metastatic NB cells. The inference is that metastatic NB cells sustain themselves the metastatic spread, by increasing expression and function of adenosinergic ectoenzymes by BM-resident cells.
Another original finding of this study is the characterization of MV released by NB cells. Indeed, exosomes have been characterized in the last years in the context of NB,32–35 whereas limited information is available regarding MV derived from NB.36 MV are reported as carrying Cu, Zn superoxide dismutase derived from parental NB cell line.42 Here, we show that MV released from metastatic NB cells and NB cell lines recapitulate the expression of a cascade of ectoenzymes, including CD38, CD39, CD203a/PC-1, and CD73 as detected on the parental cells. Even more important is the characterization of the phenotype of MV released in the BM environment in samples derived from NB patients. MV isolated from NB patients express GD2, a NB-associated antigen. This finding, bona fide, indicate that the MV we analyzed derive – at least in part – from metastatic NB cells. Secondly, MV derived from NB patients show an expression of adenosinergic ectoenzymes higher than MV from controls. The multiple peaks of fluorescence intensity, detected predominantly in samples from NB patients, may suggest that the MV studied derive from distinct cell populations (i.e., resident BM cells as well as metastatic NB cells). A further observation is that the production of ADO and the consumption of related by-products observed in MV isolated from NB patients is significantly higher than in controls, suggesting that the increased expression of adenosinergic ectoenzymes may be paralleled by higher enzymatic performances. Finally, the observation that the level of BM infiltration by NB cells alters the phenotype of MV released within BM microenvironment is of relevance for its clinical transferability. Indeed, the increased expression of CD203a/PC-1 on MV in BM samples with high infiltration suggests that these MV may produce increased amounts of ADO exploiting alternative pathway of synthesis as compared to BM with low NB infiltration.
It is known that the presence of MV endowed with complete and functional adenosinergic pathways correlates with the presence of malignant plasma cells in the BM of patients with multiple myeloma.24 Here, we observed that a high percentage of MV expressing CD38 and CD73 in the BM microenvironment of NB patients correlate with a worse event-free survival. Thus, it is tempting to speculate that the release of MV equipped with efficient adenosinergic enzymatic activities leads to increased production of ADO within the BM niche. BM is a closed system, ideal to the establishment of an immunosuppressive microenvironment that coadiuvate tumor growth and dissemination of metastasis. Previous studies performed in solid and hematological malignancies have already demonstrated a key role of tumor-derived MV in the progression of disease.30,43–45
In conclusion, based on i) the expression of functional adenosinergic ectoenzymes on malignant cells, ii) the up-regulation of these molecules on BM-resident cells, iii) the release of immunosuppressive MV able of amplify ADO production within the BM context, as depicted in Figure 9, the results of this study may delineate a potential immune escape mechanism for metastatic NB. Further studies will evaluate whether CD38, CD39, CD203a/PC-1, and CD73 may represent novel therapeutic targets for high-risk NB patients.
Figure 9.
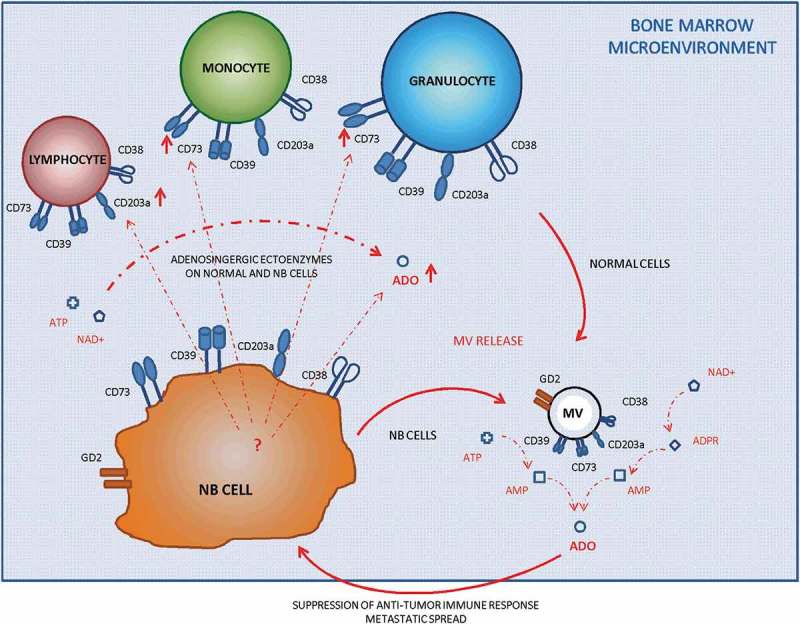
Schematic representation of the interactions between resident cells and metastatic cells in the BM.
