Summary
Aims
Serotonin (5‐HT) neurons mediate the ectopic release of dopamine (DA) induced by l‐DOPA in the Parkinsonian brain. We hypothesized that the participation of noradrenalin transporters (NET) in the clearance of DA may account for the lower effect of l‐DOPA in extrastriatal regions compared with the striatum.
Methods
Using a multisite intracerebral microdialysis approach, we tested the influence of the pharmacological blockade of NET and/or the destruction of noradrenalin (NE) fibers on DA and 5‐HT release in the striatum, hippocampus (HIPP), substantia nigra pars reticulata (SNr) and prefrontal cortex (PFC) of 6‐hydroxydopamine‐lesioned rats.
Results
l‐DOPA (12 mg/kg, i.p.) increased DA extracellular levels to a lesser extent in the SNr, PFC and HIPP compared with the striatum. The NET blockers desipramine (10 mg/kg, i.p.) and reboxetine (3 mg/kg, i.p.) potentiated l‐DOPA effect in the PFC, SNr and HIPP but not in the striatum. The NE neurotoxin N‐(2‐chloroethyl)‐N‐ethyl‐2‐bromobenzylamine (50 mg/kg, i.p. 1 week before dialysis experiment) potentiated l‐DOPA effect in the SNr and HIPP. 5‐HT extracellular levels were enhanced only when l‐DOPA was combined to NET blockers.
Conclusion
Noradrenalin neurons are indirectly involved in the mechanism of action of l‐DOPA in part through the heterologous reuptake of DA in extrastriatal regions.
Keywords: Dopamine, Intracerebral microdialysis, l‐DOPA, Noradrenergic terminals, Serotonin
Introduction
l‐DOPA is the most effective medicinal therapy of Parkinson's disease (PD) 1. Its efficacy has been associated with an increase in dopamine (DA) transmission that counteracts the loss of DAergic cells in the substantia nigra pars compacta (SNc), the hallmark of the disease 2. However, a better knowledge of the mechanism of action of l‐DOPA is still necessary to develop new therapeutic strategies against the motor and nonmotor side effects 3.
Serotonin (5‐HT) neurons have been identified as the main actor of the DA effects of l‐DOPA 4, 5, 6. Indeed, exogenous l‐DOPA enters 5‐HT neurons where it is decarboxylated into DA. The newly synthesized DA is then released as a false neurotransmitter by 5‐HT terminals in numerous brain regions due to the widespread 5‐HT innervation 6, 7, 8. In fully DA‐depleted rats, the magnitude of the increase in DA release induced by l‐DOPA is slightly higher in the striatum compared with other brain regions, not fully matching the density of 5‐HT terminals 6. Thus, the regulation of DA extracellular levels induced by l‐DOPA in each brain region could involve specific heterologous mechanisms and notably noradrenalin (NE)‐ containing neurons 9, 10.
Noradrenalin neurons did not appear to play a role in the increase in DA release induced by l‐DOPA 6, 11. Nevertheless, they are known to participate in the clearance of DA extracellular levels via the NE transporter (NET). In line with the stronger NE innervation of extrastriatal regions 12, 13, NET blockers have been shown to potentiate DA extracellular levels in the cortex but not in the striatum 14, 15. Therefore, the clearance of DA levels induced by l‐DOPA should be higher in extrastriatal regions compared with the striatum. An increase in l‐DOPA‐induced DA release has been reported in the striatum after the administration of desipramine (DMI), a nonselective NET inhibitor 16. It is possible that the region‐dependent effects of l‐DOPA on DA extracellular levels could also be related to an action of NE itself at 5‐HT neurons 17, 18.
In the present study, we used a multisite intracerebral microdialysis approach 19 to investigate the influence of NET and NE fibers on the ectopic DA release induced by l‐DOPA (3 and/or 12 mg/kg, i.p.) in the 6‐hydroxydopamine (6‐OHDA)‐lesioned rat model of PD. DA and 5‐HT extracellular levels were monitored simultaneously in the substantia nigra pars reticulata (SNr), striatum, hippocampus (HIPP) and the prefrontal cortex (PFC). The role of NET was studied with DMI and the selective NET inhibitor reboxetine 20, 21. The neurotoxin N‐(2‐chloroethyl)‐N‐ethyl‐2‐bromobenzylamine (DSP‐4) was used to investigate the involvement of NE fibers 22.
Materials and methods
Animals
Male Sprague–Dawley rats (Charles River, France) weighing 250–300 g were used. Animal room temperature was maintained at 21 ± 2°C and humidity at 60% with a 12‐light/dark cycle (obscurity at 8 pm) and ad libitum food and water. Procedures involving animals and their care were conformed to European Economic Community (86‐6091 EEC), the French National Committee (décret 87/848) and approved by the Ethical Committee of Centre National de la Recherche Scientifique, Région Aquitaine‐Limousin. All efforts were made to minimize animal suffering and reduce the number of animals used.
6‐Hydroxydopamine Injections in the Medial Forebrain Bundle
Rats were anaesthetized with chloral hydrate (400 mg/kg; intraperitoneal, i.p.) and placed on a stereotaxic apparatus. The intracerebral injections of 6‐OHDA were preceded 30–45 min before by i.p. pretreatment with DMI (25 mg/kg, calculated as the salt) to protect NE neurons. The 6‐OHDA solution, containing 6‐OHDA (3 μg/μL) and ascorbic acid (1 mM) dissolved in saline, was injected through a glass pipette placed in the left medial forebrain bundle by Picospritzer® III (Intracell Ltd, Royston, UK). The stereotaxic coordinates (in mm with respect to bregma) were the following: anteroposterior (AP) = −3.7; lateral (L) = +1.6 23. Injection sites (five times 0.5 μL/1 min) were separated by 250‐μm intervals along the dorsoventral extent. The injections were done 7–8 mm below the brain surface.
