Transplantation of human organs including the kidney, liver, heart, and lung has become a mainstay of clinical treatment. With allogeneic composite tissue transplantation (CTA), there are always the issues of practicality, immune rejection, and ethics, but joint efforts at the end of the last century at American University of Louisville and Kleinert Hand and Microsurgery Center team led to the design and completion of the first successful CTA preclinical model 1. Based on this porcine model, five stages of postoperative tissue rejection were established, based on severity 2. The immunosuppressive regimen implemented at this time included tacrolimus (FK‐506), mycophenolate mofetil, and prednisone, which successfully prevented CTA rejection 3. This study formed the basis for hand allografts in the United States and the world 4. It has been 15 years since we completed the first successful human hand allograft; the patient recovered function well, without rejection of the transplanted hand, and continues to work and lead a normal social life 5. Another type of CTA, the face transplant, has also been carried out in the clinic 6. For patients with systemic metastases of cancer, multiple organ failure, hereditary whole body muscle atrophy, or traumatic paraplegia, although brain tissue and brain function remains healthy, current medical technology has no means or strategies to save their lives 7.
History
In the early 1900s, Dr. Guthrie developed the first animal model of head transplantation using dogs 7. In the 1950s, the former Soviet Union and Chinese scientists used dogs as models 7. The upper body of the donor dog was transplanted onto the back of the neck of the recipient dog, to study allogeneic head transplantation. This study resulted in two‐headed dogs, with survival ranging from several hours to several days. In the 1960–70s, Dr. White in the United States used monkeys as animal models of head/body reconstruction, replacing the head from the donor's body with the isolated recipient head (graft) at the cervical level (C3–C4) 8, 9. White's monkey models advanced the field of allograft reconstruction of the head and body transplantation to become more clinically relevant, resulting in one donor body to the recipient head. This model was designed to focus on the anatomical and physiological aspects of transplantation, but its limitations were it did not evaluate measures to prevent immune rejection, and it lacked an effective central nervous system recovery strategy. Thus, this model cannot achieve long‐term survival after transplantation. There have been few advances in allo‐head and body reconstruction (AHBR) since that time, and as such, the field has been limited by lack of an established, effective biological animal model, which prevents completion of the necessary preclinical experiments and clinical translation of AHBR.
Applying Lessons from History to the New Model
Anticipating that the early stages of our AHBR model design process will consume a large number of animals, our group selected mice to complete the preliminary experiments to conserve resources (see Figure 1). Critical technical aspects of the surgical procedure will be evaluated in mice, and monkeys will subsequently be used to validate the AHBR design with regard to the long‐term effects on heart and lung function, and prevention of immune rejection.
Figure 1.
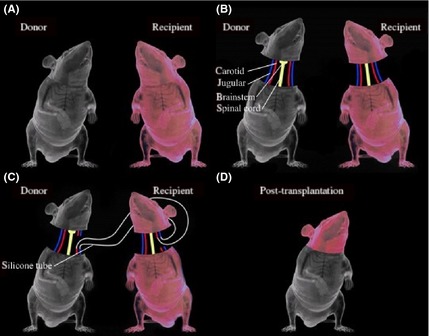
Schematic representation of the AHBR mouse model. (A) Two mice with different colors are selected. (B) All tissues have been separated including carotid artery, jugular vein, spinal cord and so on. (C) In order to ensure transplanted brain tissue does not stop blood circulation to avoid cerebral ischemia and hypoxia, cross‐circulation is established by the silicone tubes. (D) Mice after transplantation.
Tissues and organs of the body depend on adequate blood circulation to maintain normal metabolism and function. Interruption of blood supply, or hypoperfusion, often results in ischemic injury to the tissue. The brain and central nervous system are highly intolerant to hypoperfusion due to hypoxic injury of nerve cells in the brain, producing irreversible changes. The AHBR procedure makes use of an aspect of White's head transplant model to ensure that brain tissue does not undergo interrupted blood perfusion, to avoid cerebral hypoxia, which is key to the recovery of brain function after transplantation. Specifically, White's animal model includes a blood flow crossover loop which consists of anastomosis of the donor and recipient common carotid artery and jugular vein, thereby maintaining a blood pressure of at least 40 mmHg, which is sufficient to meet the metabolic needs of brain tissue, similar to normal blood perfusion 10. In our AHBR model, initial experimental observations show that with unilateral carotid and venous cross‐circulation, the cerebral blood supply is adequate, and recipient mouse EEG and ECG during surgery and postoperatively show normal electrophysiological activity.
Breathing and circulation are the two major systems required to maintain life. Spontaneous breathing and blood circulation in the central nervous system is the key aspect of the AHBR model that provides for long‐term survival. Past animal models of AHBR used cervical third or fourth level transaction 8, 9. In such studies, the donor brainstem was not preserved, resulting in loss of both independent breathing and circulation. Therefore, in our AHBR model surgery design, we chose to retain the donor brainstem. Our experimental short‐term results show that retaining the donor brainstem results in mice with spontaneous breathing, not dependent on an artificial respiration machine, along with preserved blood circulation. Future studies will continue to complete the AHBR mouse model experiments and to extend short‐term observation to long‐term efficacy studies.
