Summary
Aims
To examine the health‐related quality of life (HRQOL) in patients with transient ischemic attack (TIA) or minor stroke and assess the impact of recurrent stroke on HRQOL.
Methods
Health‐related quality of life data on patients participated in the Clopidogrel in High‐risk patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial were analyzed. The available 90‐day EuroQoL data (EQ‐5D) were used to calculate EQ‐5D index score. Poor HRQOL was defined as an EQ‐5D index score ≤0.5. The characteristics of HRQOL and factors predicting poor HRQOL in these patients were then explored.
Results
Among the total 5170 patients enrolled, 90‐day HRQOL data were obtained from 5104 patients for analysis. The mean EQ‐5D index score at day 90 was 0.88 ± 0.21 for all patients, but only 0.42 ± 0.35 for those with recurrent strokes. Poor 90‐day HRQOL was found in 294 (5.8%) patients. Patients with poor HRQOL had more strokes during follow‐up than patients with good HRQOL (94.9 vs. 4.7%, P < 0.001). Age, history of hypertension and diabetes, and NIHSS at baseline were independent risk factors for predicting poor HRQOL. Stroke recurrence, NIHSS at baseline, age, and minor stroke on admission became independent risk factors once stroke recurrence was added into the model.
Conclusions
Stroke recurrence was associated with poor HRQOL in patients with TIA or minor strokes. Interventions focusing on controlling risk factors and prevention of worsening of neurological function may prevent poor HRQOL in these patients.
Keywords: EQ‐5D, Health outcomes, Quality of life, Stroke, Transient ischemic attack
Introduction
Health‐related quality of life (HRQOL) offers a comprehensive measurement of the impact of a disease and recovery process from the perspective of the patient. It is now a commonly used outcome measurement tool in stroke trials 1, 2, 3, 4. Previous studies have described the long‐term HRQOL of stroke survivors and identified age 1, 5, 6, gender 5, baseline severity 1, 4, 5, 6, 7, diabetes 7, hypertension 1, 8, disability 4, 9, aphasia 10, depression 4, 9, 11, cognitive impairment 8, and low socioeconomic status 5 as independent risk factors predicting poor long‐term HRQOL after stroke. However, these studies were conducted in patients with either ischemic stroke 10, 11, intracerebral hemorrhage 1, subarachnoid hemorrhage 6 or all strokes combined together 4, 5, 8, 9, 12. Patients with transient ischemic attack (TIA) or minor ischemic stroke were reported to have short‐term poor prognosis 13, 14, 15. Patients with nondisabling cerebrovascular events (TIA or minor stroke) may also have poor HRQOL at 90 days after the index event because of high rates of stroke recurrence 4, 12. To our knowledge, few studies have assessed HRQOL in these patients after TIA or minor stroke.
Using data from the recently completed Clopidogrel in High‐risk patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial 16, we aimed to describe the 90‐day HRQOL and examined the potential influence of stroke recurrence on HRQOL in these patients with TIA or minor stroke.
Materials and Methods
Study Design and Subjects
The CHANCE trial (ClinicalTrials.gov number: NCT00979589) was a randomized, double‐blind, placebo‐controlled trial conducted at 114 centers in China between October 2009 and July 2012 16. Details on the rationale, design, and major results have been described in previous articles 16, 17. Briefly, 5170 patients within 24 h of onset of minor stroke or high‐risk TIA were randomized to combination therapy with clopidogrel and aspirin or placebo plus aspirin. The primary outcome was stroke (ischemic or hemorrhagic) during 90 days of follow‐up. The CHANCE protocol was approved by the ethics committee at each study center. All participants or their legal proxies provided written informed consent.
Patients enrolled in the CHANCE trial met the following inclusion criteria: age ≥ 40, diagnosis of an acute minor ischemic stroke (National Institute of Health Stroke Scale [NIHSS] ≤ 3) or high‐risk TIA (ABCD2 ≥ 4), and ability to start the study drug within 24 h after symptom onset. Patients with pre‐existing disabling conditions defined as modified Rankin Scale (mRS) >2 were excluded. For this study, 5104 patients were included after excluding patients who died (20) and were without 90‐day EQ‐5D data (46).
