ABSTRACT
Background: A higher rate of cancer in systemic sclerosis (SSc) is recognized but the role of SSc-linked autoantibodies status (positive/negative and autoantibody specificities) in the survival of SSc-patients with cancer remains poorly understood.
Methods: We utilized the Clalit-Health-Services medical database in a case-control study to evaluate the autoantibody status and specificities of SSc-patients with age- and sex-matched controls with regard to the prevalence of different cancer-subtypes and their impact on mortality. SSc-linked autoantibodies (ANA, anti-centromere, anti-RNP, anti-RNA polymerase III (RNAPIII) and anti-Scl-70) status was assessed in terms of cancer risk and outcome.
Results: 2,431 SSc-patients and 12,377 age- and sex-matched controls were included. SSc-patients had a relative risk of cancer of 1.90 (95%CI 1.62-2.24, p < 0.0001) and tended to develop malignancies earlier than controls. RNAPIII and Scl-70 autoantibody were associated with an increased overall cancer risk and after SSc diagnosis risk of cancer, respectively. As expected, SSc-patients with cancer had a risk of death of 2.15 (1.65-2.79) in comparison to SSc-patients without cancer. ANA-positive SSc-patients with cancer had a better prognosis than ANA-negative cases (p = 0.0001). Despite the benefit of ANA-positive status on survival, the anti-Scl-70-positive subgroup with cancer had a significant negative impact on the survival compared to Scl-70-positive cases without cancer, whereas anti-RNAPIII and anti-centromere had no significant impact.
Conclusion: ANA positivity is an independent predictor of favorable prognosis in SSc-patients with cancer, possibly suggesting that humoral autoimmunity in SSc with cancer may have some benefit. However, no survival benefit was discernible with the common autoantibodies.
Keywords: Systemic sclerosis, scleroderma, malignancy, cancer, autoantibodies, autoimmune diseases
Introduction
Systemic sclerosis (SSc) is a complex autoimmune disease of unknown aetiology, in which there is abnormal activation of fibroblasts and overproduction and accumulation of extracellular matrix in the skin, but also in different internal organs that may culminate in end-stage organ failure.1 The role of autoimmunity as the cardinal underlying driver in SSc is being increasingly appreciated with the recognition of shared genetic pathways with other autoimmune diseases from genome-wide association studies (GWAS) and molecular studies, especially of type-I interferon responses.2,3
Analogous to other autoimmune diseases, most notably dermatomyositis, SSc is associated with an increased age- and sex-adjusted risk of malignancy development,4,5 commonly in the lung, liver, hematologic system, and bladder.6–8 The high risk of cancer in such conditions was originally attributed to various factors including disease-related chronic inflammation, genetic predisposition for both autoimmunity and malignancy, and as a treatment complication.9
Several SSc-specific autoantibodies have been linked to specific demographic, clinical, organ system,10 risk of cancer, and survival features which first emerged with the description of anti-Scl-70 (topoisomerase-I).11 Striking advances have been made in recent years in elucidating the mechanisms linking cancer and SSc with, cancer expression of RNA polymerase III (RNAPIII) been associated with serum anti-RNAPIII autoantibodies in SSc.12–14 Furthermore, an evidence of a genetic abnormality at the RNA polymerase 3 polypeitde A (POLR3A) locus (somatic mutations and/or loss of heterozygosity) has been reported in six of eight SSc patients, but only three had somatic mutations.15 The shorter disease interval reported between RNAPIII and cancer onset powerfully supports the idea that adaptive immunity to tumors may underscore some SSc cases via humoral autoimmune paraneoplastic mechanisms15 and that other autoantibodies beyond RNAPIII might contribute to this as a mechanism of disease.16
Despite the literature concerning the risk of cancer in SSc, the role of some autoantibodies in the risk of cancer among SSc-patients is still controversial with conflicting findings in relationship to key SSc-linked autoantibodies including anti-Scl-70.17 Moreover, there is a dearth of knowledge on the outcome of SSc-patients and with respect to their autoantibody status and cancer. Also, the impact of different cancer subtypes on the mortality of SSc-patients has not been defined. Thus, we conducted a large-scale population-based study to evaluate both cancer risk and impact on survival in SSc. In particular, we sought to determine whether humoral autoimmunity as determined by anti-nuclear antigens (ANA) and autoantibody specificity, in general, impacted on patient survival, the hypothesis being that SSc associated autoimmunity might be associated with a better survival.
Results
Study population
The entire population comprised of 15,141 subjects (12,710 controls and 2,431 SSc-patients). Being an age- and gender-matched case-control study, cases and controls did not differ for age (either age at study production – 63.4 ± 18.1 years in the controls vs 62.7 ± 17.9 years in the cases – or at the diagnosis/beginning of the follow-up – 54.5 ± 18.6 vs 54.8 ± 18.7 in controls and in cases, respectively) and gender (females, representing 81.7% of the sample both for cases and controls): they differed for body mass index (BMI) (p < 0.001), socioeconomic status (with low socioeconomic status being more represented in cases – 44.4% vs 39.7% in controls, p < 0.001), occurrence of cancer (higher among cases, 23.1% vs 15.1%, p < 0.001) and all-cause mortality (being higher among cases, 26.2% vs 12.5%, p < 0.001). Further details are shown in Table 1.
Table 1.
Overall population, systemic sclerosis (SSc) patients and age-and-sex matched controls – basic characteristics. Abbreviations: NS (not statistically significant); SD (standard deviation).
| Characteristic | All population (n = 15,141) | Controls (n = 12,710) | SSc-patients (n = 2,431) | Statistical significance (p-value) |
|---|---|---|---|---|
| Age (mean±SD; median) | 63.32 ± 18.06; 66 | 63.44 ± 18.08; 66 | 62.69 ± 17.90; 66 | NS |
| Age at diagnosis or at the beginning of the follow-up (mean±SD; median) |
54.57 ± 18.64; 57 | 54.54 ± 18.63; 57 | 54.77 ± 18.67; 57 | NS |
| Gender (female; %) | 12,377 (81.7%) | 10,390 (81.7%) | 1,987 (81.7%) | NS |
| Body Mass Index (n; %)a | <0.001 | |||
| <20 kg/m2 | 1,283 (9.2%) | 1,098 (8.6%) | 185 (15.6%) | |
| 20–24.9 kg/m2 | 4,189 (30.1%) | 3,803 (29.9%) | 386 (32.5%) | |
| 25–30 kg/m2 | 4,380 (31.5%) | 4,055 (31.9%) | 325 (27.4%) | |
| >30 kg/m2 | 4,045 (29.1%) | 3,754 (29.5%) | 291 (24.5%) | |
| Socioeconomic status (n; %)b | <0.001 | |||
| Low | 5,763 (40.4%) | 4,769 (39.7%) | 994 (44.4%) | |
| Medium | 5,364 (37.6%) | 4,543 (37.8%) | 821 (36.7%) | |
| High | 3,122 (22.0%) | 2,699 (22.5%) | 423 (18.9%) | |
| Smoking (n; %) | 4,332 (28.6%) | 3,628 (28.5%) | 704 (29.0%) | NS |
| Cancer (n; %) | 2,480 (16.4%) | 1,919 (15.1%) | 561 (23.1%) | <0.001 |
| All-cause mortality (n; %) | 2,226 (14.7%) | 1,589 (12.5%) | 637 (26.2%) | <0.001 |
a Available for 91.8% of data; b Available for 94.1% of the data.