Ethical Aspects of AHBR
Ethical issues in the development of human organ transplants are an important topic. There are regulations in place in most countries to ensure that transplantation of organs is safe and fair. The donor has an irreplaceable role in human health and the continuation of life. Transplantation of organs and tissues, including adult stem cells and almost all organs of the body, has become common in modern medicine. Although current medical technology cannot guarantee that AHBR will allow the patient normal standing and walking after surgery, it can extend precious life. If AHBR is able to successfully achieve clinical translation, this new surgical treatment strategy is theoretically expected to save critically ill, incurable patients' lives.
Critical Research Model
Establishing and Standardizing the Murine AHBR Model
Allograft reconstruction of the head and body is the next frontier of CTA and must be extensively tested before its clinical application. Based on the lessons learned from previous head or body transplantation studies, we first designed an AHBR mouse model. Our team will continue to validate this model and extend short‐term observation to long‐term efficacy studies. The porcine CTA model derived from our previous hand transplant research results from the Louisville research group provided internationally recognized standards for clinical classification of tissue rejection via histopathological features and allowed for assessment of rejection window of time. We will establish a similar evaluation system for AHBR.
Through Cross‐Circulation to Avoid Cerebral Ischemia and Hypoxia
The model relies on anastomosis of the carotid arteries and veins to create a circulatory loop between the donor and recipient to ensure that blood perfusion is not interrupted, and brain tissue is not exposed to anoxia. Electrophysiological changes in the EEG are used to provide accurate, reliable, and timely detection standards to monitor the brain function and to maintain the body's normal blood pressure and cerebral perfusion pressure.
Preserving the Donor Brainstem to Guarantee Postoperative Breathing and Circulation
Many important reflex centers are located in the brain stem, cerebellum, and spinal cord. In particular, the medulla oblongata is home to the circulatory, respiratory, and swallowing functional centers. Therefore, by retaining the donor brainstem, the postoperative donor body can retain effective spontaneous breathing and blood circulatory function and allow long‐term studies. Special attention should be given to breathing regularity and frequency as well as ECG indicators because the donor brainstem may have limited physiological function. Nerve reflexes and basic sense of movement and muscle tension are regularly checked and recorded.
Conclusion
Our preliminary experiments in mice in the field of AHBR have already yielded important observations, including surgical methods for preventing cerebral ischemia and preserving donor body spontaneous breathing and circulation.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by Scientist Foundation of the Second Affiliated Hospital of Harbin Medical University (to Dr. Xiaoping Ren), Harbin, China.
References
- 1. Ren X, Shirbacheh MV, Ustuner T, et al. Osteomyocutaneous flap as a preclinical composite tissue allograft: Swine model. Microsurgery 2000;20:143–149. [DOI] [PubMed] [Google Scholar]
- 2. Zdichavsky M, Jones JW, Ustuner T, et al. Scoring of skin rejection in a swine composite tissue allograft model. J Surg Res 1999;85:1–8. [DOI] [PubMed] [Google Scholar]
- 3. Jones JW, Ustuner T, Zdichavsky M, et al. Long‐term survival of an extremity composite tissue allograft with FK506‐ mycophenolate mofetil therapy. Surgery 1999;126:384–388. [PubMed] [Google Scholar]
- 4. Francois C, Breidenbach WC, Maldonado C, et al. Hand transplantation: Comparisons and observations of the first four clinical cases. Microsurgery 2000;20:360–371. [DOI] [PubMed] [Google Scholar]
- 5. Hautz T, Engelhardt TO, Weissenbacher A, et al. World experience after more than a decade of clinical hand transplantation: Update on the Innsbruck program. Hand Clin 2011;27:423–431. [DOI] [PubMed] [Google Scholar]
- 6. Wamke P. The first facial transplant. Lancet 2005;366:1984. [DOI] [PubMed] [Google Scholar]
- 7. Ren X, Laugel M. The next frontier in composite tissues allotransplantation. CNS Neurosci Ther 2013;19:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White RJ, Wolin LR, Massopust LC Jr, Taslitz N, Verdura J. Cephalic exchange transplantation in the monkey. Surgery 1971;70:135–139. [PubMed] [Google Scholar]
- 9. White RJ, Wolin LR, Massopust LC Jr, Taslitz N, Verdura J. Primate cephalic transplantation: Neurogenic separation, vascular association. Transplant Proc 1971;3:602–604. [PubMed] [Google Scholar]
- 10. White RJ, Albin MS, Verdura J, et al. The isolation and transplantation of the brain. An historical perspective emphasizing the surgical solutions to the design of these classical models. Neurol Res 1996;18:194–203. [DOI] [PubMed] [Google Scholar]