Data Collection and Risk Factors Definition
Baseline demographics, vascular risk factors, premorbid mRS, medications, and clinical measures were collected. Vascular risk factors included history of stroke or TIA, myocardial infarction, congestive heart failure, atrial fibrillation or flutter, hypertension, diabetes mellitus, dyslipidemia, current or previous smoking, and alcohol consumption. Hypertension was defined as a systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, history of hypertension or antihypertensive drug use 18. Dyslipidemia was defined as serum triglyceride ≥150 mg/dL, low‐density lipoprotein cholesterol ≥130 mg/dL, high‐density lipoprotein cholesterol ≤40 mg/dL, history of dyslipidemia, or lipid‐lowering drug use. Diabetes was defined as fasting glucose concentration ≥7.0 mmol/L, nonfasting glucose concentration ≥11.1 mmol/L with classic symptoms of hyperglycemia or hyperglycemic crisis, history of diabetes or glucose‐lowering drugs use 18, 19. Heavy alcohol consumption was defined as consumption of ≥2 standard alcoholic beverages per day.
At day 90 after the onset of TIA or minor stroke, mRS and NIHSS scores were collected by trained and certified investigators through face‐to‐face interview. Poor functional outcome was defined as mRS 3‐5 (dependence) 20.
Quality of Life
Health‐related quality of life was assessed using the EuroQoL questionnaire 21 at day 90 follow‐up visit. EuroQoL consists of two parts: EQ‐5D and EQ visual analogue scale (EQ‐VAS). EQ‐5D comprises of the following five dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has three levels: no problems, some problems, and extreme problems. The EQ‐VAS is carried out by patient as an assessment of self‐well‐being in the vertical and visual analogue scales. A value of 100 on this scale indicates a perfect score for health but a score of 0 means death. A single utility score can be calculated using the population‐based preference weights for each dimension of EQ‐5D 22, 23, 24. We used the Chinese preference weights developed by Liu et al. 24. The EuroQoL scale was also completed by investigators through face‐to‐face interview. All investigators were trained and certified based on a shared standardized interview protocol. For those patients with dysarthria or disability caused by severe stroke, EuroQoL was completed by a proxy. Patient and proxy respondent agreement was assessed using intraclass correlation coefficient (ICC). The ICC of EQ‐5D index was 0.72 (95% confidence intervals 0.60–0.81).
Statistical Analysis
Categorical variables were presented as percentages and continuous variables as mean with standard deviation (SD) or median with interquartile (IQR). The associations between EuroQoL (EQ‐5D index score and EQ‐VAS) and clinical outcome (mRS and NIHSS) were assessed using partial correlations, adjusting for age and sex. Spearman's rank correlation coefficients were used to take into account the ordinal and nonparametric nature of the scales.
Poor HRQOL was defined as an EQ‐5D index score of ≤0.5, and good HRQOL was defined as an EQ‐5D index score of >0.5 1. Univariable and multivariable analyses were performed to identify predictors of poor HRQOL at day 90 after the onset of a TIA or minor stroke. Differences on baseline variables between the poor and good HRQOL groups were compared using chi‐square (χ 2) test for categorical variables and t‐test or Mann–Whitney U‐tests for continuous variables. All baseline variables with a P‐value of ≤0.1 were included in a stepwise multivariable logistic regression. Odds ratios (ORs) with 95% confidence intervals (CI) were calculated using the good HRQOL group as the reference. EQ‐VAS was used as sensitivity analyses, with a definition of EQ‐VAS score of ≤40 as poor HRQOL 25. Dual antiplatelet treatment was forced to be included in all models. We set up models with or without the predictor of stroke recurrence during follow‐up, respectively.
The α level of significance was P < 0.05 two‐sided. All analyses were performed with SAS software version 9.3 (SAS Institute Inc, Cary, NC, USA).
Results
Patients
Among the 5170 patients with confirmed diagnosis of TIA or minor stroke in the CHANCE study, 90‐day EQ‐5D scores from 5104 patients were obtained for analysis. Twenty patients died during the study period. HRQOL data were missing in 46 patients of whom 36 were lost to follow‐up. Among the 5104 patients, 4034 (79.0%) completed the assessment independently; 600 (11.8%) were with assistance and 470 (9.2%) completed by a proxy because of dysarthria or disability caused by severe stroke.