Independent predictors of cancer occurrence
At the multivariate logistic regression assessing covariates associated with malignancy, independent predictors of occurrence of cancer were age (odds-ratio, OR, 1.05 [95% confidence interval, CI, 1.04-1.05], p < 0.0001), socioeconomic status (medium, OR 1.25 [95%CI 1.12-1.41], p = 0.0001; high, OR 1.40 [95%CI 1.23-1.60], p < 0.0001), SSc (OR 1.90 [95%CI 1.62-2.24], p < 0.0001), and smoking (OR 1.25 [95%CI 1.12-1.39], p = 0.0001) (Table 1S).
Interaction between SSc and cancer in terms of death: independent predictors of mortality at the univariate analysis
Interaction between SSc and cancer had a significant impact on the risk of death. At the Kaplan–Meier survival analysis, controls without cancer and the SSc-patients with cancer had the best and the worst survival curves, respectively (chi-squared = 1,213.43; degrees of freedom = 3; p < 0.0001; Figure 1a). Indeed, in comparison to controls without cancer, controls with cancer (crude hazard ratio, HR, 3.86 [95%CI 3.39-4.39], p < 0.05), SSc-patients without cancer (crude HR 2.63 [95%CI 2.31-3.00], p < 0.05) and SSc-patients with cancer (crude HR 5.65 [95%CI 4.45-7.17], p < 0.05) exhibited higher risk of death (Table 2S).
Figure 1.
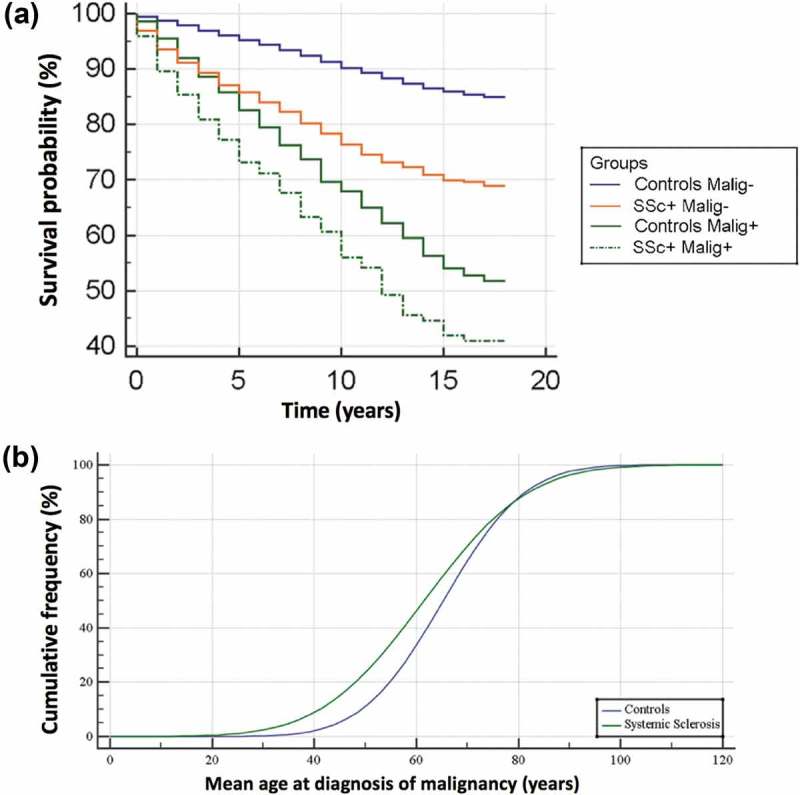
(a) Kaplan–Meyer survival curve for systemic sclerosis patients (SSc) and controls with and without cancer. (a) Cumulative frequency showing mean age at diagnosis of malignancy in systemic sclerosis in comparison to controls. Between age 30 and 70, cancers present at a younger age in SSc subjects (green line).
SSc-patients without cancer had a lower risk of death (crude HR 0.68 [95%CI 0.58-0.81], p < 0.05) in comparison with controls with cancer. SSc-patients with cancer had a higher risk of death (crude HR 1.46 [95%CI 1.13-1.90], p < 0.05) compared to controls with cancer. Finally, with respect to SSc without cancer, SSc with cancer had a higher risk of death (crude HR 2.14 [95%CI 1.65-2.79], p < 0.05) (Table 2S).
Interaction between SSc and cancer in terms of death: independent predictors of mortality at the multivariate analysis
At the Cox multivariate survival analysis, independent risk factors of death were higher age (HR 1.06 [95%CI 1.05-1.06], p < 0.0001), diagnosis of SSc (HR 2.16 [95%CI 1.89-2.48], p < 0.0001), presence of malignancy (HR 2.47 [95%CI 2.24-2.72], p < 0.0001), BMI <20 vs 20–24.9 kg/m2 (HR 1.35 [95%CI 1.15-1.60], p = 0.0003). Independent protective factors for death were BMI 25-30 vs 20–24.9 kg/m2 (HR 0.80 [95%CI 0.71-0.91], p = 0.0007), female gender (female vs male, HR 0.78 [0.69-0.87], p < 0.0001), and higher socioeconomic status (high vs low, HR 0.66 [0.57-0.75], p < 0.0001) (Table 3S).
Multivariate logistic regression analysis of types of SSc-related cancers
At the multivariate logistic regression assessing risk of different cancer subtypes in SSc in comparison to controls after adjustment for age (Table 2), oesophagus cancer (OR 5.32 [95%CI 1.37-20.55], p = 0.0154), lung cancer (OR 2.12 [95%CI 1.25-3.60], p = 0.0053), vagina and vulva cancers (OR 9.85 [4.51-21.50], p < 0.0001), multiple myeloma (OR 3.03 [95%CI 1.31-7.03], p = 0.0097), myelodysplastic syndrome (OR 8.10 [95%CI 2.11-31.08], p = 0.0023), non-Hodgkin’s lymphoma (OR 2.75 [1.70-4.45], p < 0.0001), stomach cancer (OR 2.60 [95%CI 1.13-6.00], p = 0.0249), and malignancy of unknown primary (OR 4.32 [95%CI 3.16-5.91], p < 0.0001) were significantly higher. Chronic leukemia resulted, instead, associated in a borderline way (OR 2.62 [95%CI 0.99-6.96], p = 0.0530). The reported OR is referred to the overall risk of cancer regardless its period of onset (before or after SSc diagnosis).
