Summary
Aims
Recent evidence indicates that the increased expression of calpain small subunit 1 (Capn4) is associated with tumorigenesis. This study was designed to explore the role which Capn4 plays in human glioma.
Methods
We detected the expression of Capn4 by immunohistochemistry in tissue microarrays and tissue samples. Following the down‐regulation of Capn4 in glioma cell lines by a specific short hairpin RNA, the function of Capn4 in invasion, migration, and proliferation was assessed. We then evaluated the prognostic role of Capn4 using univariate and multivariate analysis in 94 glioblastoma (GBM) patients.
Results
Glioma tissues exhibited notably higher expression of Capn4 compared with control brain tissues and was positively correlated with histological malignancy. The down‐regulation of Capn4 in glioma cells led to a decrease in invasion and migration in vitro. Through univariate analysis, the prognosis of GBM patients with Capn4 overexpression was significantly poorer with respect to progression‐free survival (PFS) and overall survival (OS). Based on the results of the multivariate analysis, Capn4high was demonstrated to be a negative independent prognostic indicator for PFS and OS in GBM patients.
Conclusion
The overexpression of Capn4 is a novel negative prognostic marker, and Capn4 may be used as a new target in therapeutic strategies for human glioma.
Keywords: Capn4, Gliomas, Invasion, Malignancy, Prognosis
Introduction
Glioma is one of the most aggressive tumors and is exceptionally difficult to treat. Although glioma therapies have improved significantly, the outcome of patients remains poor. The median survival time of these patients is only 14 months 1. Therefore, it is important to explore prognostic factors and to develop new schemes for the treatment of glioma.
Calpains, a family of calcium‐dependent neutral cysteine proteases, can selectively catalyze the controlled proteolysis of many specific substrates in response to calcium signals and are associated with cytoskeletal remodeling, cell motility, proliferation, apoptosis, and gene expression. To date, there are 16 characterized human calpain isoform genes that are defined by the presence of a protease domain, micro μ‐calpain, and milli m‐calpain. More and more studies have illustrated the importance of aberrant calpain expression and activity during tumorigenesis. For example, increased μ‐calpain expression is detected in schwannomas and meningiomas 2. Increased expression of m‐calpain occurs in colorectal adenocarcinomas as does high calpain activity in endometrial cancer 3, 4. Additionally, calpain small subunit 1 (Capn4), a subunit of calpains, has been reported to be overexpressed in hepatocellular carcinoma (HCC) 5 and intrahepatic cholangiocarcinoma (ICC) 6. Furthermore, patients with high levels of Capn4 exhibit poorer outcome. Additional studies have indicated that increased expression of Capn4 contributes to local tumor invasion and peripheral metastasis and has a major effect on tumor progression 5, 6. Based on the essential role of Capn4 in maintaining calpain activity and stability and the fact that abnormal calpain expression is a key character in cancer, identification of the role of Capn4 in glioma may be significant.
In the study, we first determined the expression of Capn4 in tissue microarray (TMA) and in formalin‐fixed and paraffin‐embedded (FFPE) tissue samples, including 279 glioma samples and 21 control brain specimens, by immunohistochemistry (IHC). Capn4 was down‐regulated in two glioma cell lines (U251 and U87) using specific shRNA to evaluate the function of Capn4 in glioma cells. Furthermore, we evaluated whether the expression of Capn4 is consistent with the prognostic role of Capn4 in 94 glioma patients who received concurrent chemoradiotherapy after tumor resection using univariate and multivariate analyses.
Materials and methods
Cell Lines and Culture
The glioma cell lines, SHG44, U251, A172, C6, and U87 (purchased from the Cell Bank of the Shanghai Branch of Chinese Academy of Sciences), were cultured in DMEM or F12K medium with 10% fetal bovine serum (GIBCO, Carlsbad, CA, USA) as previously described 7.