Quality of Life
In 5104 survivors after a TIA or minor stroke, 244 (4.8%), 268 (5.3%), and 255 (5.0%) patients reported extreme problems in mobility, self‐care, and usual activities at day 90, respectively. In contrast, only 58 (1.1%) patients reported pain/discomfort, and 73 (1.4%) reported anxiety/depression. Proxy respondents reported significantly worse problems in all domains of EQ‐5D than patients who were self‐respondents (Figure 1, P < 0.001).
Figure 1.
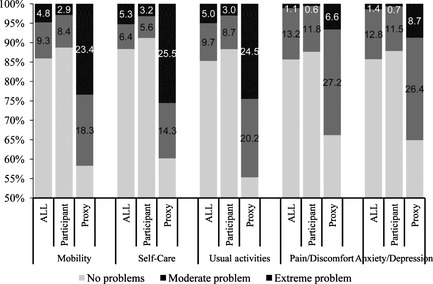
EQ‐5D domains for patients at day 90 after a transient ischemic attack or minor stroke.
The overall mean EQ‐5D index score was 0.88 ± 0.21, and overall mean EQ‐VAS was 84 ± 15 at the day 90 visit. The mean EQ‐5D index score of patients with recurrent stroke during follow‐up was much lower than those without stroke recurrence (0.42 ± 0.35 vs. 0.93 ± 0.09, P < 0.001) (Table 1). Both EQ‐5D index score and EQ‐VAS were correlated with the 90‐day mRS scores (EQ‐5D index score: Spearman r = −0.62, P < 0.001; EQ‐VAS: Spearman r = −0.47, P < 0.001) and NIHSS (EQ‐5D index score: Spearman r = −0.55, P < 0.001; EQ‐VAS: Spearman r = −0.44, P < 0.001) (Figure 2). The mean EQ‐5D index score was 0.95 ± 0.05 for patients with a mRS of 0, but decreased to 0.10 ± 0.18 for patients with a mRS of 5 at day 90 (Table 1).
Table 1.
Quality of life compared to stroke recurrence and modified Rankin scale at day 90 after a transient ischemic attack or minor stroke
| N | EQ‐5D index score | EQ visual analog scale | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| All | 5104 | 0.88 ± 0.21 | 0.96 (0.89, 0.96) | 84 ± 15 | 89 (80, 95) |
| Without stroke recurrence | 4598 | 0.93 ± 0.09 | 0.96 (0.96, 0.96) | 87 ± 11 | 90 (80, 95) |
| With stroke recurrence | 506 | 0.42 ± 0.35 | 0.35 (0.11, 0.71) | 58 ± 24 | 60 (40, 76) |
| 90‐day mRSa | |||||
| 0 | 3120 | 0.95 ± 0.05 | 0.96 (0.96, 0.96) | 89 ± 10 | 90 (85, 95) |
| 1 | 1454 | 0.89 ± 0.11 | 0.96 (0.87, 0.96) | 83 ± 12 | 85 (80, 90) |
| 2 | 196 | 0.67 ± 0.19 | 0.68 (0.54, 0.83) | 71 ± 16 | 72 (62, 80) |
| 3 | 92 | 0.44 ± 0.24 | 0.51 (0.29, 0.60) | 59 ± 19 | 60 (50, 72) |
| 4 | 213 | 0.16 ± 0.19 | 0.11 (0.09, 0.23) | 44 ± 18 | 45 (30, 57) |
| 5 | 28 | 0.10 ± 0.18 | 0.11 (−0.02, 0.21) | 36 ± 22 | 30 (20, 50) |
EQ‐5D, European quality of life scale; mRS, modified Rankin scale; SD, standard deviation; IQR, interquartile range. a1 missing value for 90‐day mRS.
Figure 2.