The presence of NB metastatic cells in the BM affects the expression of ectonucleotidases on BM-resident cells. Moreover, both cell populations released MV endowed with adenosinergic ectoenzymes within the BM. Those MV participate in ADO production, thus contributing to the immune escape of metastatic NB cells.
Materials and methods
Patients and cell lines
The patients involved in the study were diagnosed with NB in Italy during the period November 2010–April 2011, and from January to April 2018. Patient’s samples were obtained following written informed consent according to Helsinki Declaration, and were subsequently centralized at the Istituto Giannina Gaslini (Italy).
Both diagnosis and staging were made according to INRG-SS criteria.4,46 Demographic and clinical data were retrieved from the Italian NB Registry.47 The main features of the patients included in the study are summarized in Table 4. All the analyses were made using samples taken at diagnosis. As controls, samples were obtained from healthy siblings of children admitted to the Istituto Giannina Gaslini to undergo BM transplants.
Table 4.
Characteristics of NB patients.
| # | Age at diagnosis(months) | BM infiltration(% NB cells) | Stage | MYCN status | Relapse | Time of relapse(months) | Follow-up | Time of follow-up(months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | <10 | M | gain | yes | 5.3 | alive | 45.2 |
| 2 | 72 | <10 | M | single copy | no | alive | 49.8 | |
| 3 | 1 | <10 | Ms | single copy | no | alive | 42.2 | |
| 4 | 46 | <10 | M | single copy | yes | 27.4 | alive | 34.9 |
| 5 | 21 | <10 | M | amplified | no | alive | 56.9 | |
| 6 | 20 | >10 | M | amplified | no | alive | 59.4 | |
| 7 | 36 | >10 | M | single copy | yes | 24.7 | dead | 48.8 |
| 8 | 17 | >10 | M | amplified | yes | 11.4 | dead | 18.1 |
| 9 | 9 | <10 | M | single copy | no | alive | 34.5 | |
| 10 | 26 | <10 | M | gain | no | alive | 46.0 | |
| 11 | 3 | <10 | Ms | amplified | N.A. | N.A. | ||
| 12 | 52 | >10 | M | amplified | N.A. | N.A. | ||
| 13 | 32 | <10 | M | - | N.A. | N.A. | ||
| 14 | 2 | >10 | L2 | single copy | N.A. | N.A. | ||
| 15 | 46 | >10 | L2 | single copy | N.A. | N.A. | ||
| 16 | 11 | >10 | M | single copy | N.A. | N.A. | ||
| 17 | 39 | <10 | M | - | N.A. | N.A. | ||
| 18 | 18 | <10 | L2 | single copy | N.A. | N.A. | ||
| 19 | 4 | >10 | Ms | single copy | N.A. | N.A. | ||
| 20 | 8 | <10 | Ms | gain | N.A. | N.A. | ||
| 21 | 39 | <10 | L2 | - | N.A. | N.A. | ||
| 22 | 16 | >10 | M | single copy | N.A. | N.A. | ||
| 23 | 140 | >10 | M | single copy | N.A. | N.A. | ||
| 24 | 4 | >10 | M | single copy | N.A. | N.A. | ||
| 25 | 53 | <10 | M | single copy | N.A. | N.A. | ||
| 26 | 34 | <10 | L | single copy | N.A. | N.A. | ||
| 27 | 140 | <10 | M | - | N.A. | N.A. |
Plasma samples were obtained from BM aspirates and stored at −80°C until use. MV were isolated from BM plasma samples of 16 NB patients and 14 control subjects. In some experiments, whole fresh BM samples were used for flow cytometric analysis.