Implantation of Microdialysis Probes
As previously reported 6, rats were anaesthetized with isoflurane (3%) in a plastic chamber. Once anaesthetized, they were fixed on a stereotaxic apparatus. A nose mask adapted to the stereotaxic frame allowed continuous distribution of the gas anesthesia during probe implantation and microdialysis experiment (isoflurane, 1.5%). A feedback‐controlled heating pad was used to maintain body temperature at +37°C. The tips of the microdialysis probes were simultaneously and ipsilaterally implanted at the following coordinates (in mm from interaural point): HIPP: AP = 3.2; L = 5; V = 2.4; PFC: AP = 12.6; L = 0.4; V = 4; SNr: AP = 3.7; L = 2.2; V = 1.2; striatum: AP = 9.7; L = 3; V = 2.4 23.
Microdialysis
The dialysis experiments were performed 3 or 4 weeks after inducing the 6‐OHDA lesion. Perfusion of the artificial cerebrospinal fluid (aCSF) was performed as described previously 6. Briefly, the aCSF contained (in mM): 154.1 Cl−, 147 Na+, 2.7 K+, 1 Mg2+ and 1.2 Ca2+. The aCSF had a pH adjusted to 7.4 with sodium phosphate buffer (2 mM) and delivered at a constant flow rate (0.5 μL/min) with a microperfusion pump (CMA 100; Phymep, Paris, France) in the microdialysis probes (4 mm long in the PFC, HIPP and striatum and 2 mm long in the SNr; CMA/11, 240 μm outer diameter, Cuprophan; Phymep). Dialysates were collected on ice every 20 min after the implantation of the dialysis probes, a timeframe corresponding to a 2‐h stabilization period 24.
Tissue Processing for Histological Verification and Postmortem Neurochemical Analysis
At the end of each experiment, rats were removed from the stereotaxic apparatus and maintained under anesthesia in the box (1.5% isoflurane) for 2 h to limit the effect of l‐DOPA and other pharmacological treatments on postmortem indexes. Thereafter, they were sacrificed, and brains were removed and immediately frozen in isopentane (−40°C) and stored at −80°C until tissue processing. Coronal 40‐μm‐thick sections were first performed for histological verification in a cryostat at −20°C, and then, bilateral pieces of the striatum and motor cortex were dissected with a blade and placed back at −80°C pending the postmortem neurochemical analysis. The correct placement of the probes in each implanted area was verified by light microscopy examination of cresyl violet‐stained sections. Only data obtained from rats with correct probe implantation in each of the examined brain areas were included in the results (9% of animals were excluded).
Chromatographic Analyses
Reverse‐phase high performance liquid chromatography coupled with electrochemical detection (HPLC‐ECD) was used to analyze DA and 5‐HT content dialysate samples (10 μL) immediately after their collection 6. A HPLC pump (pump 116; Beckman, Paris, France) delivered the mobile phase [containing: 15% HPLC grade methanol, NaH2PO4 (70 mM), EDTA (0.1 mM), octylsulfonic acid (0.1 mM), pH 4.5 obtained with orthophosphoric acid] at 0.25 mL/min flow rate through an Equisyl‐BDS column (C18; 2 × 250 mm; particle size 5 μm; CIL‐Cluzeau, Sainte‐Foy La Grande, France). An amperometric cell Ag/AgCl (VT‐03) coupled to a programmable detector (Decade II Antec; AlphaMos, Toulouse, France) allowed detection of 5‐HT and DA. The potential of the electrode was set at +0.5 V. Output signals were recorded on a computer (System GOLD; Beckman). The sensitivity was 0.08 pg/10 μL for DA and 0.1 pg/10 μL 5‐HT, with a signal/noise ratio of 3:1.
As previously reported 6, 25, HPLC‐ECD was used to assess the degree and selectivity of the 6‐OHDA and DSP‐4 lesions by measuring tissue concentration of monoamines in the motor cortex and the striatum. The homogenization of tissues was performed in 200 μL of 0.1 N HClO4 using sonication. Homogenates were centrifuged (15,000 g; 30 min; 4°C), and supernatants (50 μL aliquots) were injected into the chromatographic system. The mobile phase containing 7% methanol, NaH2PO4 (60 mM), EDTA (0.1 mM), octane sulfonic acid (2 mM) had a pH 3.9 adjusted with orthophosphoric acid and was filtered through a 0.22‐mm Millipore filter before being pumped at a 1.2 mL/min flow rate (pump 116; Beckman). Aliquots were injected using a refrigerated (4°C) autosampler (SIL 20AC; Shimadzu, Paris, France). The mobile phase entered the HPLC column (Chromasyl C8, 150 × 4.6 mm, 5 μm; CIL‐Cluzeau) preceded by a Brownlee‐Newgard precolumn (RP‐8, 15 × 3.2 mm, 7 μm; CIL‐Cluzeau). Oxidation and reduction in compounds was obtained using a dual‐electrode analytic cell (model 5011) connected to a coulometric detector (CoulochemII; ESA, Paris, France). The optimal potentials applied for the reduction and the oxidation were −270 and +350 mV, respectively.