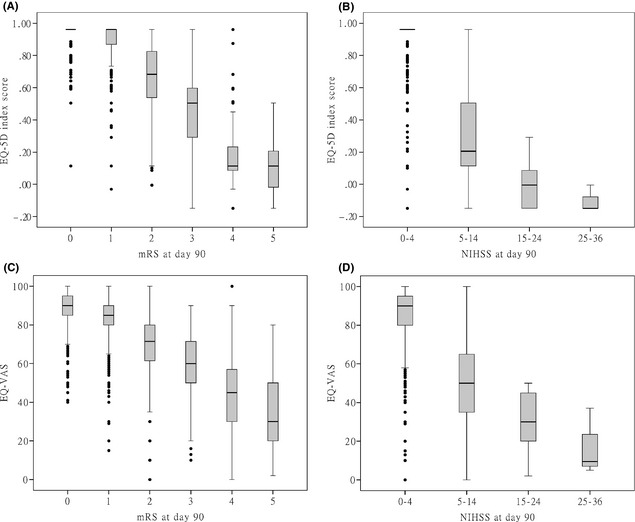
Box plot of EQ‐5D index score and EQ‐VAS by mRS and NIHSS levels at day 90 after a transient ischemic attack or minor stroke. EQ‐5D, European quality of life scale; VAS, visual analog scale; mRS, modified Rankin Scale; NIHSS, National Institutes of Health stroke scale. (A): EQ‐5D index score by mRS levels at day 90. (B): EQ‐5D index score by NIHSS levels at day 90. (C): EQ‐VAS by mRS levels at day 90. (D): EQ‐VAS by NIHSS levels at day 90.
Poor Quality of Life
Of 5104 patients, 294 (5.8%) had an EQ‐5D index score of ≤0.5 (poor HRQOL). Among patients with recurrent strokes, 279 (55.1%) had poor HRQOL. Only 15 (0.3%) had poor HRQOL in those without recurrent strokes.
The baseline demographic and medical characteristics by HRQOL category are shown in Table 2. Older age, history of hypertension and diabetes, and NIHSS at baseline were independent risk factors predicting poor HRQOL at day 90 after a TIA or minor stroke. In‐hospital treatments of risk factors (antihypertensive, hypoglycemic, and lipid‐lowering treatment) prevented poor HRQOL at day 90 (Table 3). The results were similar in sensitivity analyses using EQ‐VAS as the measure of HRQOL.
Table 2.
Baseline demographics, risk factors, and clinical measures by HRQOL category
| All (N = 5104) | Poor HRQOLa (N = 294) | Good HRQOL (N = 4810) | P | |
|---|---|---|---|---|
| Age (year), mean (SD) | 62.6 ± 10.7 | 65.2 ± 11.0 | 62.4 ± 10.7 | <0.001 |
| Female sex, n (%) | 1728 (33.9) | 117 (39.8) | 1611 (33.5) | 0.03 |
| Han ethnic, n (%) | 5034 (98.6) | 290 (98.6) | 4744 (98.6) | 1.00 |
| Body mass index, mean (SD) | 24.7 ± 3.0 | 24.8 ± 3.1 | 24.7 ± 3.0 | 0.18 |
| Vascular risk factors, n (%) | ||||
| Ischemic stroke | 1018 (20.0) | 56 (19.0) | 962 (20.0) | 0.69 |
| TIA | 172 (3.4) | 10 (3.4) | 162 (3.4) | 0.98 |
| Myocardial infarction | 92 (1.8) | 6 (2.0) | 86 (1.8) | 0.75 |
| Congestive heart failure | 77 (1.5) | 7 (2.4) | 70 (1.5) | 0.31 |
| Known atrial fibrillation or flutter | 173 (3.4) | 10 (3.4) | 163 (3.4) | 0.99 |
| Hypertension | 3358 (65.8) | 219 (74.5) | 3139 (65.3) | 0.001 |
| Diabetes mellitus | 1083 (21.2) | 82 (27.9) | 1001 (20.8) | 0.004 |
| Dyslipidemia | 560 (11.0) | 34 (11.6) | 526 (10.9) | 0.74 |
| Current or previous smoking | 2191 (42.9) | 106 (36.1) | 2085 (43.3) | 0.01 |
| Alcohol consumption | 1582 (31.0) | 85 (28.9) | 1497 (31.1) | 0.43 |
| Index event, n (%) | ||||
| Minor stroke | 3677 (72.0) | 249 (84.7) | 3428 (71.3) | <0.001 |
| TIA | 1427 (28.0) | 45 (15.3) | 1382 (28.7) | |
| Premorbid mRS, n (%) | ||||
| 0 | 4212 (82.5) | 249 (84.7) | 3963 (82.4) | 0.30 |
| 1 | 757 (14.8) | 39 (13.3) | 718 (14.9) | |
| 2 | 135 (2.6) | 6 (2.0) | 129 (2.7) | |
| NIHSS at baseline, median (IQR) | 2 (0–2) | 2 (1–3) | 1 (0–2) | <0.001 |
| In‐hospital treatment, n (%) | ||||
| Antihypertensive | 1450 (28.4) | 17 (5.8) | 1433 (29.8) | <0.001 |
| Hypoglycemic | 520 (10.2) | 6 (2.0) | 514 (10.7) | <0.001 |
| Lipid‐lowering | 1824 (35.7) | 22 (7.5) | 1802 (37.5) | <0.001 |
| Dual antiplatelet | 2550 (50.0) | 129 (43.9) | 2421 (50.3) | 0.03 |
HRQOL, Health‐related quality of life; SD, standard deviation; IQR, interquartile range; mRS, modified Rankin score; NIHSS, National Institutes of Health stroke scale; TIA, transient ischemic attack. aPoor HRQOL defined as EQ‐5D index score of 0.5 or less using the Chinese preference weights. Comparison by chi‐square test for categorical and t‐test or Mann–Whitney U‐test for continuous variables.