Table 2.
Multivariate logistic regression assessing the overall risk of different cancers in systemic sclerosis (SSc) in comparison to controls. Abbreviations: CI (confidence interval); CNS (central nervous system); OR (odds-ratio); SE (standard error).
| Variable | Overall number of cancers N (%) |
Cancer in SSc-patients N (%) |
Coefficient | SE | Wald | p-value | OR | 95%CI |
|---|---|---|---|---|---|---|---|---|
| CNS cancer | 34 (0.2%) | 7 (0.3%) | −0.83 | 1.02 | 0.66 | 0.4180 | 0.44 | 0.06 to 3.24 |
| Oropharyngeal cancer | 29 (0.2%) | 10 (0.4%) | 0.65 | 0.63 | 1.06 | 0.3033 | 1.92 | 0.56 to 6.61 |
| Larynx cancer | 20 (0.1%) | 4 (0.2%) | 0.28 | 0.77 | 0.13 | 0.7148 | 1.33 | 0.29 to 6.00 |
| Thyroid cancer | 112 (0.7%) | 26 (1.1%) | 0.13 | 0.37 | 0.11 | 0.7427 | 1.13 | 0.54 to 2.35 |
| Breast cancer | 723 (4.8%) | 125 (5.1%) | 0.28 | 0.15 | 3.71 | 0.0539* | 1.33 | 1.00 to 1.77 |
| Lung cancer | 160 (1.1%) | 48 (2.0%) | 0.75 | 0.27 | 7.79 | 0.0053** | 2.12 | 1.25 to 3.60 |
| Oesophagus cancer | 12 (0.1%) | 4 (0.2%) | 1.67 | 0.69 | 5.87 | 0.0154** | 5.32 | 1.37 to 20.55 |
| Stomach cancer | 46 (0.3%) | 13 (0.5%) | 0.96 | 0.43 | 5.03 | 0.0249** | 2.60 | 1.13 to 6.00 |
| Pancreas cancer | 51 (0.3%) | 8 (0.3%) | −0.43 | 0.73 | 0.35 | 0.5551 | 0.65 | 0.16 to 2.72 |
| Liver and bile ducts cancer | 25 (0.2%) | 3 (0.1%) | 0.10 | 0.75 | 0.02 | 0.8942 | 1.10 | 0.26 to 4.78 |
| Colorectal cancer | 287 (1.9%) | 47 (1.9%) | 0.03 | 0.26 | 0.01 | 0.9137 | 1.03 | 0.62 to 1.70 |
| Kidney cancer | 78 (0.5%) | 8 (0.3%) | 0.15 | 0.43 | 0.12 | 0.7338 | 1.16 | 0.50 to 2.70 |
| Bladder cancer | 116 (0.8%) | 19 (0.8%) | 0.27 | 0.36 | 0.55 | 0.4570 | 1.31 | 0.65 to 2.64 |
| Prostate cancer | 84 (0.6%) | 13 (0.5%) | −0.02 | 0.54 | 0.00 | 0.9708 | 0.98 | 0.34 to 2.82 |
| Uterus cancer | 113 (0.7%) | 21 (0.8%) | 0.49 | 0.34 | 2.02 | 0.1550 | 1.62 | 0.83 to 3.17 |
| Cervical cancer of the uterus | 46 (0.3%) | 11 (0.5%) | 0.42 | 0.54 | 0.63 | 0.4273 | 1.53 | 0.54 to 4.37 |
| Ovary cancer | 72 (0.5%) | 11 (0.5%) | 0.33 | 0.43 | 0.58 | 0.4449 | 1.39 | 0.59 to 3.26 |
| Vagina and vulva cancers | 37 (0.2%) | 21 (0.9%) | 2.29 | 0.40 | 33.00 | <0.0001** | 9.85 | 4.51 to 21.50 |
| Bone cancer | 13 (0.1%) | 1 (0.0%) | −18.24 | 6,264.52 | 0.00 | 0.9977 | 0.00 | |
| Sarcoma | 44 (0.3%) | 14 (0.6%) | 0.72 | 0.49 | 2.17 | 0.1405 | 2.06 | 0.79 to 5.40 |
| Melanoma | 114 (0.8%) | 25 (1.0%) | −0.36 | 0.52 | 0.48 | 0.4897 | 0.70 | 0.25 to 1.93 |
| Acute leukaemia | 75 (0.5%) | 15 (0.6%) | 0.38 | 0.41 | 0.87 | 0.3502 | 1.46 | 0.66 to 3.24 |
| Chronic leukaemia | 41 (0.3%) | 12 (0.5%) | 0.96 | 0.50 | 3.74 | 0.0530* | 2.62 | 0.99 to 6.96 |
| Hodgkin’s lymphoma | 35 (0.2%) | 10 (0.4%) | 0.75 | 0.55 | 1,86 | 0.1730 | 2.11 | 0.72 to 6.20 |
| Non-Hodgkin’s lymphoma | 159 (1.1%) | 48 (2.0%) | 1.01 | 0.25 | 16.91 | <0.0001** | 2.75 | 1.70 to 4.45 |
| Myelodysplastic syndrome | 11 (0.1%) | 6 (0.2%) | 2.09 | 0.69 | 9.30 | 0.0023** | 8.10 | 2.11 to 31.08 |
| Multiple myeloma | 44 (0.3%) | 13 (0.5%) | 1.11 | 0.43 | 6.68 | 0.0097** | 3.03 | 1.31 to 7.03 |
| Malignancy of unknown primary | 297 (2.0%) | 120 (4.9%) | 1.46 | 0.16 | 83.73 | <0.0001** | 4.32 | 3.16 to 5.91 |
| Other neoplasms | 111 (0.7%) | 28 (1.2%) | −0.38 | 0.26 | 2.12 | 0.1450 | 1.34 | 0.66 to 2.70 |
*Borderline association, **significant association.
SSc tended to develop malignancies earlier than controls (p < 0.0001, Figure 1b).