Patients and Follow‐Up
Of the 279 glioma samples, 185 samples were commercial TMAs without follow‐up data (Shanghai Biochip Co. Ltd, Shanghai,China). The other 94 samples were acquired from 94 GBM patients who underwent radical resection between February 2005 and November 2010 at the Department of Neurosurgery, Huashan Hospital, Fudan University. Patient characteristics are presented in Table S1. Control brain specimens were randomly chosen from the peritumoral tissues of the glioma patients (n = 16) or trauma/epilepsy surgery (n = 5). Ethical approval was given by the local independent Ethics Committee of Huashan Hospital. Each patient agreed to sign an informed consent form before the study began.
TMAs, FFPE Tissue Samples, and IHC
The TMAs were built as mentioned elsewhere 8. To determine Capn4 status, IHC was performed using an antibody (ab93959, Rabbit, 1:100; Abcam, Cambridge, MA, USA) that specifically recognizes Capn4 as described in previous publication 6. Two blinded independent observers examined Capn4 staining in TMAs and FFPE tissues, and an agreement was made for each score 7. A positive reaction for Capn4 was classified into four grades (0 for negative; 1 for weak; 2 for moderate; and 3 for strong) as previously described 9. The intensity of Capn4 was classified by expression level (Capn4high: moderate or strong, and Capn4low: negative or weak).
Capn4 Targeting Short Hairpin RNA
Lentiviral‐mediated pGMLV‐GFP‐shRNA‐Capn4 was purchased from Shanghai Genomeditech (China) and was used to knockdown Capn4 in GBM cells (U87‐ or U251‐vshCapn4). The most effective shRNA targeting sequence for Capn4 was 5′‐ CCGGCGCACACATTACTCCAACATTCTCGAGAATGTTGGAGTAATGTGTGCGTTTTTG‐3′ (sense) and 5′‐ AATTCAAAAACGCACACATTACTCCAACATTCTCGAGAATGTTGGAGTAATGTGTGCG‐3′ (antisense). We authenticated the stably transfected cells using qRT‐PCR and immunoblotting.
qRT‐PCR and Western Blotting Analysis
qRT‐PCR and western blotting were performed to determine Capn4 expression in glioma cell lines. qRT‐PCR was performed using the ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) starting with 1 ng cDNA and SYBR Green real‐time PCR Master Mix (TaKaRa, Dalian, China). The primers for Capn4 were 5′‐ACCCACTCCGTAACCTC‐3′ and 5′‐GGGTAGCAACCGTGAA‐3′; the primers for GAPDH were: 5′‐TCCACCACCCTGTTGCTGTA‐3′ and 5′‐ACCACAGTCCATGCCATCAC‐3′. Western blotting was performed as described in a previous publication 6. Rabbit anti‐human Capn4 (ab92333,1:1000; Abcam) was used to test the expression of Capn4. GAPDH (ab8245, Mouse, 1:10,000; Abcam) was used as a control. Procedures were performed thrice.
Wound Healing Assay
Cells were seeded on 24‐well plates, and after 24 h of growth, the cell had reached 80–90% density. A wound was made across the center of the well. The remaining cells were washed three times with phosphate‐buffered saline and then cultured in a serum‐free medium. At times 0, 24 h, and 48 h after wounding, we digitally photographed images of the healing process. Three separate experiments were performed.
Transwell Invasion Assay
Transwell invasion assays were performed using 24‐well transwell plates precoated with Matrigel (Falcon354480; BD Biosciences, Franklin Lakes, NJ, USA). The lower chambers were filled with complete media, and the upper chambers were filled with 1% FBS media. U87 and U251 cells were seeded in the upper chamber of inserts at 1 × 105 cells per well. After 24 h, the inserts were removed and washed. The upper layer was removed with cotton rods, and the membrane was fixed and stained with crystal violet. Cells in five microscopic fields were counted, photographed, and analyzed. All experiments were repeated three times.
Cell Proliferation Assays
U87 and U251 cells were placed in a 96‐well microtiter plate with 5 × 103 cell per well. The number of cells was checked after 24 and 48 h later using the 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazoliumbromide (MTT) assay. Cells were incubated with MTT (20 μL/well [Amresco, Solon, OH, USA]), and 200 μL DMSO was added. The absorbance of each wells was tested at 490 nm. Every experiment was replicated at least three times.