Table 3.
Independent risk factors for poor quality of life at day 90 after a transient ischemic attack or minor stroke
| EQ‐5D indexa | EQ‐VASb | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Model 1 | ||||
| Age, per 10 years | 1.26 (1.12–1.41) | <0.001 | 1.19 (1.02–1.38) | 0.03 |
| History of hypertension | 1.74 (1.32–2.31) | <0.001 | 1.60 (1.10–2.33) | 0.01 |
| History of diabetes | 1.65 (1.24–2.20) | <0.001 | 1.93 (1.33–2.78) | <0.001 |
| NIHSS at baseline | 1.65 (1.48–1.86) | <0.001 | 1.58 (1.36–1.84) | <0.001 |
| Antihypertensive treatment | 0.20 (0.12–0.33) | <0.001 | 0.31 (0.17–0.57) | <0.001 |
| Hypoglycemic treatment | 0.27 (0.11–0.63) | 0.002 | 0.37 (0.14–0.96) | 0.04 |
| Lipid‐lowering treatment | 0.19 (0.12–0.29) | <0.001 | 0.19 (0.11–0.35) | <0.001 |
| Dual antiplatelet treatment | 0.78 (0.61–1.004) | 0.054 | 0.84 (0.60–1.16) | 0.28 |
| c‐statistic | 0.81 | 0.79 | ||
| Model 2 | ||||
| Stroke recurrence | 402.11 (232.96–694.07) | <0.001 | 109.72 (63.55–189.45) | <0.001 |
| NIHSS at baseline | 1.40 (1.16–1.70) | <0.001 | 1.38 (1.17–1.64) | <0.001 |
| Age, per 10 years | 1.26 (1.07–1.49) | 0.006 | – | – |
| Index event is minor stroke | 1.98 (1.20–3.29) | 0.008 | – | – |
| History of diabetes | – | – | 1.62 (1.08–2.44) | 0.02 |
| Dual antiplatelet treatment | 1.16 (0.81–1.65) | 0.42 | 1.11 (0.76–1.61) | 0.60 |
| c‐statistic | 0.97 | 0.94 | ||
EQ‐5D, European quality of life scale; VAS, visual analog scale; OR, odds ratio; CI, confidence intervals. aPoor HRQOL was defined as an EQ‐5D index score of 0.5 or less using the Chinese preference weights. bPoor HRQOL was defined as an EQ‐VAS score of 40 or less.
More patients with poor HRQOL had recurrent stroke during follow‐up than patients with good HRQOL (94.9% vs. 4.7%, P < 0.001). Older age, high body mass index, NIHSS at baseline, history of hypertension and diabetes, and failure to initiate in‐hospital treatments of risk factors (antihypertensive, hypoglycemic, lipid‐lowering, and dual antiplatelet treatment) were independent risk factors of stroke recurrence within 90 days after a TIA or minor stroke (Tables S1 and S2). Stroke recurrence, NIHSS at baseline, older age, and minor stroke as index event were independent risk factors of poor HRQOL if stroke recurrence during follow‐up was added into the analytic model predicting HRQOL (Table 3, model 2). The models explained a large amount of the variation in the utility score (c‐statistic 0.97; 0.94).