Pharmacological Treatments
Dialysis experiments were performed three/four weeks after inducing the 6‐OHDA lesion. On the day of the experiment, pharmacological treatments started at least 3 h after the implantation of the dialysis probes, a timeframe corresponding to a 2‐h stabilization period 24 plus a 1‐h assessment period of 5‐HT baseline 11, 26. An injection of benserazide (15 mg/kg, i.p.) preceded (20 min before) each i.p. injection of l‐DOPA to limit the peripheral decarboxylation of the latter. Rats that had no detectable DA levels in striatal dialysates, revealing a complete lesion of DA neurons, were used in our study.
The NET inhibitors DMI (10 mg/kg, i.p.) or reboxetine (3 mg/kg subcutaneously, s.c.) were administered 20 min before the administration of l‐DOPA (12 mg/kg, i.p.). Drugs were dissolved (as the free base) in sterile 0.9% NaCl in a volume of 2 mL/kg. DSP‐4 (50 mg/kg, i.p.) was administered once, 1 week before the microdialysis experiment. In DSP‐4‐treated rats, l‐DOPA was administered at 3 mg/kg followed 2 h later by 12 mg/kg, and its effect was monitored for an additional 3‐h period.
Drugs
Benserazide hydrochloride, desipramine hydrochloride, l‐DOPA methylester hydrochloride, N‐(2‐chloroethyl)‐N‐ethyl‐2‐bromobenzylamine hydrochloride, reboxetine hydrochloride, 6‐hydroxydopamine hydrobromide were purchased from Sigma (Illkirch, France). The other chemicals and reagents used in the study were purchased from VWR, Strasbourg, France and Sigma.
Data and Statistical Analysis
The extracellular levels of DA were expressed in pg/10 μL and corresponded in each group to the mean ± SEM values. The concentration of extracellular 5‐HT in each sample was expressed in pg/10 μL or as the percentage of the average baseline level calculated from the three fractions preceding any treatment 11, 26. 5‐HT concentration corresponded in each group to the mean ± SEM values. Tissue concentration of monoamines was expressed in ng/mg of tissue and corresponded to the mean ± SEM values in each group.
The statistical significance of the effect of 6‐OHDA and DSP‐4 lesions on basal values of tissue and extracellular concentrations of monoamines was evaluated using the Student's t‐test. Statistical analysis was performed on the overall effect of each group that corresponds to the average of DA or 5‐HT extracellular levels in dialysates collected after the administration of treatments over a period of 2 or 3 h monitoring. The statistical significance of the effects of treatment with DMI, reboxetine and DSP‐4 on l‐DOPA‐stimulated extracellular DA or those with DMI and DSP‐4 on l‐DOPA‐induced extracellular 5‐HT, was assessed using the Student's t‐test. The statistical significance of the effect of reboxetine on the extracellular levels of 5‐HT induced by l‐DOPA was evaluated by a two‐way ANOVA (pretreatment × treatment) followed in case of significance (P < 0.05) by a post hoc Fisher's protected least significance difference (PLSD) test to allow multiple comparisons between groups.
Results
Effect of NET Inhibitors on l‐DOPA‐induced Changes of DA and 5‐HT Extracellular Levels and Tissue DA, 5‐HT and NE Levels
As previously reported 6, 26, the 6‐OHDA lesion suppressed both extracellular and tissue levels of DA in the ipsilateral striatum without affecting 5‐HT and NE tissue levels in the striatum and/or the cortex (Table 1). l‐DOPA (12 mg/kg, i.p.) increased DA in the striatum (overall effect: 9.7 ± 1.4 pg/10 μL over 3 h monitoring), SNr (4.3 ± 0.7 pg/10 μL), PFC (3.8 ± 0.9 pg/10 μL) and HIPP (3.6 ± 0.8 pg/10 μL; Figure 1). Pretreatment with DMI (10 mg/kg, i.p.), without modifying the effect of l‐DOPA in the striatum, induced at least a twofold increase in l‐DOPA‐induced DA release in the SNr (P < 0.05, Student's t‐test), PFC (P < 0.01) and HIPP (P < 0.01). In the presence of DMI, l‐DOPA also enhanced 5‐HT levels in the SNr (P < 0.05; Student's t‐test), HIPP (P < 0.05), PFC (P < 0.05) but not in the striatum (Figure 1).
Table 1.
Tissue concentrations of DA, 5‐HT and NE in the striatum and the cortex of 6‐OHDA‐lesioned rats
| Tissue levels in 6‐OHDA rats (ng/mg of tissue) | ||
|---|---|---|
| Ipsilateral | Contralateral | |
| Striatum | ||
| DA | ND | 7147 ± 155 |
| 5‐HT | 293 ± 90 | 336 ± 32 |
| Cortex | ||
| NE | 236 ± 22 | 258 ± 19 |
| 5‐HT | 164 ± 16 | 195 ± 12 |
Each value, expressed in ng/mg of tissue, represents the mean ± SEM of 48 rats in unilaterally 6‐hydroxydopamine (6‐OHDA)‐lesioned rats. Brain tissues were collected 2 h after the end of microdialysis experiments that were performed 3–4 weeks after the unilateral injection of 6‐OHDA in the median forebrain bundle.
ND, not detectable.
Figure 1.