NB cell lines GI-ME-N, GI-LI-N, HTLA 230, IMR-32, LAN-5, SKNAS and SH-SY-5Y were purchased from ATCC. NB cells were isolated from BM samples as previously described10 and frozen until use. NB cell lines were cultured in DMEM medium, supplemented with 10% fetal bovine serum, 1 mM non-essential amino acids, 4 mM L-glutamine (all from Sigma-Aldrich) and antimycotic/antibacterial. Cells were detached using Non-enzymatic Cell Dissociation Solution solution (Sigma-Aldrich) and subjected to flow cytometric analysis.
Isolation of microvesicles
MV were isolated from BM plasma samples by differential centrifugation, as reported.48 Briefly, 500 μl of each BM plasma sample was diluted (1:3) in PBS and centrifuged (3,000 g for 15 min at 4°C) to pellet large cell debris and remove remaining platelets. The supernatant was collected in a suitable centrifugation tube and centrifuged (20,000 g for 1 h at 4°C) in a fixed-angle rotor, washed once in PBS and suspended in 50 μl of binding buffer [PBS containing 0.5% BSA and 2 mM EDTA (both from Sigma Aldrich)]. MV size and polydispersity were analyzed using the Zetasizer Nano ZS90 particle sizer at a 90° fixed angle (Malvern Instruments, Worcestershire, UK), as described.49 The expression of PS, a marker that identifies MV, was investigated by flow cytometry on MV preparation, using FITC-conjugated Annexin V (Beckman Coulter), as reported.24
Flow cytometric analysis
The expression of ectoenzymes was evaluated on MV, BM cells and NB cells using anti-CD38 (#IB4), anti-CD73 (#CB73) and anti-CD26 mAb (#CB26) monoclonal (m)Abs generated in our Lab and conjugated with FITC-, PE- or APC-fluorochromes by Aczon (Bologna, Italy). Anti-CD203a(PC-1) (#3E8) was kindly provided by J. Goding, and anti-CD39 PE-Cy7 mAb was purchased from eBiosciences. PE-conjugated anti-GD2 mAb (#14.G2a) was purchased from BD Biosciences. FITC- or APC-conjugated irrelevant isotype-matched mAbs were purchased from Beckman Coulter.
MV were suspended in binding buffer, incubated with specific mAbs (20 min in the dark, at 4°C), and then washed with 500 µl of binding buffer. Samples were then centrifuged (20,000 g for 1 h at 4°C). MV suspended in staining buffer (400 µl) were then subjected to flow cytometric analysis.
BM whole blood samples (50 µl) were incubated with specific mAbs (20 min in the dark at 4°C). BM infiltration by NB cells was evaluated using anti-CD45 PC7 mAb (Beckman Coulter). Erythrocytes were then lysed using BD FACS lysis buffer (BD Biosciences, 15 min at RT in the dark). After one additional washing step, cells were suspended in binding buffer and then analyzed by flow cytometry.
NB cells were washed in PBS, suspended in binding buffer and incubated with specific mAbs (20 min in the dark at 4°C). Cells were then washed, suspended in binding buffer and then analyzed by flow cytometry.
In some experiments, BM whole blood samples were treated with BD FACS lysis buffer (BD Biosciences, 15 min at RT in the dark) to eliminate erythrocytes. After one additional washing step, BM MNC were suspended in binding buffer, counted and then cultured with metastatic NB cells at 1:1 MNC:NB ratio for 48 h in DMEM 10% FBS. Cells were then washed and stained with anti-CD45, anti-CD38, anti-CD203a/PC-1, anti-CD39 and anti-CD73 mAbs (20 min in the dark at 4°C). Cells were then washed, suspended in binding buffer and then analyzed by flow cytometry.
Flow cytometric analysis was performed using a Gallios cytometer and Kaluza software (Beckman Coulter). Results related to MV were expressed as i) percentage of positive MV and as ii) percentage of MV presenting high expression of specific molecules. In detail, the first peak of mean fluorescence intensity (MFI) for each molecule was used as a marker to define the percentage of MV with high expression of the selected molecule (MV with high expression are above the first peak of fluorescence) or iii) mean relative of fluorescence intensity (MRFI), obtained as follows: mean fluorescence obtained with specific mAb/mean fluorescence obtained with irrelevant isotype-matched mAb. Data related to BM cells and NB cells were expressed as MRFI. For BM cells, analysis was performed gating on different cell subsets on the basis of CD45 expression, gating on i) CD45− cells (infiltrating NB cells) and on ii) CD45+ cells (BM-resident cells), using side scatter to discriminate between lymphocytes, monocytes, and granulocytes. In some experiments, analysis was performed by comparing two subgroups of NB patients, on the basis of NB infiltration. Cut-off levels were calculated by ROC curves analysis, using MedCalc (MedCalc software).