Interaction between SSc and SSc-related cancers in terms of death: independent predictors of mortality at the multivariate analysis
Assessing the impact of different cancer subtypes on the survival of SSc-patients after adjustment for SSc, the following cancers exhibited a high risk of death: lung cancer (HR 4.59 [95%CI 3.65-5.76], p = 0.0064), oesophagus cancer (HR 3.62 [95%CI 1.87-6.99], p = 0.0001), stomach cancer (HR 3.41 [95%CI 2.29-5.07], p < 0.0001), liver cancer (HR 5.30 [95%CI 3.37-8.34], p < 0.0001), pancreas cancer (HR 5.86 [95%CI 4.22 −8.14], p < 0.0001), vagina and vulvar cancer (HR 3.23 [95%CI 1.96-5.30], p < 0.0001), Hodgkin’s lymphoma (HR 3.72 [95%CI 2.23-6.20], p < 0.0001), and multiple myeloma (HR 3.55 [95%CI 2.30-5.48], p < 0.0001). Further details are reported in Table 3.
Table 3.
Cox multivariate survival analysis assessing the impact of different cancer subtypes on mortality of systemic sclerosis patients. Abbreviations: CI (confidence interval); CNS (central nervous system); HR (hazard ratio).
| Cancer | HR | 95%CI | p-value |
|---|---|---|---|
| CNS cancer | 2.86 | 1.62 to 5.05 | 0.0003 |
| Oropharynx cancer | 0.93 | 0.38 to 2.24 | 0.8665 |
| Thyroid cancer | 1.14 | 0.70 to 1.87 | 0.5979 |
| Larynx cancer | 2.39 | 1.28 to 4.47 | 0.0064 |
| Sarcoma | 1.43 | 0.81 to 2.52 | 0.2223 |
| Melanoma | 1.18 | 0.77 to 1.79 | 0.4502 |
| Breast cancer | 1.75 | 1.48 to 2.06 | <0.0001 |
| Lung cancer | 4.59 | 3.66 to 5.77 | <0.0001 |
| Oesophagus cancer | 3.62 | 1.88 to 6.99 | 0.0001 |
| Stomach cancer | 3.42 | 2.30 to 5.08 | <0.0001 |
| Liver cancer | 5.30 | 3.37 to 8.34 | <0.0001 |
| Pancreas cancer | 5.87 | 4.23 to 8.14 | <0.0001 |
| Colorectal cancer | 1.63 | 1.31 to 2.02 | <0.0001 |
| Kidney cancer | 1.97 | 1.35 to 2.87 | 0.0004 |
| Bladder cancer | 2.27 | 1.71 to 3.03 | <0.0001 |
| Prostate cancer | 0.92 | 0.58 to 1.46 | 0.7272 |
| Ovarian cancer | 2.67 | 1.92 to 3.80 | <0.0001 |
| Uterus cancer | 1.61 | 1.13 to 2.28 | 0.0081 |
| Cancer of the cervix uteri | 1.85 | 1.05 to 3.26 | 0.0346 |
| Vagina and vulva cancer | 3.23 | 1.97 to 5.31 | <0.0001 |
| Acute leukemia | 1.53 | 0.97 to 2.40 | 0.0667 |
| Chronic leukemia | 2.09 | 1.21 to 3.61 | 0.0083 |
| Hodgkin’s lymphoma | 3.72 | 2.23 to 6.21 | <0.0001 |
| Non-Hodgkin’s lymphoma | 2.20 | 1.64 to 2.96 | <0.0001 |
| Multiple myeloma | 3.56 | 2.31 to 5.48 | <0.0001 |
| Myelodysplastic syndrome | 2.48 | 0.93 to 6.63 | 0.0709 |
| Cancer of unknown primary | 1.65 | 1.27 to 2.13 | 0.0001 |
| Other neoplasms | 3.14 | 2.33 to 4.23 | <0.0001 |
Impact of autoantibody status on cancer risk: subgroup analyses
In this cohort, 1,651 patients were tested for at least one autoantibody, namely, 78.7% were tested for ANA, 61.1% for anti-Scl-70, 49.9% for anti-centromere, anti-RNP 41.5%, and only 10% for anti-RNAPIII. Among these, 84.1% were ANA positive, 39.4% were positive for anti-Scl-70, 32.0% were anti-centromere positive, 15.0% were anti-RNAPIII positive and 3.3% were anti-RNP positive. Double positivity at any time of study period (not necessarily at the same time) was low and reported in Table 4S.
Among the negative ANA group, 31 patients were found to be positive for anti-Scl-70, three were positive for anti-centromere, one was positive for anti-RNP and one was positive for RNAPIII. After the exclusion of these “false negative ANA” patients, the percentage of ANA positivity increased to 86%. In this cohort, only Scl-70 and RNAPIII auto-antibodies were associated with a higher risk of cancer. Specifically, Scl-70 antibody positivity was found to confer risk of having cancer after SSc diagnosis (HR 1.41 [95%CI 1.05-1.90], P = 0.0224) whereas RNAPIII antibody positivity conferred an overall (before and after SSc diagnosis) risk of cancer diagnosis (HR 1.94 [1.00-3.73], p = 0.0488) (Table 4).
Table 4.
Multivariate Cox proportional-hazard regression analysis for assessing the risk of cancer and of death related to different systemic sclerosis (SSc)-autoantibodies. Abbreviations: ANA (anti-nuclear antigens); BMI (body mass index); CI (confidence interval); HR (hazard ratio); RNAPIII (RNA polymerase III); RNP (ribonucleoproteins); Scl-70 (topoisomerase-I); SES (socioeconomic status).
| Risk of cancer development in SSc-Patients |
Risk of death in SSc-patients with cancer |
|||||
|---|---|---|---|---|---|---|
| Autoantibody | HRa | 95%CI | p-value | HRa | 95%CI | p-value |
| ANA | ||||||
| Overall risk | 0.84 | 0.66 to 1.08 | 0.1666 | 0.64 | 0.50 to 0.83 | 0.0007 |
| Risk after SSc diagnosis | 0.83 | 0.59 to 1.17 | 0.2894 | |||
| Risk in ±36 months of SSc diagnosis | 0.81 | 0.57 to 1.16 | 0.2565 | |||
| High titre vs low | 0.90 | 0.63 to 1.27 | 0.5385 | |||
| RNAPIII | ||||||
| Overall risk | 1.94 | 1.00 to 3.73 | 0.0488 | 1.53 | 0.60 to 3.88 | 0.3763 |
| Risk after SSc diagnosis | 1.96 | 0.70 to 5.52 | 0.2022 | |||
| Risk in ±36 months of SSc diagnosis | 1.97 | 0.67 to 5.79 | 0.2160 | |||
| Scl-70 | ||||||
| Overall risk | 1.13 | 0.90 to 1.43 | 0.2872 | 1.39 | 1.08 to 1.80 | 0.0106 |
| Risk after SSc diagnosis | 1.41 | 1.05 to 1.90 | 0.0224 | |||
| Risk in ±36 months of SSc diagnosis | 1.23 | 0.89 to 1.72 | 0.2113 | |||
| Centromere | ||||||
| Overall risk | 1.28 | 0.94 to 1.74 | 0.1116 | 1.42 | 0.99 to 2.03 | 0.0545 |
| Risk after SSc diagnosis | 0.95 | 0.59 to 1.53 | 0.8324 | |||
| Risk in ±36 months of SSc diagnosis | 1.10 | 0.67 to 1.81 | 0.7192 | |||
| RNP | ||||||
| Overall risk | 0.97 | 0.64 to 1.45 | 0.8734 | 0.50 | 0.23 to 1.09 | 0.0796 |
| Risk after SSc diagnosis | 1.26 | 0.77 to 2.07 | 0.3620 | |||
| Risk in ±36 months of SSc diagnosis | 0.90 | 0.48 to 1.70 | 0.7414 | |||
aHR was computed adjusting for age, gender, BMI, SES, and smoking status.