Statistical Analysis
SPSS 19.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. Values are described as the mean ± SD. The Student's t‐test and Fisher's exact probability test were used for comparisons between groups. The overall survival (OS) and progression‐free survival (PFS) were evaluated using the Kaplan–Meier method for univariate analysis, and differences were tested using the log‐rank test. For multivariate analysis, Cox's proportional hazards regression model was used. Values of P < 0.05 were considered statistically significant.
Results
Express of Capn4 was High in Gliomas, and High Level of Capn4 Expression Correlates with Histological Malignancy
Following the identification of primary gliomas using H&E staining, the expression of Capn4 was detected by IHC in 279 glioma and 21 control tissues. We observed immunoreactivity of Capn4 protein in the cytoplasm (Figure 1A), and a very low level of Capn4 expression was observed in control tissues compared with glioma tissues. There was a statistically significant difference in Capn4 expression between glioma samples and nontumorous samples (P < 0.01; Figure 1B).
Figure 1.
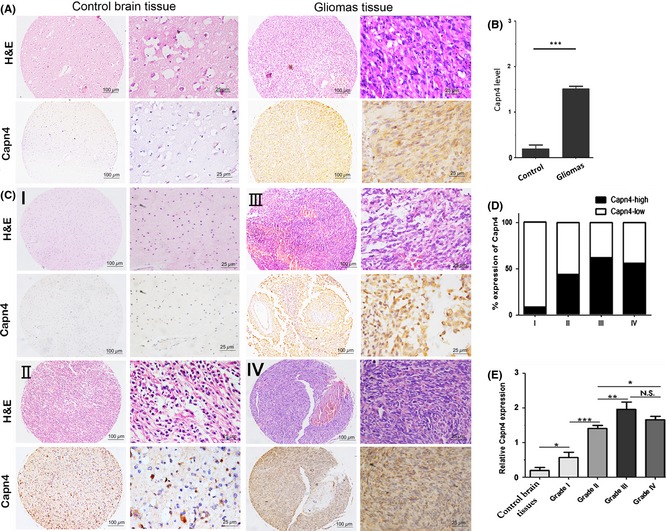
Calpain small subunit 1 (Capn4) expression is increased in gliomas and positively correlated with histological malignancy. Tissue microarrays (TMAs) and formalin‐fixed and paraffin‐embedded (FFPE) tissue containing control brain (n = 21) and glioma specimens (n = 279) were subjected to immunohistochemical staining with the Capn4 antibody as described in the Methods section. (A) Hematoxylin‐eosin and immunohistochemistry. Weak Capn4 staining was observed in control brain tissues. Capn4 protein expression was higher in tumor samples. Scale bar: 100 μm and 25 μm. (B) Capn4 expression was significantly higher in gliomas than in control brain tissues. (C) Capn4 expression varied with glioma grade. (D) The level of Capn4 was 8.70% (2/23) in grade I, 44.23% (46/104) in grade II, 62.96% (17/27) in grade III, and 56.00% (70/125) in grade IV. (E) Capn4 expression was positively correlated with glioma grade.*P < 0.05; **P < 0.01; ***P < 0.001. N.S. indicates no significance.
We observed positive staining of Capn4 in the cytoplasm of glioma tissues of different grades (Figure 1C). In total, 135 of the 279 glioma tissues expressed a high level of Capn4 (135/279, 48.39%). In accordance with previous reports, patients with Capn4high expression exhibited more aggressive features. The level of Capn4 was 8.70% (2/23) in grade I, 44.23% (46/104) in grade II, 62.96% (17/27) in grade III, and 56.00% (70/125) in grade IV (Figure 1D and E). These differences were significant. However, we did not observe relationships between the expression of Capn4 with other clinical characteristics, including age and sex (Table S1).
