Summary
Aims
Positron emission tomography (PET) imaging using 5‐HT 1A receptor radioligands shows a decreased expression of this serotonin receptor in the hippocampus of patients with Alzheimer's disease (AD) at advanced stages. However, previous 5‐HT 1A receptor radioligands used in human imaging were antagonists, thought to bind to 5‐HT 1A receptors in different functional states (i.e., both the one which displays high affinity for agonists and is thought to mediate receptor activation, as well as the functional state which has low affinity for agonists). Comparing the PET imaging obtained using an agonist radioligand, which binds selectively to the functional state of the receptors, with the PET imaging obtained using an antagonist radioligand would therefore provide original information on 5‐HT 1A receptor impairment during AD.
Methods
Quantitative autoradiography using 18F‐F15599 and 18F‐MPPF, a 5‐HT 1A agonist and antagonist, respectively, was measured in hippocampi of 18 patients with AD.
Results
Functional 5‐HT 1A receptors, labeled by 18F‐F15599, represented ~35% of total receptors, as estimated by 18F‐MPPF labeling. 18F‐F15599 binding decreased in dentate gyrus of patients with AD, as indicated by Braak's stages. In contrast, binding of 18F‐MPPF was statistically unchanged.
Conclusion
These in vitro results support testing the concept of functional PET imaging using agonist radioligands in clinical studies.
Keywords: 5‐HT1A receptor, Alzheimer's disease, F1559, MPPF, PET, Serotonin
Introduction
Positron emission tomography (PET) is a nuclear medicine imaging modality that can be used to study brain function and neurochemistry of small animals 1, medium‐sized animals 2, and human subjects 3. The development of PET radioligands allows the in vivo exploration of an increasing variety of central nervous system targets. These include numerous monoamine receptor subtypes, such as serotonin 5‐HT1A receptors which are highly expressed in the hippocampus and are known to be important for regulation of memory processes 4. Because early cognitive deficits in Alzheimer's disease (AD) concern episodic memory associated with neurofibrillary tangles in the hippocampus 5, several PET studies have focused on the status of 5‐HT1A receptors in the hippocampus of patients with AD. While a majority of these studies, using the radiolabelled antagonist 18F‐MPPF, have shown a decrease of hippocampal 5‐HT1A receptor density in patients with advanced AD 6, 7, divergent results have been reported in the case of patients at predementia stages of the disease 8, 9. One hypothesis to account for the observations is that compensatory mechanisms of 5‐HT1A receptor regulation may intervene at early stages of AD. If so, this receptor may constitute a procognitive target in AD 10.
The present study is based on the fact that 5‐HT1A receptors have been shown to exist in different G‐protein‐coupling states: (1) a state that has high affinity for agonists (i.e., in an “active state”) when the receptor is coupled with G‐proteins; and (2) a state that has low affinity for agonists when the receptor is uncoupled from G‐proteins 11, 12, 13, 14. The binding of agonists to G‐protein‐coupled receptors is considered to elicit a functional response and growing evidence suggests that changes in receptor/G‐protein coupling may be involved in neurodegenerative disorders 15. In this context, comparing the receptor binding of an antagonist (which indiscriminately labels the receptor in different states) with that of an agonist (which preferentially labels G‐protein‐coupled receptors, Figure 1) could therefore provide information concerning the proportion of “functional receptors” 16. To our knowledge, no imaging study has compared in patients with AD the binding of an agonist versus an antagonist 5‐HT1A PET radiotracer, both of which are amenable to further imaging studies in vivo.
Figure 1.
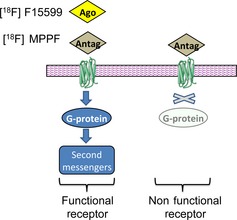
Schematic representation of 18F‐MPPF antagonist binding (Antago) indiscriminately labeling 5‐HT 1A receptors in a G‐coupled receptor state (functional and linked to the second messengers) and in uncoupled state (nonfunctional state). The agonist, 18F‐F15599 (Ago), binds specifically the G‐coupled receptor state (functional receptors) 19. Note that PET radioligands, at tracer dose, occupy only a small fraction of the available receptors.
The aim of this study was to investigate on human brain tissue whether it is possible to obtain distinct 5‐HT1A‐binding profiles at different stages of AD, depending on the pharmacological profile of the PET radioligand. We hypothesized that an antagonist radioligand, which labels the entire population of 5‐HT1A receptors, would have a different binding pattern to that of an agonist radioligand which would be anticipated to label only those 5‐HT1A receptors that are coupled to G‐proteins (i.e., in their “active state”).