Discussion
Based on the results of the CHANCE trial, TIA or minor ischemic stroke survivors had a mean utility score of 0.88 (mean EQ‐VAS, 84) at day 90 after symptom onset, and 5.8% of these patients had a poor HRQOL (i.e., EQ‐5D index score of 0.5 or less). The 90‐day HRQOL in patients after a TIA or minor stroke was better than those with ischemic stroke 4, intracerebral hemorrhage 1, 4, 26, or subarachnoid hemorrhage 6. However, recurrence of stroke after TIA or minor stroke may be associated with poor HRQOL.
As expected, age, history of hypertension and diabetes, and NIHSS at baseline were independent predictors of poor HRQOL at day 90 for patients with a TIA or minor stroke, which was similar to the findings in the literature 1, 4, 5, 6, 7, 8, 9, 10, 11. Previous studies have reported that older age 1, 5, 6, female sex 5, baseline severity 1, 4, 5, 6, 7, diabetes 7, hypertension 1, 8, disability 4, 9, aphasia 10, depression 4, 9, 11, cognitive impairment 8, and low socioeconomic status 5 were independent predictors of poor long‐term HRQOL after stroke. Our data indicated that poor HRQOL after a TIA or minor stroke was largely attributable to recurrence of stroke within 90 days after the onset of the index events as 94.9% of those with poor HRQOL had another stroke. Hypertension and diabetes lost predictive power for poor HRQOL after recurrence of stroke was added into the model. These risk factors may influence HRQOL through affecting the recurrence of stroke 12.
About 9.2% of patients had severe strokes at day 90; therefore, their HRQOL questionnaires were completed by proxies. EuroQol that completed by proxy was validated in our study, with an acceptable agreement (ICC = 0.72) similar to previous studies 27, 28. However, another previous study showed that HRQOL could be reported differently by proxy from patients. The explanation was that patient could be depressed and proxy was overburdened by the need to provide care 29. Proxy respondents reported more problems of self‐care and mobility but less problems of pain or discomfort 4, 27. In our study, proxy respondents reported significantly worse problems in all domains of EQ‐5D than self‐respondents. The explanation was that in our study, proxies were only used when patients had disability or inability to complete the assessment independently.
Our study shows that HRQOL after stroke/TIA highly correlated with the neurological and functional outcome measures (NIHSS, mRS). Modified Rankin Scale and Barthel Index emphasize on the functional outcome but are not generated by patient's self‐perceptions 7. There is an overall correlation between disability and HRQOL, and these measurements translated into HRQOL utility values in patients with stroke/TIA 1, 2, 30. It appeared that mRS correlated well with stroke survivors’ perceptions and captured more information on HRQOL than either NIHSS or Barthel Index 2. Given the frequent use of mRS as an outcome measurement tool in managing patients with stroke/TIA 20, 31, it could be used as proxy for HRQOL when it is unavailable. In fact, many cost‐effectiveness analyses on stroke management employ quality‐adjusted life years (QALYs) as the outcome measurement tool, with utility weights predicted by mRS 32, 33. The HRQOL scores generated by mRS category in our study may be used as utility weights in future cost‐effectiveness analyses for stroke/TIA care in China 33.
A major strength of our study was the prospective assessment of HRQOL in a large TIA or minor stroke population with rather thorough follow‐ups. We selected easily identifiable risk factors at baseline and in the acute phase of treatment to illustrate the potential for clinicians to affect the HRQOL of patients in the acute phase after the onset of a TIA or minor stroke in clinical practice. Our data showed that controlling risk factors (hypertension, diabetes, and dyslipidemia) and preventing neurological worsening can improve the HRQOL in these patients.