Impact of autoantibody status on survival in cancer in SSc: subgroup analyses
Negativity of ANA was significantly associated with a worse survival of SSc patients with cancer (chi-squared = 16.12, degrees of freedom = 1, p = 0.0001) (Figure 2). After the exclusion of ANA-negative patients but positive for other SSc-linked autoantibodies “false negative ANA”, the p-value became even more significant (p < 0.0001).
Figure 2.
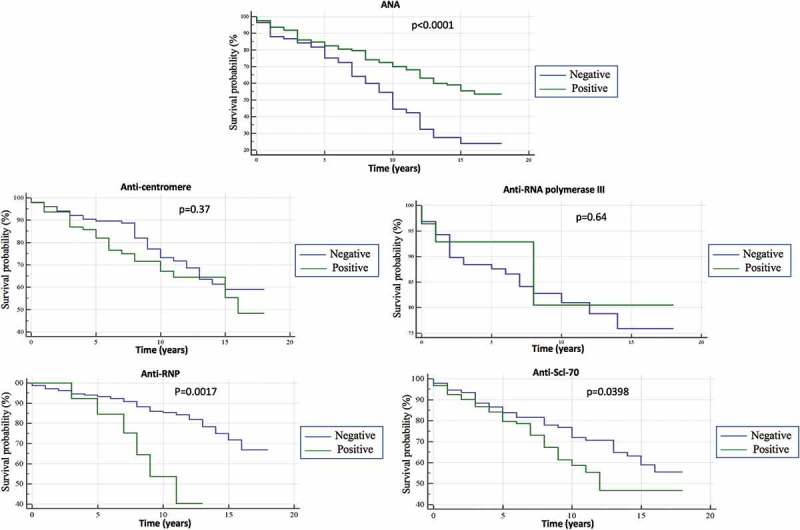
Kaplan–Meyer survival curve analysis for systemic sclerosis with cancer stratified according to positivity/negativity for a panel of autoantibodies (ANA, anti-centromere, RNA polymerase III, anti-RNP, anti-Scl-70. SSc-patients with cancer and positive for an SSc-related autoantibody were compared to overall SSc cohort with cancer but negative for the same antibody in terms of survival.
Concerning the impact of different SSc-linked autoantibodies on SSc-patients with cancer survival, anti-Scl-70 (chi-squared = 4.23, degrees of freedom = 1, p = 0.0398), anti-RNP (chi-squared = 9.90, degrees of freedom = 1, p = 0.0017) were associated with a worse survival (Figure 2). Anti-centromere (chi-squared 0.82, degrees of freedom = 1, p = 0.37) and RNAPIII (chi-squared 0.22, degrees of freedom = 1, p = 0.64) had no significant impact on the survival of SSc-patients (Figure 2). HR for death (adjusted for confounders) was statistically significant only for ANA (HR of 0.64, 95%CI 0.50-0.83, p = 0.0007) and Scl-70 (HR of 1.39, 95%CI 1.08-1.80, p = 0.0106).
To assess the interplay between anti-Scl-70 positivity and cancer in terms of mortality in SSc, we stratified SSc-patients with positive anti-Scl-70 according to malignancy status and we found that SSc-patients with cancer and positive for anti-Scl-70 had a higher risk of death (HR of 1.93, 95%CI, 1.21-3.09, p = 0.0058) than those positive to this antibody but without cancer. However, stratifying patients with positive anti-RNP according to malignancy status, no significant differences were found in terms of survival rate (HR of 4.38, 95%CI, 0.86-22.18, p = 0.0743).
Discussion
This study is the first to test the hypothesis that humoral autoimmunity as determined by autoantibody status in SSc cases with cancer might impact on patient survival. Indeed, SSc-patients with cancer and ANA negativity both by immunofluorescence and the common performed autoantibody specificities had a worse survival than those exhibiting ANA positivity. This points towards a potential survival benefit at the population level in SSc cases with cancer and discernible autoimmunity compared to the patient group with SSc and cancer without discernible autoimmunity. Despite this, the “cardinal” autoantibody specificities within ANA including Scl-70 and RNAPIII antibodies were not linked to a better survival and indeed the former had a worse survival.
At the population level, these novel findings on the presence of ANA being linked to a better survival may represent effective immune system and a better anti-tumor immune reaction. In agreement with this interpretation, one study has found that positivity of ANA in lung cancer, not linked to autoimmune disease, was associated with a prolonged survival.18 Furthermore, some patients develop ANA after immune checkpoint inhibitors such as PD-1 inhibitors, representing an enhanced immune activity against cancer.19
In our study, we were not able to link any ANA-specific autoantibodies to the better survival noted in the ANA-positive group. In the group negative for ANA by immunofluorescence we identified over 30 cases who had SSc-linked autoantibodies and when these were included in the ANA-positive group the link with ANA positivity and cancer survival remained strong.
The possibilities include the presence of other ANA subtypes that are linked to a worse survival in the so-called ANA-negative SSc-patients that are yet to be defined. A second possibility is of a cell-mediated autoimmunity mechanism accounting for paraneoplastic SSC autoimmunity, but poor anti-tumor immune responses.
Whilst ANA-positive status, in general, was linked to a better survival over ANA-negative status, the Scl-70 link to poor survival in cancer is noteworthy. Indeed, the present study showed that SSc-patients with cancer and anti-Scl-70 positivity had a worse prognosis than SSc-patients without this antibody. This finding remains difficult to explain and could relate to the impact of therapy of more severe SSc or an adverse effect of the tumor in aggravating autoimmune responses in other organs, particularly the lungs but defining specific mechanisms of death were beyond the scope of this study. It will be important to determine in future studies whether the mortality in ANA-negative cases was due directly to tumor mortality rather than the SSC disease process itself.