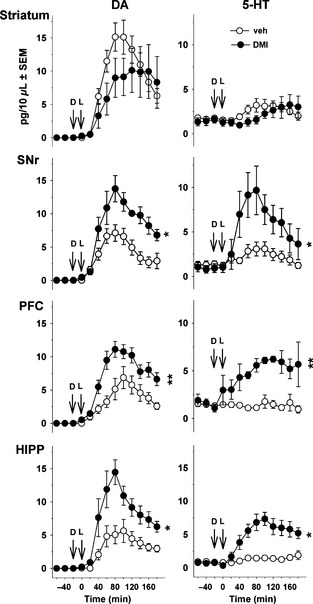
Effect of desipramine (DMI) on dopamine (DA) and serotonin (5‐HT) release induced by l‐DOPA in 6‐OHDA‐lesioned rats. Data points represent mean ± SEM (n = 8–9 rats/group) of the extracellular concentration (pg/10 μL dialysate) of DA (left panel) and 5‐HT (right panel) versus time, observed in striatum, substantia nigra pars reticulata (SNr), prefrontal cortex (PFC) and hippocampus (HIPP). Microdialysis experiments were performed 3–4 weeks after the unilateral injection of 6‐hydroxydopamine (6‐OHDA). l‐DOPA (L) was administered intraperitoneally (i.p.) at 12 mg/kg (time 0) and was preceded 20 min before by the i.p. administration of benserazide (15 mg/kg). Desipramine (D or DMI; 10 mg/kg, i.p.) or its vehicle (veh) was administered 20 min before l‐DOPA. Asterisks refer to the probability level of statistical significance of the overall effect of the DMI group versus the vehicle group: *P < 0.05, **P < 0.01 (Student's t‐test).
Figure 2 illustrates the effect of reboxetine on l‐DOPA‐stimulated DA release. Reboxetine (3 mg/kg, s.c.) potentiated l‐DOPA‐stimulated DA extracellular levels in the SNr (P < 0.05, Student's t‐test), PFC (P < 0.01) and HIPP (P < 0.05) but not in the striatum (NS). In this experiment, we have also addressed the effect of NET blockade per se on 5‐HT levels in 6‐OHDA rats that received reboxetine or its vehicle without l‐DOPA (Figure 3). Reboxetine did not alter 5‐HT extracellular levels by itself, but enhanced 5‐HT extracellular levels in the presence of l‐DOPA, as shown for DMI. Indeed, 5‐HT extracellular levels were altered by the combination of the drugs in the SNr [two‐way ANOVA, reboxetine × l‐DOPA, F(1,18) = 6.6; P < 0.05], PFC [F(1,18) = 5.3; P < 0.05] and HIPP [F(1,18) = 12.3; P < 0.01] but not in the striatum [F(1,18) = 1.62; NS]. Correspondingly, 5‐HT extracellular levels were enhanced in the PFC (P < 0.01 vs. the veh+l‐DOPA group, PLSD test), SNr (P < 0.001) and HIPP (P < 0.001; Figure 3).
Figure 2.
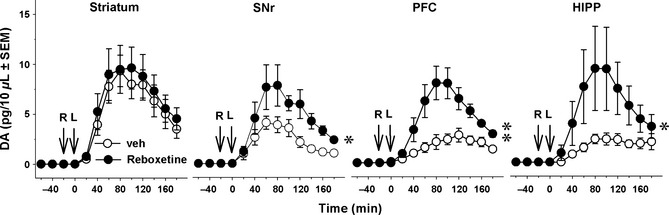
Effect of reboxetine on the extracellular levels of dopamine (DA) induced by l‐DOPA in 6‐OHDA‐lesioned rats. Data represent mean ± SEM (n = 5–6 rats/group) of extracellular concentration (pg/10 μL) of DA versus time, observed in striatum, substantia nigra pars reticulata (SNr), prefrontal cortex (PFC) and hippocampus (HIPP; panels from left to right, respectively). Microdialysis experiments were performed 3–4 weeks after the unilateral injection of 6‐hydroxydopamine (6‐OHDA). l‐DOPA (L) was administered intraperitoneally (i.p.) at 12 mg/kg (time 0) and was preceded 20 min before by the i.p. administration of benserazide (15 mg/kg). Reboxetine (R, 3 mg/kg, subcutaneously) or its vehicle (veh) was administered 20 min before l‐DOPA. Asterisks refer to the probability level of statistical significance of the overall effect in the reboxetine group versus the vehicle group: *P < 0.05, **P < 0.01 (Student's t‐test).
Figure 3.
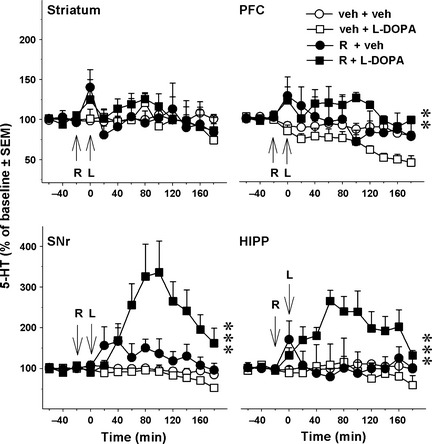
Effect of reboxetine on the extracellular levels of serotonin (5‐HT) induced by l‐DOPA in 6‐OHDA‐lesioned rats. Data represent mean ± SEM (n = 4–6 rats/group) of extracellular concentration of 5‐HT (in percentage of baseline) versus time, observed in striatum, substantia nigra pars reticulata (SNr), prefrontal cortex (PFC) and hippocampus (HIPP). Microdialysis experiments were performed 3–4 weeks after the unilateral injection of 6‐hydroxydopamine (6‐OHDA). l‐DOPA (L) or its vehicle (veh) was administered intraperitoneally (i.p.) at 12 mg/kg (time 0) and was preceded 20 min before by the administration of benserazide (15 mg/kg, i.p.). Reboxetine (R, 3 mg/kg, subcutaneously) or its vehicle (veh) was administered 20 min before l‐DOPA. Asterisks represent probability level of statistical significance of the R+l‐DOPA group versus the veh+l‐DOPA group (**P < 0.01, ***P < 0.001; Fisher's PLSD test after significant two‐way ANOVA).