ADO production by MV
MV pools, each composed by MV obtained from five different individuals (NB patients or controls), were suspended in PBS + 0.1% glucose and incubated at 37°C and 5% CO2 in 96-well plates (Costar Corning), in the presence (or absence) of ATP, NAD+, ADPR or AMP (all at a concentration of 50 µM). Supernatants were collected after 15 or 45 min incubation and acetonitrile (ACN, Sigma Aldrich) immediately added (1:2 supernatant: ACN ratio) to stabilize ADO. Samples were then centrifuged at 12,000 g and supernatants were collected and stored at −80°C until use. The presence of substrates and byproducts was analyzed by HPLC assay.
HPLC analysis
MV supernatants were evaporated by Speed-vac (Eppendorf), reconstituted in the mobile-phase buffer, and assayed by HPLC, as described.50 Chromatographic analysis was performed with an HPLC System (Beckman Gold 126/166NM, Beckman Coulter) equipped with a reverse-phase column (Synergi Fusion C18, 5 µm; 150 × 4.5 mm, Phenomenex). Substrates and products of the enzymatic reactions were separated using a pH 5.1 mobile-phase buffer (0.125 M citric acid and 0.025 M KH2PO4) containing 8% ACN over 10 min, at a flow rate of 0.8 mL/min. UV absorption spectra were measured at 254 nm. HPLC-grade standards used to calibrate the signals were dissolved in AIM V serum-free medium (Invitrogen, Paisley, UK), pH 7.4, 0.2 μm sterile-filtered and injected in a buffer volume of 20 μL. The retention times (Rt, in min) of standards were: ATP, 1.9; AMP, 2.15; NAD+, 2.8; ADPR, 3.2; NIC, 4.5; and ADO; 5.56. Peak integration was performed using a Karat software (Beckman Coulter).
The qualitative identity of HPLC peaks was confirmed by co-migration with known reference standards. The presence of ADO was also confirmed by spiking standard (50 μM ADO), followed by chromatography. Quantitative measurements were inferred by comparing the peak area of samples with calibration curves for peak areas of each standard compound. Concentrations were expressed as nmol of substrates and products.
Cell proliferation assay
Cell proliferation was assessed using carboxyfluorescein succinimidyl ester (CFSE) dilution assay. Briefly, PB MNC from four different normal donors were stained with 1 µg/ml CFSE (Invitrogen), incubated for 15 min at 37°C, washed and then cultured in RPMI medium supplemented with 10% FBS. Cells were kept at 37°C and 5% CO2, alone or in the presence of beads coated with anti-CD3/anti-CD28 mAb (T cell activation/expansion kit, Miltenyi Biotec). Stimulated cells were cultured in 96 flat-bottom well plates (Costar Corning) in the presence or absence of MV isolated from 300 μl of BM plasma samples obtained from four different NB patients. MNC were harvested after 6 days, washed, and then stained with APC-conjugated anti-CD3 mAb (Beckman Coulter). After additional washes, cells were run on Gallios cytometer, and CFSE dilution was analyzed gating on APC+ cells, using Kaluza software (Beckman Coulter). Data were expressed as the percentage of proliferating cells.
Statistical analysis
Statistical analysis was performed using Prism 5.03 software (GraphPad Software). Gaussian distribution of data was analyzed using Kolmogorov-Smirnov test. The Student t test or the Mann-Whitney test was used to compare data, depending on data distribution. The significance range as follows: *p < 0.05 (significant), **p < 0.005, and ***p < 0.0005. Cut-off levels were determined by ROC curves analysis, using MedCalc (MedCalc Software).
Funding Statement
This work was supported by grants from Compagnia di San Paolo, Fondo per gli investimenti della ricerca di base (FIRB), Fondazione Ricerca Molinette and Fondazione CRT to F. Malavasi.