Knockdown of Capn4 Decreased the Invasion and Migration of Glioma Cells
To examine the function of Capn4 in glioma cells, the expression of Capn4 in SHG44, U251, A172, C6, and U87 cells was determined by qRT‐PCR and western blot. As shown in Figure 2A, a high level of Capn4 was detected in U251, A172, C6, and U87 cells, whereas a low level was detected in SHG44 cells. The U251 and U87 cells were then transfected with pGMLV‐GFP‐vshRNA‐Capn4, and knockdown of Capn4 was verified by qRT‐PCR and western blot (Figure 2B). Transwell assays revealed that knockdown of Capn4 expression correlated with the impairment in the invasiveness of U87 and U251 cells (Figure 2C). The wound healing assay demonstrated an obvious delay in the rate of wound repairing U87‐vshRNA‐Capn4 and U251‐vshRNA‐Capn4 cells at 24 h and 48 h, compared with U87‐vshRNA‐control and U251‐vshRNA‐control (Figure 2D). Moreover, the down‐regulation of Capn4 exhibited no significant effect on the proliferation of the cells at 24 and 48 h (P > 0.05, Figure 2E).
Figure 2.
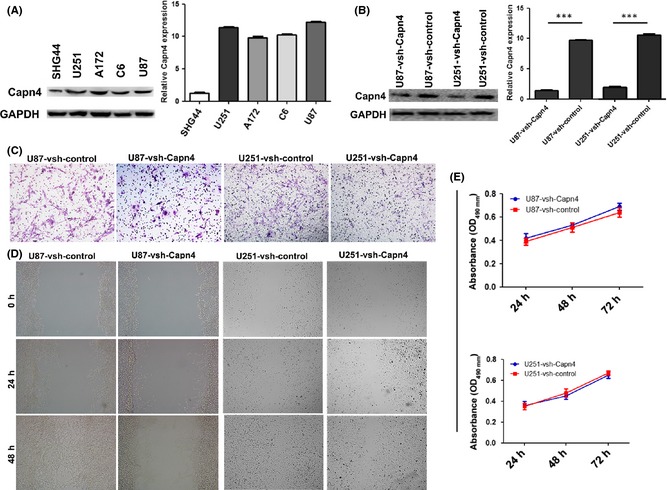
Functional analysis after knockdown Calpain small subunit 1 (Capn4) in glioma cells in vitro. (A) The expression of Capn4 was determined by immunoblotting and qRT‐PCR in glioma cell lines. (B) The interference of Capn4 in U87 and U251 cells was successful using lentiviral‐mediated pGMLV‐GFP‐vshRNA‐Capn4. The knockdown of Capn4 expression was tested by immunoblotting and qRT‐PCR (C) The transwell assays revealed that knockdown Capn4 expression was correlated with decreased cell invasion (original magnification, ×100). (D) The U87 and U251 cells transfected with vsh‐Capn4 migrated more slowly than the vsh‐control at 24 and 48 h in the wound healing assay. (E) Cell proliferation was detected by MTT assay. These differences were not significant.
Capn4 Overexpression in GBM was Associated with Poor Prognosis
Of the 279 patients, 94 cases of GBM had complete follow‐up data. As presented in Table 1, Capn4 was not obviously related to other clinical or pathological characteristics including gender, age, extent of resection, the expression of mindbomb E3 ubiquitin protein ligase 1 (MIB‐1) or TP53.
Table 1.
Correlation of Capn4 expression with clinical and molecular characteristics in archival glioblastoma (GBM) patients
| Clinical characteristics | High levels of Capn4 | Low levels of Capn4 | P | Molecular characteristics | High levels of Capn4 (no.) | Low levels of Capn4 (no.) | P |
|---|---|---|---|---|---|---|---|
| Age (year) | 0.549 | MIB‐1 | 0.47 | ||||
| Median | 52 | 54 | High | 22 | 17 | ||
| Range | 13–77 | 13–77 | Low | 30 | 25 | ||
| Sex (no.) | 0.636 | TP53 | 0.38 | ||||
| Male | 32 | 27 | Mutated | 22 | 19 | ||
| Female | 20 | 15 | Wild‐type | 30 | 23 | ||
| Preoperative KPS (no.)a | 0.873 | ||||||
| ≥70 | 47 | 39 | |||||
| <70 | 5 | 3 | |||||
| Extent of resection (no.)a | 0.877 | ||||||
| GTR | 48 | 39 | |||||
| STR | 4 | 3 |
PS, Karnofsky scoring; GTR, gross total resection; STR, subtotal resection; MIB‐1, mindbomb E3 ubiquitin protein ligase 1; KPS, Karnofsky score; and Capn4, calpain small subunit 1.