In both experiments, tissue concentrations of DA in the striatum of 6‐OHDA‐lesioned rats were similar between saline‐ and NET blockers‐treated rats. 5‐HT and NE tissue levels in the cortex and/or the striatum were also similar between groups (data not shown).
Effect of DSP‐4 on l‐DOPA‐induced Changes of DA and 5‐HT Extracellular Levels
l‐DOPA enhanced DA release at 3 plus 12 mg/kg in the four brain regions (Figure 4). In DSP‐4‐treated rats, the effect of l‐DOPA on DA release was enhanced in the SNr and HIPP (P < 0.05; Student's t‐test), whereas the trend toward an increase observed in striatum and PFC did not reach significance.
Figure 4.
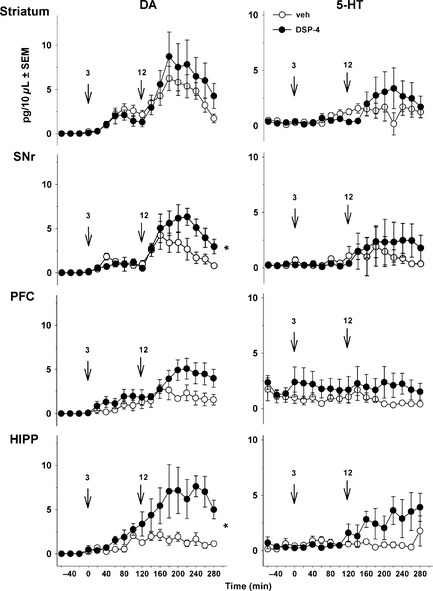
Effect of DSP‐4 on the extracellular levels of dopamine (DA) and 5‐HT induced by l‐DOPA in 6‐OHDA‐lesioned rats. Data represent mean + SEM (n = 5–6 rats/group) of extracellular concentration (pg/10 μL dialysate) of DA (left panel) and 5‐HT (right panel) versus time, observed in striatum, substantia nigra pars reticulata (SNr), prefrontal cortex (PFC) and hippocampus (HIPP). Microdialysis experiments were performed 3–4 weeks after the unilateral injection of 6‐OHDA. One week before the microdialysis experiments, 6‐OHDA‐lesioned rats received an intraperitoneal (i.p.) administration of 50 mg/kg DSP‐4 or its vehicle (veh). l‐DOPA was administered i.p. at 3 mg/kg (time 0, first arrow) and was preceded 20 min before by the administration of benserazide (15 mg/kg, i.p.). Two hours after the first l‐DOPA injection, rats received another i.p. injection of l‐DOPA at 12 mg/kg (time 120, second arrow). Asterisks refer to the probability level of statistical significance of the overall effect of l‐DOPA at 12 mg/kg in the DSP‐4 group versus the veh group: *P < 0.05 (Student's t‐test).
Basal 5‐HT extracellular levels did not differ between 6‐OHDA‐lesioned rats and 6‐OHDA/DSP‐4‐lesioned rats in the four brain regions (Figure 4; Student's t‐test, NS for all brain regions). l‐DOPA did not significantly enhance 5‐HT extracellular levels in any of the examined brain areas of DSP‐4‐treated rats, even in the HIPP where a trend toward an increase was observed.
DA tissue levels were suppressed in the 6‐OHDA‐lesioned side in both experimental groups (Table 2). Cortical tissue levels of NE were homogeneously reduced by DSP‐4 by almost 80% in both sides. 5‐HT tissue levels were not altered in the striatum and the cortex, whatever the neurotoxin used or the side of the brain considered.
Table 2.
Effect of DSP‐4 in 6‐OHDA‐lesioned rats on tissue concentrations of DA, 5‐HT and NE in the striatum and the cortex
| Vehicle group (n = 6) | DSP‐4 group (n = 5) | |||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| Striatum | ||||
| DA | ND | 5944 ± 313 | ND | 6769 ± 716 |
| 5‐HT | 349 ± 68 | 472 ± 91 | 302 ± 54 | 434 ± 78 |
| Cortex | ||||
| NE | 247 ± 26 | 257 ± 20 | 54 ± 15* | 39 ± 10** |
| 5‐HT | 133 ± 26 | 132 ± 20 | 126 ± 18 | 168 ± 24 |
Each value, expressed in ng/mg of tissue, represents the mean ± SEM of 5–6 rats/group in unilaterally 6‐hydroxydopamine (6‐OHDA)‐lesioned rats. Brain tissues were collected 2 h after the end of microdialysis experiments that were performed 3–4 weeks after the unilateral injection of 6‐OHDA in the median forebrain bundle. One week before the microdialysis experiments, 6‐OHDA‐lesioned rats received 50 mg/kg DSP‐4 or its vehicle.
*P < 0.05 and **P < 0.01 versus the corresponding side of the vehicle group (Student's t‐test).
ND, not detectable.
Discussion
The results show that NE fibers and/or the NET participate in the effects of l‐DOPA on both DA and 5‐HT releases in extrastriatal regions. NE mechanisms can be targeted to modify l‐DOPA‐stimulated DA release elsewhere than in the striatum. This could limit the deleterious consequences attributed to excessive striatal DA release induced by l‐DOPA 27.