Methods
Human Subjects, Tissue Selection, and Neuropathological Staging
Frozen samples were obtained from the brain banks of two University Hospitals (Hospices Civils de Lyon and Assistance Publique ‐ Hôpitaux de Paris). These subjects were selected from specific individuals with cognitive deficits or premortem suspected AD cases (all came from Alzheimer brain collections). Their use was approved by the brain bank's scientific review committee. In total, samples of 18 subjects were dissected from each hemisphere (Table 1).
Table 1.
Demographic data of the brains
| Braak stages | 0 | I–II–III | IV–V–VI |
|---|---|---|---|
| n (total = 16) | 4 | 6 | 8 |
| Age (years) | 61.5 ± 5.4 | 74.8 ± 7.7 | 81.7 ± 12.2 |
| Gender (M/F) | 4/0 | 4/2 | 4/4 |
| PMI (h) | 29.8 ± 3.9 | 30.5 ± 12.9 | 27.8 ± 5.9 |
n, number of patients; M/F, male/female; PMI, postmortem interval.
Anterior hippocampal sections from one hemisphere were fixed in formalin solution and cut into blocks for neuropathological staging. Briefly, after rehydration, 5‐μm‐thick sections were incubated overnight at 4°C with a monoclonal primary antibody directed against hyperphosphorylated tau (Innogenetics Br‐03, clone AT8, dilution 1:500; Zymed Lab‐SA detection system). Each case was given a Braak's stage based on the intensity of staining of neuropil threads (NTs), according to the BrainNet Europe consortium 17, an adaptation of the Braak and Braak classification 18. For autoradiography experiments (see below), the contralateral hemisphere was cut at the level of anterior hippocampus and immediately stored at −80°C (without formalin fixation). Consecutive 30‐μm‐thick sections were cut at −20°C (10/subject), mounted on gelatin‐coated slides, and stored at −80°C.
PET Radioligand Synthesis and Quality Controls
Because of the short half‐life of fluorine‐18 (110 min), 18F‐MPPF and 18F‐F15559 were synthesized in an automated radiosynthesizer (NEPTIS Synthesizer, ORA, Philippeville, Belgium) on the same days when autoradiography experiments were carried out (see below), according to previously described radiochemical pathways 19. Their chemical and radiochemical purities were higher than 98%, as determined by HPLC, and their specific activity at time of autoradiography was systematically calibrated at 37 GBq/μmol (1 Ci/μmol).
Quantitative Autoradiography with PET Radiotracers
Defrosted slides were incubated for 20 min in Tris phosphate‐buffered saline buffer (138 mM NaCl, 2.7 mM KCl, pH adjusted to 7.5) containing 111 kBq/mL of 18F‐MPPF or 18F‐F15599 (i.e., 3 nM). Nonspecific binding was determined in duplicate serial sections co‐incubated with 10 μM serotonin (Sigma‐Aldrich, Saint‐Quentin Fallavier, France). For verification of the agonistic binding of 18F‐F15599, the corresponding buffer was supplemented with Gpp(NH)p (10 μM), a nonhydrolysable analogue of GTP decoupling G‐protein‐coupled receptors.
After incubation, the slides were dipped in cold buffer and distilled water (4°C) then dried and juxtaposed to a phosphor imaging plate for 60 min (BAS‐5000, Fujifilm, Tokyo, Japan). All films were analyzed by a computer‐assisted image analysis system (Multigauge, Fujifilm), and regions of interest (CA1, dentate gyrus) were drawn manually, according to a human brain atlas 20 and confirmed by a following eosin–hematoxylin staining. Quantitation in each region of interest was performed by measuring the average optical density in adjacent brain sections.
In parallel, calibration standards were prepared from rat brain tissue homogenates. Briefly, Potter‐homogenization of freshly extracted rat brains was performed in controlled temperature. Proteins of the homogenates were quantified by a chemistry analyzer (Architect Ci8200, Abbott Diagnostics, Lake Forest, IL, USA). Their exact wet mass was determined after aliquoting in micro‐vials, before −80°C congelation. On the day of radioligand syntheses, increasing activities of 18F‐radioligand (3.13, 6.25, 12.5, and 25 μCi) were mixed with 50 μL of these defrosted homogenates in micro‐vials (from 25 to 300 fmol/mg, ligand/protein). These radioactive micro‐vials were immediately frozen in 2‐methylbutane cooled with dry ice; the frozen core samples were extracted and cut into 30 μm coronal sections using a −20°C cryostat. These extemporaneous standards were juxtaposed to the same imaging plates used for the human tissue, and signal to concentration curves were generated. Nonspecific binding was subtracted from the total binding to determine the specific binding, and measurements were converted into fmoles/mg of protein, according to the calibration curve obtained from the standards. In preliminary studies, homogenized human brain tissue was used concurrently to rat brain tissue for calibration standard preparation. As concentration curves with human tissue were strictly superimposable on curves with rat tissue, the latter was then used for the all experiments.