Fisher's exact test.
In the 94 patients, the median OS was 17 months and the average OS (±SD) was 18.57 ± 11.34 months. The median PFS was 10 months, and the average PFS (±SD) was 12.05 ± 10.20 months.
The expression of Capn4 was also correlated with OS and PFS. The 1‐ and 2‐year OS rates of the Capn4low group were much higher than those in the Capn4high group (88.1% vs. 55.8% and 42.9% vs. 17.3%, respectively). Furthermore, the 1‐ and 2‐year PFS rates in the Capn4low group were significantly lower than those in the Capn4high group (54.8% vs. 30.8% and 19.0% vs. 15.4%, respectively).
Following univariate analysis by the Kaplan–Meier method, patients with a Karnofsky score (KPS) ≥70 exhibited better prognosis (OS: P = 0.020, PFS: P = 0.038). Overexpression of Capn4 was observed in 52 patients (55%), and the number of patients with Capn4low was 42 (45%). Capn4high correlated with shorter OS (P = 0.032, Figure 3A) and PFS (P = 0.013, Figure 3B). Other clinicopathological factors did not exhibit significant differences between the subgroups (Table S2).
Figure 3.
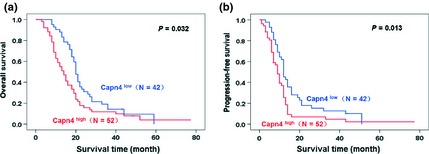
Overall survival (OS) and progression‐free survival (PFS) analysis of 94 glioma patients with respect to Calpain small subunit 1 (Capn4) expression. (A, B) The patients with Capn4high expression exhibited poorer prognosis with respect to OS and PFS.
Following multivariate analysis using Cox's proportional hazard regression model, Capn4high was observed to have a prognostic value for poorer OS (P = 0.048, HR = 1.541, 95% CI: 1.004–2.055) and PFS (P = 0.008, HR = 1.828, 95% CI: 1.174–2.847). A KPS ≥70 was also a prognostic factor for OS (0 = 0.034, HR = 2.059 95% CI: 1.055–4.017) and PFS (P = 0.019, HR = 2.234 95% CI: 1.151–4.757).
Discussion
Malignant gliomas are the most common and deadliest of the brain tumors. These tumors are highly invasive and are characterized by rapid tumor cell proliferation and exhibit poor outcome. Understanding the molecular mechanisms of gliomagenesis mechanisms and tumor progression is necessary to discover useful diagnostic and therapeutic strategies. Our study revealed that alterations in Capn4 expression seemed to be correlated with the grade of glioma and patient survival.
Capn4 is a protein belonging to the family of calcium‐dependent proteases 10. Although the detailed physiological role of Capn4 remains poorly understood in tumorigenesis and tumor progression, excessive calpain expression and activation have been associated with a number of diseases due to the hydrolysis of numerous substrates, such as inhibitors of Iκ‐B, focal adhesion kinase, talin, and proto‐oncogenes 11. Recently, Capn4 was reported to be overexpressed in HCC and ICC, and high levels of Capn4 have been associated with poor patient outcome 4, 5. Here, we demonstrated that Capn4 was overexpressed in glioma tissues and that its expression positively correlated with histological malignancy (Figure 1). Therefore, we conclude that high levels of Capn4 are correlated with the progression of glioma.
The migration of tumor cells, which contributes to local tumor invasion and peripheral metastasis, has a major effect on tumor progression. Although metastasis of GBM is rare, recurrences at nonoriginal intracranial areas at a distance from the initial location are not uncommon. In our experiments, we demonstrated that knockdown of Capn4 impaired U87 and U251 cell invasion and migration. Initial research implicated calpain in the regulation of integrin‐mediated cell adhesion, as m‐calpain was found to localize to integrin‐associated focal adhesion structures and directly cleaved the focal adhesion protein talin 12. Furthermore, pharmacological inhibition and RNAi to suppress calpain activity further implicated m‐calpain in the regulation of focal adhesion turnover and cell migration in a variety of tumor‐derived and oncogene‐transformed cell models in 2D in vitro culture 13, 14, 15, including the U87 GBM cell line 16, 17. The functional mutation analysis of focal adhesion substrates revealed the role of calpain‐mediated proteolytic cleavage of focal adhesion kinase and talin in the dynamic turnover of focal adhesion structures. This indicates that Capn4 may have a significant effect on integrin‐mediated cell migration 2, 18, 19, 20, 21, and the overexpression of Capn4 may be associated with the malignancy of human glioma.