One of the main findings is the potentiation of l‐DOPA‐stimulated DA release by the blockade of NET in extrastriatal regions only. In the presence of DMI or reboxetine, the magnitude of the effect of l‐DOPA‐stimulated DA release became similar in all brain regions. Although an increase in striatal DA release induced by 50 mg/kg l‐DOPA has been reported in the presence of 25 mg/kg DMI 16, this effect of DMI could reflect its nonselective binding at various other sites than the NET 28. Using 10 mg/kg DMI 29 and 3 mg/kg reboxetine, doses inducing a high and selective level of NET blockade 21, we did not report any modification of l‐DOPA‐stimulated DA release in the striatum. These doses were centrally active because they potentiated the action of l‐DOPA in other brain regions. Therefore, the region‐dependent changes reported here are likely to reflect pharmacodynamics, rather than pharmacokinetic interactions between the drugs.
We further confirmed a role for NE fibers in the above effects with the NE neurotoxin DSP‐4 30. In 6‐OHDA‐lesioned rats treated with DSP‐4, which decreased NE tissue concentrations by 80% as previously reported 22, the efficacy of l‐DOPA to enhance DA release was potentiated in the SNr and HIPP but not in the striatum. These effects were, however, less pronounced after DSP‐4 treatment compared with NET blockers and did not reach significance in the PFC. Moreover, we found that the NE lesion did not potentiate the effect elicited by 3 mg/kg l‐DOPA in any brain region suggesting a threshold effect related to the amount of extracellular DA concentrations. Therefore, the contribution of NE fibers in the neurochemical effects of l‐DOPA may depend on the dose of l‐DOPA.
These results are compatible with the fact that NE terminals regulate extracellular DA concentrations in areas densely innervated by NE neurons through the uptake of DA by the NET 14, 15, 31, 32. Indeed, the local infusion of NET blockers increased DA extracellular levels in the cortex, HIPP but not in the striatum 32, 33. The density of NE fibers, NET expression levels and NE tissue concentrations are higher in the SNr, PFC or HIPP compared with the striatum 13, 34, 35. Consequently, the NE system plays a powerful role in the clearance of l‐DOPA‐derived DA extracellular levels in extrastriatal brain regions through the NET as extrastriatal DA levels reach similar striatal DA levels upon NET blockade.
The fact that the effect of l‐DOPA is of lower magnitude in DSP‐4‐treated rats compared with DMI‐ or reboxetine‐treated rats suggests the existence of other mechanisms. Interestingly, we found that l‐DOPA enhanced 5‐HT extracellular levels only in the presence of NET blockers and in brain regions where DA levels were also enhanced (i.e., all brain regions but not the striatum). As NET blockers enhance NE but not 5‐HT release 21, 36, it is possible that NE favors the increase in both DA and 5‐HT coming from 5‐HT terminals. This effect of NE could not operate at the level of the dorsal raphe nucleus (DRN) as reboxetine alone or combined with l‐DOPA tends to inhibit 5‐HT firing rate 37. More likely, the increase in 5‐HT extracellular levels induced by l‐DOPA only when NET is impaired may occur at the level of 5‐HT terminals independently of DRN 5‐HT neuron activity. Notably, the increase in 5‐HT levels could reflect a competition at the level of 5‐HT transporters (SERT). Indeed, DA levels stimulated by l‐DOPA and NE levels raised by NET blockers could saturate SERT, which constitute one important mechanism able to clear up monoamines from the extracellular space. In support of this hypothesis, SERT has been previously shown to participate in the regulation of DA and 5‐HT extracellular levels induced by l‐DOPA 6, 38. Furthermore, SERT is also involved in the reuptake of NE in the frontal cortex and HIPP 39. This hypothesis deserves further investigation.
These results add further insight into the role of monoaminergic systems in the mechanism of action of l‐DOPA on DA extracellular levels. Exogenous l‐DOPA enters all monoaminergic cells 40 but the resulting effect on DA levels may differ regarding the monoaminergic system considered. Spared DA neurons in DA‐depleted conditions are presumably inhibited due to the stimulation of DA autoreceptors by extracellular DA levels 8, 41. The increase in DA extracellular levels depends on the integrity of 5‐HT neurons in the Parkinsonian brain 6. Indeed, 5‐HT neurons decarboxylate l‐DOPA into DA, which enters exocytosis vesicles via the vesicular transporter VMAT2. Although it is generally thought that the release of l‐DOPA‐derived DA occurs in an impulse‐dependent manner from 5‐HT neurons 4, neurochemical studies suggest that impulse‐independent mechanisms are also involved in the increase in extracellular DA levels [present study; 6, 42, 43]. Finally, the present data with DSP‐4 directly confirms that NA fibers are not involved in l‐DOPA‐stimulated DA release, in line with the fact that the newly synthesized DA from l‐DOPA may be further converted into NA inside NA terminals. Nevertheless, we showed that NA fibers play a major role in the clearance of extracellular DA in areas enriched with NA terminals. The mechanisms of clearance of l‐DOPA‐stimulated DA release deserve additional studies especially in extrastriatal regions.