Statistical Analysis
Statistically significant variations in radioligand binding were measured by Mann–Whitney nonparametric test (GraphPad Prism 6 for Mac OS X, San Diego, CA, USA).
Results
The neuropathological staging centered on the characteristic distribution of neurofibrillary tangles and NTs and the corresponding six stages were reassembled according to the BrainNet Europe consortium 17. Stage 0 was characterized by a total absence of neurofibrillary pathology and was used as control group (n = 4); stages I, II, and III had changes confined to the transentorhinal and entorhinal regions, and a beginning of AT8 immunopositive NTs in temporo‐occipital and temporal cortices, respectively (n = 6); stages IV, V, and VI had marked destruction of these regions, extending to isocortical association areas, namely the occipital cortex (n = 8).
For quantitative autoradiography, the addition of Gpp(NH)p or of an excess of serotonin in the buffer led to a 70% and 90% decreases of the 18F‐F15599 binding in the dentate gyrus and the CA1 area tissues, respectively (experiments in duplicate for the 18 subjects). In the same conditions, no significant modification of 18F‐MPPF binding was measured after addition of Gpp(NH)p. At each Braak's stage, the total amount of 5‐HT1A binding sites (18F‐MPPF) was significantly 3‐fold higher in the CA1 area than in the dentate gyrus (Figure 2). During the first Braak's stage (0), the proportion of 5‐HT1A agonist binding sites measured by the ratio of 18F‐MPPF/18F‐F15599 was 40% and 35% in the CA1 area and in the dentate gyrus, respectively. In the dentate gyrus, 18F‐F15599 labeling fell significantly by 40% and 50% during the I/II/III and IV/V/VI Braak's stages, respectively (P < 0.01), but remained unchanged in the CA1 area during all Braak's stages (Figure 3).
Figure 2.
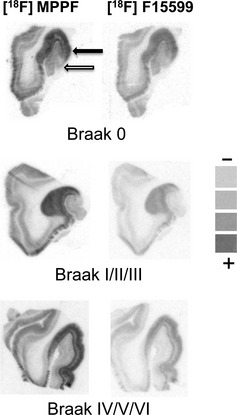
The regional distribution of 18F‐MPPF and 18F‐F15599 binding sites in hippocampi of Alzheimer's disease patients at different Braak's stages. Black and white arrows show the prominent signal in CA1 and dentate gyrus, respectively. Pseudocolor scale obtained from extemporaneous standards is shown from low (−) to high radioactive level (+).
Figure 3.
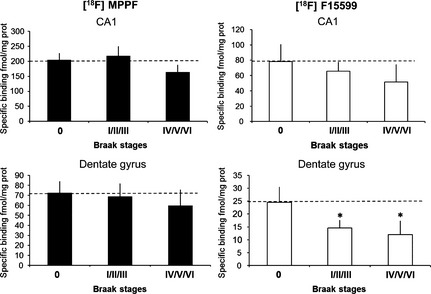
5‐HT 1A receptor‐binding site densities in the CA1 area and the dentate gyrus of Alzheimer's disease patients at different Braak's stages determined from film autoradiography with 18F‐MPPF and 18F‐F15599. *P < 0.01.
Discussion and Conclusion
This study compared for the first time in patients with AD the binding of two PET radioligands directed toward the same serotonin receptor, but differing in their agonist or antagonist pharmacological properties. We hypothesized that an antagonist radioligand (18F‐MPPF), which labels the entire population of 5‐HT1A receptors, would display a different binding pattern to that of an agonist radioligand (18F‐F15599) which was anticipated to label only those 5‐HT1A receptors which are G‐coupled and linked to the second messengers (i.e., in their “active” or “functional” state).
Although the present study utilized brain tissue samples from a modest number of patients, the validity of its results is supported by its robust methodology: Braak's staging adapted by the BrainNet Europe consortium was chosen because of the better correlation of hyperphosphorylated tau deposits than β‐amyloid with the cognitive status 17, 18; 18F‐F15599 and 18F‐MPPF bindings were compared in serial sections limiting interindividual variability; 18F‐F15599 and 18F‐MPPF are both highly selective for 5‐HT1A receptors and display similar nanomolar affinity for this target (approximately 3 nM in each case) 21, 22, allowing a direct comparison of their binding (if identical experimental conditions are used) 19; their incubation concentrations were optimally chosen for autoradiography so that they were three times their Kd values in human brain 23; the fact that 18F‐F15599 binds preferentially to G‐protein‐coupled “functional” 5‐HT1A receptors 19 was confirmed by the receptor/G‐protein decoupling action of Gpp(NH)p; and, finally, autoradiography data were obtained in a quantitative manner (calibration curves).