Whether the differential expression of this molecule detected in gliomas has a diagnostic, prognostic or predictive value will determine its significance in clinical application. A meaningful molecule can be designated as a glioma biomarker if it provides particular diagnostic, prognostic, or predictive data beyond that obtained by mere histological classification 22, such as IDH‐1 and O‐6‐methylguanine‐DNA methyltransferase (MGMT) promoter methylation. IDH‐1, which was first detected during the analysis of GBM genes in 2008 23, was identified as a diagnostic biomarker that distinguished pilocytic astrocytoma (WHO I) from diffuse astrocytoma (WHO II) and distinguished primary GBM from secondary GBM 24, 25, 26. IDH1 also became a prognostic biomarker in gliomas after rigorous verification of numerous clinical data 23, 26, 27, 28. Controversy regarding its value in predicting response to a particular type of therapy, such as TMZ, still exists 28, 29, 30, 31. The status of MGMT promoter methylation is recognized as a predictive biomarker for GBM response to TMZ 32. Our clinic‐pathological data demonstrated that high expression of Capn4 may predict poorer prognosis. Thus, Capn4high is likely to be a candidate negative prognostic biomarker and a potential target in glioma therapy. Further studies will test the relationship between Capn4 and IDH1 and MGMT promoter methylation, explore the value of Capn4 high in diagnosis, prognosis, and prediction, and verify its potency in clinical use.
By employing both in vivo and in vitro experiments, we observed that high levels of Capn4 promoted glioma progression. Additionally, the Capn4 excessive expression of Capn4 is a predictor of poor prognosis in glioma patients and could be a new target of molecular therapeutic for glioma.
Financial Disclosure
This work was supported by the National Science Foundation for Distinguished Young Scholars of China (grant No. 81025013), the “Dawn” Tracking program of Shanghai Education Commission, China (grant No. 10GG01. The Project for National 985 Engineering of China (985III‐YFX0102). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Table S1. High level of Capn4 is associated with the grade of human gliomas.
Table S2. Univariate and multivariate Cox regression analyses of OS and PFS in archival GBM patients.
The first two authors contributed equally to this work.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 2. Kimura Y, Koga H, Araki N, et al. The involvement of calpain‐dependent proteolysis of the tumor suppressor NF2 (merlin) in schwannomas and meningiomas. Nat Med 1998;4:915–922. [DOI] [PubMed] [Google Scholar]
- 3. Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 2003;83:731–801. [DOI] [PubMed] [Google Scholar]
- 4. Saez ME, Ramirez‐Lorca R, Moron FJ, Ruiz A. The therapeutic potential of the calpain family: New aspects. Drug Discov Today 2006;11:917–923. [DOI] [PubMed] [Google Scholar]
- 5. Bai DS, Dai Z, Zhou J, et al. Capn4 overexpression underlies tumor invasion and metastasis after liver transplantation for hepatocellular carcinoma. Hepatology 2009;49:460–470. [DOI] [PubMed] [Google Scholar]
- 6. Zhang C, Bai DS, Huang XY, et al. Prognostic significance of Capn4 overexpression in intrahepatic cholangiocarcinoma. PLoS One 2013;8:e54619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hua W, Yao Y, Chu YW, et al. The CD133+ tumor stem‐like cell‐associated antigen may elicit highly intense immune responses against human malignant glioma. J Neurooncol 2011;105:149–157. [DOI] [PubMed] [Google Scholar]
- 8. Ke AW, Shi GM, Zhou J, et al. CD151 amplifies signaling by integrin alpha6beta1 to PI3K and induces the epithelial‐mesenchymal transition in HCC cells. Gastroenterology 2011;140:1629–1641. [DOI] [PubMed] [Google Scholar]
- 9. Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: A clinicopathologic study of 292 cases. J Clin Oncol 2005;23:1473–1482. [DOI] [PubMed] [Google Scholar]