Our study has several limitations. First, our findings may not be externally generalizable to non‐Chinese population. This analysis was based on data from the CHANCE trial, which was performed in Chinese patients. HRQOL may be affected by patient's ethnicity and culture. Second, the data came from a randomized, controlled trial that selected patients only with high‐risk TIA (ABCD2 ≥ 4, able to start drug within 24 h after onset, and mRS < 3). Our finding is limited to this population of patients. Third, our study used the available data from the CHANCE trial and only estimated HRQOL at 3 months after the onset of a TIA or minor stroke. Previous population‐based studies showed that HRQOL declined annually up to 5 years after stroke 12, 34. The results of our study require more verification. Finally, other significant factors that may impact HRQOL of patients, such as socioeconomic status 5, possible cognitive impairment 8, or depression 1, 4, 29, 35, were not assessed in this trial.
In summary, recurrent stroke was associated with poor HRQOL in patients with high‐risk TIA or minor strokes. Aggressive risk factor control and prevention of recurrence of stroke or neurological worsening may be potential strategies to improve HRQOL in patients after TIA or minor stroke.
Sources of Funding
The CHANCE study is supported by Grants (2008ZX09312‐008, 2011BAI08B02, 2012ZX09303, 200902004 and 2013BAI09B03) from the Ministry of Science and Technology of the People's Republic of China and Grant (BIBD‐PXM2013_014226_07_000084) from Beijing Institute for Brain Disorders.
Conflict of Interest
Dr. Johnston is the principal investigator of the POINT trial, a NIH‐sponsored trial with clopidogrel and placebo donated by Sanofi.
Supporting information
Table S1. Baseline Demographics and Risk Factors of Stroke Recurrence at 90 days.
Table S2. Independent Predictors of Stroke Recurrence at 90 days after a Transient Ischemic Attack or Minor Stroke.
Appendix S1. The CHANCE investigators.
Acknowledgments
We thank all participating hospitals, relevant clinicians and patients, and imaging and laboratory technicians.
CHANCE investigators are given in the Appendix S1.
The first two authors contributed equally to this work.
References
- 1. Christensen MC, Mayer S, Ferran JM. Quality of life after intracerebral hemorrhage: results of the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke 2009;40:1677–1682. [DOI] [PubMed] [Google Scholar]
- 2. Ali M, Fulton R, Quinn T, Brady M; VISTA Collaboration . How well do standard stroke outcome measures reflect quality of life? A retrospective analysis of clinical trial data. Stroke 2013;44:3161–3165. [DOI] [PubMed] [Google Scholar]
- 3. Alli O, Doshi S, Kar S, et al. Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;61:1790–1798. [DOI] [PubMed] [Google Scholar]
- 4. Sprigg N, Selby J, Fox L, Berge E, Whynes D, Bath PM. Very low quality of life after acute stroke: data from the Efficacy of Nitric Oxide in Stroke trial. Stroke 2013;44:3458–3462. [DOI] [PubMed] [Google Scholar]
- 5. Sturm JW, Donnan GA, Dewey HM, et al. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2004;35:2340–2345. [DOI] [PubMed] [Google Scholar]
- 6. Ronne‐Engström E, Enblad P, Lundström E. Outcome after spontaneous subarachnoid hemorrhage measured with the EQ‐5D. Stroke 2011;42:3284–3286. [DOI] [PubMed] [Google Scholar]
- 7. Fischer U, Anca D, Arnold M, et al. Quality of life in stroke survivors after local intra‐arterial thrombolysis. Cerebrovasc Dis 2008;25:438–444. [DOI] [PubMed] [Google Scholar]
- 8. Patel MD, McKevitt C, Lawrence E, Rudd AG, Wolfe CD. Clinical determinants of long‐term quality of life after stroke. Age Ageing 2007;36:316–322. [DOI] [PubMed] [Google Scholar]
- 9. Ahlsiö B, Britton M, Murray V, Theorell T. Disablement and quality of life after stroke. Stroke 1984;15:886–890. [DOI] [PubMed] [Google Scholar]
- 10. Kwa VI, Limburg M, de Haan RJ. The role of cognitive impairment in the quality of life after ischaemic stroke. J Neurol 1996;243:599–604. [DOI] [PubMed] [Google Scholar]
- 11. Naess H, Lunde L, Brogger J. The effects of fatigue, pain, and depression on quality of life in ischemic stroke patients: The Bergen Stroke Study. Vasc Health Risk Manag 2012;8:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luengo‐Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Quality of life after TIA and stroke: ten‐year results of the Oxford Vascular Study. Neurology 2013;81:1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnston SC, Gress DR, Browner WS, Sidney S. Short‐term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901–2906. [DOI] [PubMed] [Google Scholar]