These data are important in the context of l‐DOPA therapy in PD because NE neurons also degenerate in the disease 44 and the additional loss of NE is involved in the expression of motor and nonmotor deficits 9, 22. Together with the present findings, this means that the loss of NE fibers may already potentiate the effect of l‐DOPA on DA release in various brain areas including the SNr, known to convey the efficacy of l‐DOPA‐induced rotations 45. On the other hand, our data indicate that the ability of NET blockers to enhance 5‐HT extracellular levels in the presence of l‐DOPA may provide higher efficacy than selective SERT blockers for an antidepressant action 37. Altogether, the present study further stresses the importance of 5‐HT and NE terminals in the mechanism of action of l‐DOPA, a point that should not be underestimated when using strategies aimed at modulating the firing rate of 5‐HT neurons.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
The authors would like to thank the “Fondation de France,” “Centre National de la Recherche Scientifique” and COST action CM1103 European for having supported the study. The study was also supported by the exchange grants of the GDRI N198 (CNRS and INSERM, France and CNRST, Morocco) and NEUROMED.
References
- 1. Hauser RA. Levodopa: Past, present, and future. Eur Neurol 2009;62:1–8. [DOI] [PubMed] [Google Scholar]
- 2. Esposito E, Di Matteo V, Di Giovanni G. Death in the substantia nigra: A motor tragedy. Expert Rev Neurother 2007;7:677–697. [DOI] [PubMed] [Google Scholar]
- 3. Meissner WG, Frasier M, Gasser T, et al. Priorities in Parkinson's disease research. Nat Rev Drug Discov 2011;10:377–393. [DOI] [PubMed] [Google Scholar]
- 4. Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5‐HT terminals is the cause of L‐DOPA‐induced dyskinesia in parkinsonian rats. Brain 2007;130:1819–1833. [DOI] [PubMed] [Google Scholar]
- 5. Hollister AS, Breese GR, Mueller RA. Role of monoamine neural systems in L‐dihydroxyphenylalanine‐stimulated activity. J Pharmacol Exp Ther 1979;208:37–43. [PubMed] [Google Scholar]
- 6. Navailles S, Bioulac B, Gross C, De Deurwaerdere P. Serotonergic neurons mediate ectopic release of dopamine induced by L‐DOPA in a rat model of Parkinson's disease. Neurobiol Dis 2010;38:136–143. [DOI] [PubMed] [Google Scholar]
- 7. Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 1978;179:641–667. [DOI] [PubMed] [Google Scholar]
- 8. Navailles S, Carta M, Guthrie M, De Deurwaerdere P. L‐DOPA and serotonergic neurons: Functional implication and therapeutic perspectives in Parkinson's disease. Cent Nerv Syst Agents Med Chem 2011;11:305–320. [DOI] [PubMed] [Google Scholar]
- 9. Delaville C, Deurwaerdere PD, Benazzouz A. Noradrenaline and Parkinson's disease. Front Syst Neurosci 2011;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eskow Jaunarajs KL, Angoa‐Perez M, Kuhn DM, Bishop C. Potential mechanisms underlying anxiety and depression in Parkinson's disease: Consequences of l‐DOPA treatment. Neurosci Biobehav Rev 2011;35:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navailles S, Bioulac B, Gross C, De Deurwaerdere P. Chronic L‐DOPA therapy alters central serotonergic function and L‐DOPA‐induced dopamine release in a region‐dependent manner in a rat model of Parkinson's disease. Neurobiol Dis 2011;41:585–590. [DOI] [PubMed] [Google Scholar]
- 12. Aston‐Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: Restricted afferent control of a broad efferent network. Science 1986;234:734–737. [DOI] [PubMed] [Google Scholar]
- 13. Fitoussi A, Dellu‐Hagedorn F, De Deurwaerdere P. Monoamines tissue content analysis reveals restricted and site‐specific correlations in brain regions involved in cognition. Neuroscience 2013;255:233–245. [DOI] [PubMed] [Google Scholar]
- 14. Mazei MS, Pluto CP, Kirkbride B, Pehek EA. Effects of catecholamine uptake blockers in the caudate‐putamen and subregions of the medial prefrontal cortex of the rat. Brain Res 2002;936:58–67. [DOI] [PubMed] [Google Scholar]
- 15. Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci 1997;9:2077–2085. [DOI] [PubMed] [Google Scholar]
- 16. Arai A, Tomiyama M, Kannari K, et al. Reuptake of L‐DOPA‐derived extracellular DA in the striatum of a rodent model of Parkinson's disease via norepinephrine transporter. Synapse 2008;62:632–635. [DOI] [PubMed] [Google Scholar]
- 17. Miguelez C, Grandoso L, Ugedo L. Locus coeruleus and dorsal raphe neuron activity and response to acute antidepressant administration in a rat model of Parkinson's disease. Int J Neuropsychopharmacol 2011;14:187–200. [DOI] [PubMed] [Google Scholar]
- 18. O'Leary OF, Bechtholt AJ, Crowley JJ, Valentino RJ, Lucki I. The role of noradrenergic tone in the dorsal raphe nucleus of the mouse in the acute behavioral effects of antidepressant drugs. Eur Neuropsychopharmacol 2007;17:215–226. [DOI] [PubMed] [Google Scholar]
- 19. Navailles S, Lagiere M, Contini A, De Deurwaerdere P. Multisite intracerebral microdialysis to study the mechanism of L‐DOPA induced dopamine and serotonin release in the parkinsonian brain. ACS Chem Neurosci 2013;4:680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Umehara M, Ago Y, Fujita K, Hiramatsu N, Takuma K, Matsuda T. Effects of serotonin‐norepinephrine reuptake inhibitors on locomotion and prefrontal monoamine release in spontaneously hypertensive rats. Eur J Pharmacol 2013;702:250–257. [DOI] [PubMed] [Google Scholar]