- 10. Sorimachi H, Hata S, Ono Y. Expanding members and roles of the calpain superfamily and their genetically modified animals. Exp Anim 2010;59:549–566. [DOI] [PubMed] [Google Scholar]
- 11. Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. The calpain system and cancer. Nat Rev Cancer 2011;11:364–374. [DOI] [PubMed] [Google Scholar]
- 12. Carragher NO, Fonseca BD, Frame MC. Calpain activity is generally elevated during transformation but has oncogene‐specific biological functions. Neoplasia 2004;6:53–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mamoune A, Luo JH, Lauffenburger DA, Wells A. Calpain‐2 as a target for limiting prostate cancer invasion. Cancer Res 2003;63:4632–4640. [PubMed] [Google Scholar]
- 14. Postovit LM, Dutt P, Dourdin N, et al. Calpain is required for MMP‐2 and u‐PA expression in SV40 large T‐antigen‐immortalized cells. Biochem Biophys Res Commun 2002;297:294–301. [DOI] [PubMed] [Google Scholar]
- 15. Carragher NO, Westhoff MA, Riley D, et al. v‐Src‐induced modulation of the calpain‐calpastatin proteolytic system regulates transformation. Mol Cell Biol 2002;22:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jang HS, Lal S, Greenwood GA. Calpain 2 is required for glioblastoma cell invasion: Regulation of matrix metalloproteinase 2. Neurochem Res 2010;35:1796–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma J, Cui W, He SM, et al. Human U87 astrocytoma cell invasion induced by interaction of betaig‐h3 with integrin alpha5beta1 involves calpain‐2. PLoS One 2012;7:e37297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuevas BD, Abell AN, Witowsky JA, et al. MEKK1 regulates calpain‐dependent proteolysis of focal adhesion proteins for rear‐end detachment of migrating fibroblasts. EMBO J 2003;22:3346–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dourdin N, Bhatt AK, Dutt P, et al. Reduced cell migration and disruption of the actin cytoskeleton in calpain‐deficient embryonic fibroblasts. J Biol Chem 2001;276:48382–48388. [DOI] [PubMed] [Google Scholar]
- 20. Chan KT, Bennin DA, Huttenlocher A. Regulation of adhesion dynamics by calpain‐mediated proteolysis of focal adhesion kinase (FAK). J Biol Chem 2010;285:11418–11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franco SJ, Rodgers MA, Perrin BJ, et al. Calpain‐mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol 2004;6:977–983. [DOI] [PubMed] [Google Scholar]
- 22. Riemenschneider MJ, Jeuken JWM, Wesseling P, Reifenberger G. Molecular diagnostics of gliomas: State of the art. Acta Neuropathol 2010;120:567–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008;321:1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 2008;116:597–602. [DOI] [PubMed] [Google Scholar]
- 25. Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol 2009;11:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res 2009;15:6002–6007. [DOI] [PubMed] [Google Scholar]
- 27. Dubbink HJ, Taal W, van Marion R, et al. IDH1 mutations in low‐grade astrocytomas predict survival but not response to temozolomide. Neurology 2009;73:1792–1795. [DOI] [PubMed] [Google Scholar]
- 28. van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: A report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res 2010;16:1597–1604. [DOI] [PubMed] [Google Scholar]
- 29. Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low‐grade gliomas. Neurology 2010;75:1560–1566. [DOI] [PubMed] [Google Scholar]
- 30. Houillier C, Wang X, Kaloshi G, et al. IDH mutations occur frequently in Chinese glioma patients and predict longer survival but not response to concomitant chemoradiotherapy in anaplastic gliomas. Oncol Rep 2011;26:1479–1485. [DOI] [PubMed] [Google Scholar]
- 31. Combs SE, Rieken S, Wick W, et al. Prognostic significance of IDH‐1 and MGMT in patients with glioblastoma: One step forward, and one step back? Radiat Oncol 2011;6:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. High level of Capn4 is associated with the grade of human gliomas.
Table S2. Univariate and multivariate Cox regression analyses of OS and PFS in archival GBM patients.