- 14. Coull AJ, Lovett JK, Rothwell PM; Oxford Vascular Study . Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population‐based study. Neurology 2004;62:2015–2020. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Johnston SC; CHANCE Investigators . Rationale and design of a randomized, double‐blind trial comparing the effects of a 3‐month clopidogrel‐aspirin regimen versus aspirin alone for the treatment of high‐risk patients with acute nondisabling cerebrovascular event. Am Heart J 2010;160:380–386. [DOI] [PubMed] [Google Scholar]
- 18. Pan YS, Jing J, Wang YL, et al. Use of statin during hospitalization improves the outcome after intracerebral hemorrhage. CNS Neurosci Ther 2014;20:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li SY, Zhao XQ, Wang CX, et al. One‐year clinical prediction in Chinese ischemic stroke patients using the CHADS2 and CHA2DS2‐VASc scores: the China National Stroke Registry. CNS Neurosci Ther 2012;18:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538–1541. [DOI] [PubMed] [Google Scholar]
- 21. Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the EuroQol a valid measure of health‐related quality of life after stroke? Stroke 1997;28:1876–1882. [DOI] [PubMed] [Google Scholar]
- 22. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–1108. [DOI] [PubMed] [Google Scholar]
- 23. Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ‐5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–220. [DOI] [PubMed] [Google Scholar]
- 24. Liu GG, Wu H, Li M, Gao C, Luo N. Chinese time trade‐off values for EQ‐5D health states. Value Health 2014;17:597–604. [DOI] [PubMed] [Google Scholar]
- 25. Lunde L. Can EQ‐5D and 15D be used interchangeably in economic evaluations? Assessing quality of life in post‐stroke patients. Eur J Health Econ 2013;14:539–550. [DOI] [PubMed] [Google Scholar]
- 26. Diringer MN, Ferran JM, Broderick J, et al. Impact of recombinant activated factor VII on health‐related quality of life after intracerebral hemorrhage. Cerebrovasc Dis 2007;24:219–225. [DOI] [PubMed] [Google Scholar]
- 27. Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Are proxy assessments of health status after stroke with the EuroQol questionnaire feasible, accurate, and unbiased? Stroke 1997;28:1883–1887. [DOI] [PubMed] [Google Scholar]
- 28. Pickard AS, Johnson JA, Feeny DH, Shuaib A, Carriere KC, Nasser AM. Agreement between patient and proxy assessments of health‐related quality of life after stroke using the EQ‐5D and Health Utilities Index. Stroke 2004;35:607–612. [DOI] [PubMed] [Google Scholar]
- 29. Williams LS, Bakas T, Brizendine E, et al. How valid are family proxy assessments of stroke patients’ health‐related quality of life? Stroke 2006;37:2081–2085. [DOI] [PubMed] [Google Scholar]
- 30. Rivero‐Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo‐Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ‐5D) health outcome. Med Decis Making 2010;30:341–354. [DOI] [PubMed] [Google Scholar]
- 31. Duncan PW, Jorgensen HS, Wade DT. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke 2000;31:1429–1438. [DOI] [PubMed] [Google Scholar]
- 32. Tung CE, Win SS, Lansberg MG. Cost‐effectiveness of tissue‐type plasminogen activator in the 3‐ to 4.5‐hour time window for acute ischemic stroke. Stroke 2011;42:2257–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan Y, Wang A, Liu G, et al. Cost‐effectiveness of clopidogrel‐aspirin versus aspirin alone for acute transient ischemic attack and minor stroke. J Am Heart Assoc 2014;3:e000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhamoon MS, Moon YP, Paik MC, et al. Quality of life declines after first ischemic stroke. The Northern Manhattan Study. Neurology 2010;75:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vilhauer JS, Young S, Kealoha C, et al. Treating major depression by creating positive expectations for the future: a pilot study for the effectiveness of future‐directed therapy (FDT) on symptom severity and quality of life. CNS Neurosci Ther 2012;18:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Demographics and Risk Factors of Stroke Recurrence at 90 days.
Table S2. Independent Predictors of Stroke Recurrence at 90 days after a Transient Ischemic Attack or Minor Stroke.
Appendix S1. The CHANCE investigators.