- 21. Valentini V, Frau R, Di Chiara G. Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: Differences with mianserin and clozapine. J Neurochem 2004;88:917–927. [DOI] [PubMed] [Google Scholar]
- 22. Delaville C, Chetrit J, Abdallah K, et al. Emerging dysfunctions consequent to combined monoaminergic depletions in Parkinsonism. Neurobiol Dis 2012;45:763–773. [DOI] [PubMed] [Google Scholar]
- 23. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th edn New York: Academic Press, 1998. [Google Scholar]
- 24. Bonhomme N, De Deurwaerdere P, Le Moal M, Spampinato U. Evidence for 5‐HT4 receptor subtype involvement in the enhancement of striatal dopamine release induced by serotonin: A microdialysis study in the halothane‐anesthetized rat. Neuropharmacology 1995;34:269–279. [DOI] [PubMed] [Google Scholar]
- 25. De Deurwaerdere P, Bonhomme N, Le Moal M, Spampinato U. d‐fenfluramine increases striatal extracellular dopamine in vivo independently of serotonergic terminals or dopamine uptake sites. J Neurochem 1995;65:1100–1108. [DOI] [PubMed] [Google Scholar]
- 26. Navailles S, Benazzouz A, Bioulac B, Gross C, De Deurwaerdere P. High‐frequency stimulation of the subthalamic nucleus and L‐3,4‐dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson's disease. J Neurosci 2010;30:2356–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navailles S, De Deurwaerdere P. Contribution of serotonergic transmission to the motor and cognitive effects of high‐frequency stimulation of the subthalamic nucleus or levodopa in Parkinson's disease. Mol Neurobiol 2012;45:173–185. [DOI] [PubMed] [Google Scholar]
- 28. Wong EH, Sonders MS, Amara SG, et al. Reboxetine: A pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry 2000;47:818–829. [DOI] [PubMed] [Google Scholar]
- 29. Tanda G, Carboni E, Frau R, Di Chiara G. Increase of extracellular dopamine in the prefrontal cortex: A trait of drugs with antidepressant potential? Psychopharmacology 1994;115:285–288. [DOI] [PubMed] [Google Scholar]
- 30. Fritschy JM, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: Compensatory response to neurotoxin‐induced cell death in the adult rat brain. J Comp Neurol 1992;321:421–441. [DOI] [PubMed] [Google Scholar]
- 31. Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock‐out mouse lines. J Neurosci 2002;22:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 1998;71:274–280. [DOI] [PubMed] [Google Scholar]
- 33. Borgkvist A, Malmlof T, Feltmann K, Lindskog M, Schilstrom B. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int J Neuropsychopharmacol 2012;15:531–540. [DOI] [PubMed] [Google Scholar]
- 34. De Deurwaerdere P, Stinus L, Spampinato U. Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: Role of 5‐HT3 receptors. J Neurosci 1998;18:6528–6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine‐ and antidepressant‐sensitive l‐norepinephrine transporter. J Comp Neurol 2000;420:211–232. [PubMed] [Google Scholar]
- 36. Bel N, Artigas F. In vivo effects of the simultaneous blockade of serotonin and norepinephrine transporters on serotonergic function. Microdialysis studies. J Pharmacol Exp Ther 1996;278:1064–1072. [PubMed] [Google Scholar]
- 37. Miguelez C, Berrocoso E, Mico JA, Ugedo L. L‐DOPA modifies the antidepressant‐like effects of reboxetine and fluoxetine in rats. Neuropharmacology 2013;67:349–358. [DOI] [PubMed] [Google Scholar]
- 38. Yamato H, Kannari K, Shen H, Suda T, Matsunaga M. Fluoxetine reduces L‐DOPA‐derived extracellular DA in the 6‐OHDA‐lesioned rat striatum. NeuroReport 2001;12:1123–1126. [DOI] [PubMed] [Google Scholar]
- 39. Vizi ES, Zsilla G, Caron MG, Kiss JP. Uptake and release of norepinephrine by serotonergic terminals in norepinephrine transporter knock‐out mice: Implications for the action of selective serotonin reuptake inhibitors. J Neurosci 2004;24:7888–7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tison F, Mons N, Geffard M, Henry P. The metabolism of exogenous L‐dopa in the brain: An immunohistochemical study of its conversion to dopamine in non‐catecholaminergic cells of the rat brain. J Neural Transm Park Dis Dement Sect 1991;3:27–39. [DOI] [PubMed] [Google Scholar]
- 41. Reed MC, Nijhout HF, Best JA. Mathematical insights into the effects of levodopa. Front Integr Neurosci 2012;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. L‐DOPA‐induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson's disease: Temporal and quantitative relationship to the expression of dyskinesia. J Neurochem 2010;112:1465–1476. [DOI] [PubMed] [Google Scholar]
- 43. Navailles S, Di Giovanni G, De Deurwaerdere P. Predicting dopaminergic effects of L‐DOPA in the treatment of Parkinson's disease. CNS Neurosci Ther 2014; doi: 10.1111/cns.12252, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McMillan PJ, White SS, Franklin A, et al. Differential response of the central noradrenergic nervous system to the loss of locus coeruleus neurons in Parkinson's disease and Alzheimer's disease. Brain Res 2011;1373:240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robertson GS, Robertson HA. Evidence that L‐dopa‐induced rotational behavior is dependent on both striatal and nigral mechanisms. J Neurosci 1989;9:3326–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]