Disclosure of Potential Conflicts of Interest
The authors disclose any conflict of interest.
References
- 1.Louis CU, Shohet JM.. Neuroblastoma: molecular pathogenesis and therapy. Annu Rev Med. 2015;66:49–63. doi: 10.1146/annurev-med-011514-023121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008. October 16;455(7215):930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwab M. MYCN amplification in neuroblastoma: a paradigm for the clinical use of an oncogene. Pathol Oncol Res. 1997;3:3–7. [DOI] [PubMed] [Google Scholar]
- 4.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009. January 10;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garaventa A, Parodi S, De Bernardi B, Dau D, Manzitti C, Conte M, Casale F, Viscardi E, Bianchi M, D’Angelo P, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009. November;45(16):2835–2842. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 6.London WB, Castel V, Monclair T, Ambros PF, Pearson AD, Cohn SL, Berthold F, Nakagawara A, Ladenstein RL, Iehara T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011. August 20;29(24):3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celia-Terrassa T, Kang Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016. April 15;30(8):892–908. doi: 10.1101/gad.277681.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016. April 8;352(6282):169–175. doi: 10.1126/science.aaf2784. [DOI] [PubMed] [Google Scholar]
- 9.Morandi F, Scaruffi P, Gallo F, Stigliani S, Moretti S, Bonassi S, Gambini C, Mazzocco K, Fardin P, Haupt R, et al. Bone marrow-infiltrating human neuroblastoma cells express high levels of calprotectin and HLA-G proteins. PLoS One. 2012;7(1):e29922. doi: 10.1371/journal.pone.0029922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scaruffi P, Morandi F, Gallo F, Stigliani S, Parodi S, Moretti S, Bonassi S, Fardin P, Garaventa A, Zanazzo G, et al. Bone marrow of neuroblastoma patients shows downregulation of CXCL12 expression and presence of IFN signature. Pediatr Blood Cancer. 2012. July 15;59(1):44–51. doi: 10.1002/pbc.23339. [DOI] [PubMed] [Google Scholar]
- 11.Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P, et al. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol. 2015. December;35 Suppl:SS244–SS275. doi: 10.1016/j.semcancer.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Bottino C, Dondero A, Bellora F, Moretta L, Locatelli F, Pistoia V, Moretta A, Castriconi R. Natural killer cells and neuroblastoma: tumor recognition, escape mechanisms, and possible novel immunotherapeutic approaches. Front Immunol. 2014;5:56. doi: 10.3389/fimmu.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pistoia V, Morandi F, Bianchi G, Pezzolo A, Prigione I, Raffaghello L. Immunosuppressive microenvironment in neuroblastoma. Front Oncol. 2013;3:167. doi: 10.3389/fonc.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A. 2013;110(27):11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadej R, Spychala J, Skladanowski AC. Expression of ecto-5ʹ-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res. 2006;16(3):213–222. doi: 10.1097/01.cmr.0000215030.69823.11. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Lee S, Nigro CL, Lattanzio L, Merlano M, Monteverde M, Matin R, Purdie K, Mladkova N, Bergamaschi D, et al. NT5E (CD73) is epigenetically regulated in malignant melanoma and associated with metastatic site specificity. Br J Cancer. 2012;106(8):1446–1452. doi: 10.1038/bjc.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q, Du J, Zu L. Overexpression of CD73 in prostate cancer is associated with lymph node metastasis. Pathol Oncol Res. 2013;19(4):811–814. doi: 10.1007/s12253-013-9648-7. [DOI] [PubMed] [Google Scholar]