Our data confirm earlier observations that SSc-patients positive for RNAPIII are at higher risk of cancer.12,20 A recent and important study by Igusa and collaborators13 have found an increased risk of cancer at SSc onset among anti-RNAPIII positive and those negative for all three anti-centromere/RNAPIII/Scl-70 antibodies patients. It is generally known that the relationship between cancer and autoimmunity is complex and bidirectional. Indeed, a study from Joseph et al.15 has shown that genetic alterations in the POLR3A gene, encoding for RNAPIII polypeptide A, and humoral and cell-mediated immune response against this mutated antigen were demonstrated in patients who are positive for anti-RNAPIII, but not in patients with other SSc-specific antibodies and cancer.15 Given the link between ANA positivity and cancer survival in SSc and the emergent biological understanding of RNAPIII in cancer in SSc then it was surprising that putative RNAPIII directed anti-tumor immunity did not translate into a better survival in this antibody subgroup.
We also found that positivity of Scl-70 antibody is associated with the risk of cancer after SSc diagnosis. Similar results regarding anti-Scl-70 were reported previously, in particular with lung cancer.21,22 However, other studies have not reported such an anti-Scl-70 association with cancer.12,13 The variability and heterogeneity of findings regarding the role of SSc-related autoantibodies in the risk of cancer might be related to the complex interplay between genetic predisposition, environmental factors and epigenetic modifications in different geographical regions resulting in different rates of cancer in SSc generally and, in autoantibodies-related SSc subgroups in particular.
Surprisingly, there is very little data regarding the impact of different cancer subtypes on the outcome of SSc-patients, and to the best of our knowledge, this is the first study to address this outcome. Whilst many cancer subtypes have high rates of mortality in SSc-patients such as pancreas, liver and bile ducts, esophagus, and lung cancer, the substantially increased mortality of hematological malignancies such as Hodgkin’s lymphoma is not completely understood. This may be attributed to the high comorbidity and low-performance status in SSc-patients preventing them of undergoing intensive chemotherapy or deleterious effects of tyrosine kinase inhibitors or immune checkpoint inhibitors given to these individuals, which resulted in enhancement of the autoimmune disorders.
The estimated risk of cancer in SSc-patients varies from one report to another even though most of the studies reported a relative risk (RR) for all sites malignancy of 1.5-2-fold4,23,24 which is similar to the risk obtained in the current study (1.9-fold). Other cohorts reported a RR of cancer in SSc above 4.25,26 We evaluated cancer subtypes and the leading cancer subtypes in our patient cohort were vagina and vulva, esophagus, lung, and hematological system. In our cohort, vagina and vulva cancers were found to be with the highest risk in the region of 10 (CI 95% 4.51-21.5). Genital organs malignancies in SSc-patients are not well described in the literature. There are more reports regarding cervical cancer rather than vagina and vulva in SSC and it has been found that atypical cytological findings on pap smears of SSc-patients are higher than in the general population.27 In our study, the risk of lung cancer was significantly higher with a value of 2.12 although it is slightly lower to what previously has been reported.28 We also found a higher prevalence of stomach as well as esophagus cancer in SSc-patients. The higher risk of oesophageal cancer in SSc-patients is well reported with a variable RR that ranges between 2.86 to 35.029,30 although, others reported no significant increased risk.30 A plausible mechanism that may explain the increasing rate of oesophageal cancer is the higher prevalence of peptic disease and Barrett’s esophagus in SSc-patients, both known to be linked with oesophageal cancer.31 Concerning hematological malignancies in SSc, variable RR have been reported according to the study design and population, yet, a metanalysis has showed an overall RR of hematological cancer in SSc of 2.2.30 In our study, in terms of specific hematological cancer, the highest risk was found for myelodysplastic syndrome, multiple myeloma, and non-Hodgkin’s lymphoma.
Our study has several strengths, mainly the sample size and its population-based design, which avoid the potential referral bias that often afflicts center-based studies. However, there are limitations that need to be acknowledged such as the inability to explore different SSc phenotypes including interstitial lung disease, cause of death, and effect of therapies on the cancer risk and survival. Finally, as some of these serological tests, such as anti-RNP and anti-RNAPIII are not routinely performed and are relatively recent tests, they were not available for the entire study population and therefore the data needs to be interpreted with caution. It is also important to mention the possibility of misclassification of SSc related to the big data real-life data-based studies.
In conclusion, our study confirms earlier observation on the increased rate of cancer in SSc-patients, especially for those positive for RNAPIII and Scl-70 antibodies. In terms of cancer subtypes, genital organs, lung, esophagus, stomach, and hematological malignancies were the most commonly SSc related malignancy reported and tended to appear earlier during the course of life in comparison to the general population. SSc-patients with cancer and ANA negativity seem to have a less favorable outcome than those positive for this antibody. Moreover, the mortality of cancer in SSc may be different than that in the general population. These findings show that the association between SSc and cancer and autoimmunity extends beyond disease risk but also has a complex effect on disparate factors including age of onset and types of cancers and the impact of autoimmunity at the population and cancer survival.
Material and methods
Design, sample, and procedures
This study is based on the chronic diseases registry of the Clalit-Health-Services (CHS), the largest healthcare maintenance organization in Israel which provides services for approximately half of the Israeli population where data on SSC in routinely collected.
With the use of massive data-mining techniques, patient data can be automatically retrieved and extracted from the database, enabling scholars to perform a wide-scale epidemiological study on a real-time heterogeneous population in an effective and accurate manner. Using the CHS’s computerized database, we extracted a cohort consisting of SSc-patients and compared them with age- and sex-matched controls. The data drawn from the database were recorded continuously since the beginning of the utilization of computerized systems in the CHS, approximately from the year 2000 until the year 2017.
Measures
SSc-patients were defined as such if they had at least one documented diagnosis of SSc in their medical records as an outpatient, either by a primary care physician or a specialist, or if they were diagnosed with SSc in their hospital discharge papers. All SSc-patients detected in the CHS database were considered eligible and, as such, enrolled in this study. Controls were randomly selected from the CHS database, with the exclusion of SSc-patients (that is to say, they may have other diseases and not necessarily healthy controls). Approximately five controls were matched by age and gender for each SSc patient. Data available from the CHS database included an array of variables, such as age, sex, socioeconomic status, BMI, smoking status (ever smokers, or never smokers by the time of entry in the study), and diagnoses of chronic diseases. More in detail, socioeconomic status was defined according to the poverty index of the member’s residence area as defined during the 2008 National Census. More specifically, the poverty index was computed based on household income, education, crowding, material conditions, and car ownership, among others. This composite index can range from 1 to 20, based on cluster analysis, with 1 as the lowest socioeconomic status and 20 as the highest. We divided the population into three categories according to their socioeconomic status, based on tertile distribution.