- 18.Lu XX, Chen YT, Feng B, Mao XB, Yu B, Chu XY. Expression and clinical significance of CD73 and hypoxia-inducible factor-1alpha in gastric carcinoma. World J Gastroenterol. 2013;19(12):1912–1918. doi: 10.3748/wjg.v19.i12.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013. June;19(6):355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horenstein AL, Chillemi A, Quarona V, Zito A, Roato I, Morandi F, Marimpietri D, Bolzoni M, Toscani D, Oldham RJ, et al. NAD(+)-metabolizing ectoenzymes in remodeling tumor-host interactions: the human myeloma model. Cells. 2015. September 17;4(3):520–537. doi: 10.3390/cells4030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedele G, Sanseverino I, D’Agostino K, Schiavoni I, Locht C, Horenstein AL, Malavasi F, Ausiello CM. Unconventional, adenosine-producing suppressor T cells induced by dendritic cells exposed to BPZE1 pertussis vaccine. J Leukoc Biol. 2015. October;98(4):631–639. doi: 10.1189/jlb.3A0315-101R. [DOI] [PubMed] [Google Scholar]
- 22.Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, Serra S, Malavasi F. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology. 2013. September 01;2(9):e26246. doi: 10.4161/onci.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM. The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood. 2012. September 20;120(12):2365–2375. doi: 10.1182/blood-2012-04-422378. [DOI] [PubMed] [Google Scholar]
- 24.Morandi F, Marimpietri D, Horenstein AL, Bolzoni M, Toscani D, Costa F, Castella B, Faini AC, Massaia M, Pistoia V, et al. Microvesicles released from multiple myeloma cells are equipped with ectoenzymes belonging to canonical and non-canonical adenosinergic pathways and produce adenosine from ATP and NAD+. Oncoimmunology. 2018;7(8):e1458809. doi: 10.1080/2162402X.2018.1458809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, László V, Pállinger E, Pap E, Kittel A, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011. August;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyiadzis M, Whiteside TL. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia. 2017. June;31(6):1259–1268. doi: 10.1038/leu.2017.91. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto T, Ohga N, Akiyama K, Hirata N, Kitahara S, Maishi N, Osawa T, Yamamoto K, Kondoh M, Shindoh M, et al. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS One. 2012;7(3):e34045. doi: 10.1371/journal.pone.0034045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci. 2015;112(12):E1433–E1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF, Welm A, Antonyak MA, Cerione RA. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun. 2017. February;16(8):14450. doi: 10.1038/ncomms14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010. March 4;115(9):1755–1764. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Luca L, D’Arena G, Simeon V, Trino S, Laurenzana I, Caivano A, La Rocca F, Villani O, Mansueto G, Deaglio S, et al. Characterization and prognostic relevance of circulating microvesicles in chronic lymphocytic leukemia. Leuk Lymphoma. 2017. June;58(6):1424–1432. doi: 10.1080/10428194.2016.1243790. [DOI] [PubMed] [Google Scholar]
- 32.Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T, Kennedy R, Ivan C, Zhang X, Vannini I, et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst. 2015. July;107(7). doi: 10.1093/jnci/djv135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colletti M, Petretto A, Galardi A, Di Paolo V, Tomao L, Lavarello C, Inglese E, Bruschi M, Lopez AA, Pascucci L, et al. Proteomic analysis of neuroblastoma-derived exosomes: new insights into a metastatic signature. Proteomics. 2017. December;17(23–24):1600430. doi: 10.1002/pmic.201600430. [DOI] [PubMed] [Google Scholar]
- 34.Haug BH, Hald OH, Utnes P, Roth SA, Lokke C, Flaegstad T, Einvik C. Exosome-like extracellular vesicles from MYCN-amplified neuroblastoma cells contain oncogenic miRNAs. Anticancer Res. 2015. May;35(5):2521–2530. [PubMed] [Google Scholar]
- 35.Nakata R, Shimada H, Fernandez GE, Fanter R, Fabbri M, Malvar J, Zimmermann P, DeClerck YA. Contribution of neuroblastoma-derived exosomes to the production of pro-tumorigenic signals by bone marrow mesenchymal stromal cells. J Extracell Vesicles. 2017;6(1):1332941. doi: 10.1080/20013078.2017.1332941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar D, Manek R, Raghavan V, Wang KK. Protein characterization of extracellular microvesicles/exosomes released from cytotoxin-challenged rat cerebrocortical mixed culture and mouse N2a Cells. Mol Neurobiol. 2018. March;55(3):2112–2124. doi: 10.1007/s12035-017-0474-x. [DOI] [PubMed] [Google Scholar]