Concerning BMI, in order to reflect a nonlinear relation between BMI and dependent variables, BMI was classified into four categories: <20, 20–24.9, 25–30, and >30 kg/m2. The normal category (BMI 20–24.9 kg/m2) was used as a reference category.
The definition of malignancy, similar to that of SSc, was based on a documented diagnosis of malignancy in medical records, as registered in the CHS database. The validity and reliability of the diagnoses in the registry were found to be high, as shown in our previously published studies.32–35
Serum samples were taken and analyzed in SSc-patients by indirect immunofluorescence or enzyme-linked immunosorbent assay to identify SSc-specific autoantibodies during the diagnosis approach or follow-up and were available for the years 2010–2017. These tests were performed as part of the clinical routine and were not research assays. In the current study, the following autoantibodies were considered and assessed: ANA, anti-centromere, anti-Scl-70 (topoisomerase-I), anti-RNAPIII and anti-RNP. Tests positivity was defined as supplied by the kit assay insert and manufacturer’s instructions. The tests could have been performed any time point during the study period regardless of SSc disease onset. In case of multiple/serial assessment of autoantibodies (exams performed at different time-points during the study period), patients were considered positive for an autoantibody if they were ever positive based on clinically obtained assays.
Statistical analyses
Before commencing any statistical analysis and data manipulation, figures were visually inspected for potential outliers. The normality of data distribution was checked using the D’Agostino-Pearson omnibus test.
Rates of malignancies (overall and stratified for single disease) were compared between SSc-patients and controls in the study sample group. For overall we mean the rate of having at least one malignant condition either solid or hematological. The Chi-squared test was used to assess the distribution of categorical socio-demographic and clinical parameters, such as socioeconomic status and gender, between SSc-patients and controls. The Student’s t-test and one-way analysis of variance (ANOVA) or their non-parametric versions, were applied for continuous parameters, such as age at study production or age at diagnosis/beginning of the follow-up (between two and more groups, respectively), based on the normality of data distribution.
The association between SSc and malignancies was evaluated by a standard unconditional multivariate logistic regression model, in that matching was loose, that is to say performed on a small number of demographic variables (namely, age and gender). In this situation, Mantel–Haenszel matched-pair conditional regression logistic analyses are not necessary36,37 and may result in inaccurate and non-robust estimates. Performed multivariate logistic regression analyses were adjusted for possible confounders, including age and calendar time. The former adjustment was carried out considering that matching for age was done at study entry, but throughout the study period, SSc patients tended to develop malignancies earlier than controls. As such, it was necessary to adjust for age in order to minimize the risk of underestimation.36,38 Matching was also performed by the calendar of time to reduce the bias due to changes of cancer incidence over time or changes in the screening methods.
Dates of registration in the medical records of SSc (or alternatively, start of follow-up for controls), malignancy and death, as well as anthropometric information and medical co-morbidities, were extracted from the database when available.
Survival analysis using Kaplan–Meier curves, log-rank test, and multivariate Cox proportional hazards method was performed to detect variables associated with an increased risk of all-cause mortality, adjusting for possible risk factors and confounders, including SSc disease duration.
Multivariate Cox proportional-hazard regression analysis was used to assess the risk of cancer and death stratified according to autoantibody positivity for three different time-points (overall risk, after SSc diagnosis, and 36 months prior and after SSc onset). The HR was computed after adjusting for age, gender, BMI, socioeconomic status, and smoking status.
All statistical analyses were performed on the entire sample, except for the analyses concerning autoantibody positivity, which were carried out as sub-group analyses.
All statistical analyses were carried out with the commercial software “Statistical Package for the Social Sciences” (SPSS version 24.0, IBM, USA). Graphs were obtained with the commercial software MedCalc Statistical Software (version 17.9.7).
All figures with a p-value of less than 0.05 were considered statistically significant.
Funding Statement
This study was supported by a grant from the Israel Cancer Association (to ST and HA).
Disclosure of potential conflict of interest
No potential conflicts of interest were disclosed.
Competing interest
Cohen AD received research grants from Janssen, Novartis, AbbVie, Janssen, and Sanofi. Cohen AD served as a consultant, advisor or speaker to AbbVie, Amgen, Boehringer Ingelheim, Dexcel pharma, Janssen, Kamedis, Lilly, Neopharm, Novartis, Perrigo, Pfizer, Rafa, Samsung Bioepis, Sanofi, Sirbal and Taro.
Ethical statement
Authors confirm that their study’s involvement with human subjects complies with the Declaration of Helsinki.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T.. Scleroderma. New Eng J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE.. Pathogenesis of systemic sclerosis. Front Immunol. 2015;6:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsumoto TR, Whitfield ML, Connolly MK. The pathogenesis of systemic sclerosis. Annu Rev Pathol. 2011;6:509–537. doi: 10.1146/annurev-pathol-011110-130312. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Shakra M, Guillemin F, Lee P. Cancer in systemic sclerosis. Arthritis Rheum. 1993;36:460–464. [DOI] [PubMed] [Google Scholar]
- 5.Shah AA, Rosen A. Cancer and systemic sclerosis: novel insights into pathogenesis and clinical implications. Curr Opin Rheumatol. 2011;23:530–535. doi: 10.1097/BOR.0b013e32834a5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jedlickova H, Durcanska V, Vasku V. Paraneoplastic scleroderma: are there any clues? Acta Dermatovenerol Croat. 2016;24:78–80. [PubMed] [Google Scholar]
- 7.Monfort JB, Lazareth I, Priollet P. Paraneoplastic systemic sclerosis: about 3 cases and review of literature. J Mal Vasc. 2016;41:365–370. doi: 10.1016/j.jmv.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Onishi A, Sugiyama D, Kumagai S, Morinobu A. Cancer incidence in systemic sclerosis: meta-analysis of population-based Cohort studies. Arthritis Rheumatol. 2013;65:1913–1921. doi: 10.1002/art.37969. [DOI] [PubMed] [Google Scholar]
- 9.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev. 2017;16:1049–1057. doi: 10.1016/j.autrev.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Derk CT, Sakkas LI, Rasheed M, Artlett C, Jimenez SA. Autoantibodies in patients with systemic sclerosis and cancer: a case-control study. J Rheumatol. 2003;30:1994–1996. [PubMed] [Google Scholar]
- 11.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Moinzadeh P, Fonseca C, Hellmich M, Shah AA, Chighizola C, Denton CP, Ong VH. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res Ther. 2014;16:R53. doi: 10.1186/ar4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igusa T, Hummers LK, Visvanathan K, Richardson C, Wigley FM, Casciola-Rosen L, Rosen A, Shah AA. Autoantibodies and scleroderma phenotype define subgroups at high-risk and low-risk for cancer. Ann Rheum Dis. 2018. doi: 10.1136/annrheumdis-2018-212999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazzaroni MG, Cavazzana I, Colombo E, Dobrota R, Hernandez J, Hesselstrand R, Varju C, Nagy G, Smith V, Caramaschi P, et al. Malignancies in patients with anti-RNA polymerase III antibodies and systemic sclerosis: analysis of the EULAR scleroderma trials and research cohort and possible recommendations for screening. J Rheumatol. 2017;44:639–647. doi: 10.3899/jrheum.160817. [DOI] [PubMed] [Google Scholar]
- 15.Joseph CG, Darrah E, Shah AA, Skora AD, Casciola-Rosen LA, Wigley FM, Boin F, Fava A, Thoburn C, Kinde I, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science (New York, NY). 2014;343:152–157. doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AA, Rosen A, Hummers L, Wigley F, Casciola-Rosen L. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum. 2010;62:2787–2795. doi: 10.1002/art.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlagea A, Falagan S, Gutierrez-Gutierrez G, Moreno-Rubio J, Merino M, Zambrana F, Casado E, Sereno M. Antinuclear antibodies and cancer: a literature review. Crit Rev Oncol Hematol. 2018;127:42–49. doi: 10.1016/j.critrevonc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Madrid F, VandeVord PJ, Yang X, Karvonen RL, Simpson PM, Kraut MJ, Granda JL, Tomkiel JE. Antinuclear antibodies as potential markers of lung cancer(). Clin Cancer Res. 1999;5:1393–1400. [PMC free article] [PubMed] [Google Scholar]
- 19.Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17:610–616. doi: 10.1016/j.autrev.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Boonstra M, Huizinga TWJ, de Vries-Bouwstra JK. Auto-antibodies and cancer in systemic sclerosis. Autoimmun Rev. 2017;16:883–884. doi: 10.1016/j.autrev.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Weiner ES, Earnshaw WC, Senecal JL, Bordwell B, Johnson P, Rothfield NF. Clinical associations of anticentromere antibodies and antibodies to topoisomerase I. A study of 355 patients. Arthritis Rheum. 1988;31:378–385. [DOI] [PubMed] [Google Scholar]
- 22.Rothfield N, Kurtzman S, Vazques-Abad D, Charron C, Daniels L, Greenberg B. Association of anti-topoisomerase I with cancer. Arthritis Rheum. 1992;35:724. doi: 10.1002/art.1780350621. [DOI] [PubMed] [Google Scholar]
- 23.Hill C, Nguyen A, Roder D, Roberts-Thomson P. Risk of cancer in patients with scleroderma: a population based cohort study. Ann Rheum Dis. 2003;62:728–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo CF, Luo SF, Yu KH, Chou IJ, Tseng WY, Chang HC, Fang Y-F, Chiou M-J, See L-C. Cancer risk among patients with systemic sclerosis: a nationwide population study in Taiwan. Scand J Rheumatol. 2012;41:44–49. doi: 10.3109/03009742.2011.618145. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi M, Horiuchi T, Ishibashi N, Yoshizawa S, Niho Y, Nagasawa K. Anticentromere antibody as a risk factor for cancer in patients with systemic sclerosis. Clin Rheumatol. 2000;19:123–126. [DOI] [PubMed] [Google Scholar]
- 26.Kang KY, Yim HW, Kim IJ, Yoon JU, Ju JH, Kim HY, Park S-H. Incidence of cancer among patients with systemic sclerosis in Korea: results from a single centre. Scand J Rheumatol. 2009;38:299–303. doi: 10.1080/03009740802642062. [DOI] [PubMed] [Google Scholar]
- 27.Bernatsky S, Hudson M, Pope J, Markland J, Robinson D, Jones N, Docherty P, Abu-Hakima M, Leclerc S, Dunne J, et al. Reports of abnormal cervical cancer screening tests in systemic sclerosis. Rheumatology (Oxford). 2009;48:149–151. doi: 10.1093/rheumatology/ken442. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal AK, McLaughlin JK, Gridley G, Nyren O. Incidence of cancer among patients with systemic sclerosis. Cancer. 1995;76:910–914. [DOI] [PubMed] [Google Scholar]
- 29.Segel MC, Campbell WL, Medsger TA Jr., Roumm AD. Systemic sclerosis (scleroderma) and esophageal adenocarcinoma: is increased patient screening necessary? Gastroenterology. 1985;89:485–488. [DOI] [PubMed] [Google Scholar]
- 30.Bonifazi M, Tramacere I, Pomponio G, Gabrielli B, Avvedimento EV, La Vecchia C, Negri E, Gabrielli A. Systemic sclerosis (scleroderma) and cancer risk: systematic review and meta-analysis of observational studies. Rheumatology. 2013;52:143–154. doi: 10.1093/rheumatology/kes303. [DOI] [PubMed] [Google Scholar]
- 31.Tian X-P, Zhang X. Gastrointestinal complications of systemic sclerosis. World J Gastroenterol. 2013;19:7062–7068. doi: 10.3748/wjg.v19.i41.7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watad A, Bragazzi NL, Adawi M, Aljadeff G, Amital H, Comaneshter D, Cohen AD, Amital D. Anxiety disorder among rheumatoid arthritis patients: insights from real-life data. J Affect Disord. 2017;213:30–34. doi: 10.1016/j.jad.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Watad A, Tiosano S, Grysman N, Comaneshter D, Cohen AD, Shoenfeld Y, Amital H. The association between systemic lupus erythematosus and valvular heart disease: an extensive data analysis. Eur J Clin Invest. 2017;47:366–371. doi: 10.1111/eci.12744. [DOI] [PubMed] [Google Scholar]
- 34.Watad A, Tiosano S, Yahav D, Comaneshter D, Shoenfeld Y, Cohen AD, Amital H. Behcet’s disease and familial Mediterranean fever: two sides of the same coin or just an association? A cross-sectional study. Eur J Intern Med. 2017;39:75–78. doi: 10.1016/j.ejim.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Watad A, Abu Much A, Bracco D, Mahroum N, Comaneshter D, Cohen AD, Amital H. Association between ischemic heart disease and systemic lupus erythematosus-a large case-control study. Immunol Res. 2017;65:459–463. doi: 10.1007/s12026-016-8884-9. [DOI] [PubMed] [Google Scholar]
- 36.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo C-L, Duan Y, Grady J. Unconditional or conditional logistic regression model for age-matched case-control data? Front Public Health. 2018;6:57. doi: 10.3389/fpubh.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah AA, Hummers LK, Casciola-Rosen L, Visvanathan K, Rosen A, Wigley FM. Examination of autoantibody status and clinical features associated with cancer risk and cancer-associated scleroderma. Arthritis Rheumatol. 2015;67:1053–1061. doi: 10.1002/art.39022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