- 37.Morandi F, Corrias MV, Pistoia V. Evaluation of bone marrow as a metastatic site of human neuroblastoma. Ann N Y Acad Sci. 2015. January;1335:23–31. doi: 10.1111/nyas.12554. [DOI] [PubMed] [Google Scholar]
- 38.Morandi F, Barco S, Stigliani S, Croce M, Persico L, Lagazio C, Scuderi F, Belli ML, Montera M, Cangemi G, et al. Altered erythropoiesis and decreased number of erythrocytes in children with neuroblastoma. Oncotarget. 2017. August 8;8(32):53194–53209. doi: 10.18632/oncotarget.18285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stigliani S, Scaruffi P, Lagazio C, Persico L, Carlini B, Varesio L, Morandi F, Morini M, Gigliotti AR, Esposito MR, et al. Deregulation of focal adhesion pathway mediated by miR-659-3p is implicated in bone marrow infiltration of stage M neuroblastoma patients. Oncotarget. 2015. May 30;6(15):13295–13308. doi: 10.18632/oncotarget.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horenstein AL, Quarona V, Toscani D, Costa F, Chillemi A, Pistoia V, Giuliani N, Malavasi F. Adenosine generated in the bone marrow niche through a CD38-mediated pathway correlates with progression of human myeloma. Mol Med. 2016. October;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horenstein AL, Morandi F, Bracci C, Pistoia V, Malavasi F. Functional insights into nucleotide-metabolizing ectoenzymes expressed by bone marrow-resident cells in patients with multiple myeloma. Immunol Lett. 2018;November:15. pii: S0165-2478(18)30484-X. doi: 10.1016/j.imlet.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 42.Mondola P, Ruggiero G, Seru R, Damiano S, Grimaldi S, Garbi C, Monda M, Greco D, Santillo M. The Cu,Zn superoxide dismutase in neuroblastoma SK-N-BE cells is exported by a microvesicles dependent pathway. Brain Res Mol Brain Res. 2003. January 31;110(1):45–51. [DOI] [PubMed] [Google Scholar]
- 43.Boysen J, Nelson M, Magzoub G, Maiti GP, Sinha S, Goswami M, Vesely SK, Shanafelt TD, Kay NE, Ghosh AK. Dynamics of microvesicle generation in B-cell chronic lymphocytic leukemia: implication in disease progression. Leukemia. 2017. February;31(2):350–360. doi: 10.1038/leu.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quezada C, Torres A, Niechi I, Uribe D, Contreras-Duarte S, Toledo F, San Martín R, Gutiérrez J, Sobrevia L. Role of extracellular vesicles in glioma progression. Mol Aspects Med. 2018. April;60:38–51. doi: 10.1016/j.mam.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014. August 5;111(31):E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK, Nuchtern JG, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009. January 10;27(2):298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haupt R, Garaventa A, Gambini C, Parodi S, Cangemi G, Casale F, Viscardi E, Bianchi M, Prete A, Jenkner A, et al. Improved survival of children with neuroblastoma between 1979 and 2005: a report of the Italian Neuroblastoma Registry. J Clin Oncol. 2010. May 10;28(14):2331–2338. doi: 10.1200/JCO.2009.24.8351. [DOI] [PubMed] [Google Scholar]
- 48.Ferretti E, Tripodo C, Pagnan G, Guarnotta C, Marimpietri D, Corrias MV, Ribatti D, Zupo S, Fraternali-Orcioni G, Ravetti JL, et al. The interleukin (IL)-31/IL-31R axis contributes to tumor growth in human follicular lymphoma. Leukemia. 2015. April;29(4):958–967. doi: 10.1038/leu.2014.291. [DOI] [PubMed] [Google Scholar]
- 49.Marimpietri D, Petretto A, Raffaghello L, Pezzolo A, Gagliani C, Tacchetti C, Mauri P, Melioli G, Pistoia V, Ribatti D. Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS One. 2013;8(9):e75054. doi: 10.1371/journal.pone.0075054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morandi F, Morandi B, Horenstein AL, Chillemi A, Quarona V, Zaccarello G, Carrega P, Ferlazzo G, Mingari MC, Moretta L, et al. A non-canonical adenosinergic pathway led by CD38 in human melanoma cells induces suppression of T cell proliferation. Oncotarget. 2015. September 22;6(28):25602–25618. doi: 10.18632/oncotarget.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]