The binding of the antagonist, 18F‐MPPF, which provides a measure of total 5‐HT1A receptor density, revealed a similar distribution and quantitative labeling at Braak's stage 0 as in previous postmortem brain studies in non‐AD subjects 24. This indicates that, in terms of serotonin neurotransmission, the Braak's stage 0 can be considered as a control group. It has to be mentioned that in terms of patient demography, there was a tendency for an effect of Braak staging on age of the subjects, that is, higher Braak staging for higher age of patients. This nonhomogeneity of patient ages (particularly for the Braak's stage 0 vs. other Braak's stages) is inherent in studies using brain banks (indeed, availability of brain tissue from very old patients without neurodegenerative diseases is rare). Nevertheless, even if the age effect has an influence on 5‐HT1A receptor density 25, 26, it should not be a source of bias in the present study because the two radioligands (18F‐MPPF and 18F‐F15599) were directly compared on brain tissue from the same patients. Differences in the densities of receptors labeled by the two radioligands can therefore be attributed to their distinct agonist/antagonist properties rather than to the ages of the patients with AD.
Interestingly, the ratio between the number of receptors labeled by the antagonist (total receptor density) and the number of receptors labeled by the agonist (receptors coupled to G‐proteins only) provides an indication of the level of 5‐HT1A receptor G‐protein‐coupling in different areas of human hippocampus, in accordance with results obtained in a feline model 15. Whereas total 5‐HT1A receptor sites measured by the antagonist, 18F‐MPPF, were unchanged at all AD stages, there was a significant decrease in the density of binding sites labeled by the 5‐HT1A agonist, 18F‐F15599, at the early Braak's stages I/II/III and at the advanced stages IV/V/VI. Our results revealed therefore an early decrease of functional 5‐HT1A hippocampus receptors, undetectable at the same stages by the prototypical radiolabelled MPPF. Other postmortem studies have described a decrease of G‐protein‐mediated GTP hydrolysis with the progression of AD 27, 28, but the in vitro approaches of those studies (i.e., [35S]GTPγS or [3H]8‐OH‐DPAT autoradiography) were unsuitable for transfer to in vivo studies. In contrast, our approach using PET radioligands is directly transferable to imaging studies in vivo. The present results therefore support a new concept consisting in comparing the binding profiles of a PET agonist and an antagonist in patients to determine, in vivo, the extent of G‐protein coupling of 5‐HT1A receptors and to follow its modifications in functionality during the neurodegenerative process.
The biological underpinnings of an early loss of 5‐HT1A receptor functionality in AD brain could be linked with the up‐regulation of 5‐HT1A receptors associated with preserved cognitive function during dementias 29. This suggests that comparison of agonist and antagonist PET measures could shed light on why 5‐HT1A agonist therapies have been unsuccessful thus far in the treatment of AD 10. Finally, future in vitro studies should not only focus on replication of the present findings in larger patient populations but also on exploring other brain regions, that is, cortical regions, and on carrying out full quantitation of binding parameters (i.e., Kd and Bmax quantitation using Scatchard analysis), before adaptation of this paradigm to in vivo imaging studies in patients with AD.
Conflict of Interest
Dr. Newman‐Tancredi is an employee and stockholder of Neurolixis but has no financial disclosures associated with this project. The other authors report no conflict of interest and have nothing to disclose.
Acknowledgments
The technical assistances in neuropathology of Ms. Elisabeth Delolme (Cardiobiotec, Groupement Hospitalier Est – Hospices Civils de Lyon), Ms. Sabrina Leclere‐Turbant (GIE‐Neuro‐CEB, La Pitié‐Salpêtrière Hospital, Paris) and in autoradiography of M. Sylvain Fieux (BioRaN, CRNL) are greatly acknowledged.
References
- 1. Lancelot S, Zimmer L. Small‐animal positron emission tomography as a tool for neuropharmacology. Trends Pharmacol Sci 2010;9:411–417. [DOI] [PubMed] [Google Scholar]
- 2. Aznavour N, Cendres‐Bozzi C, Lemoine L, et al. MPTP animal model of Parkinsonism: dopamine cell death or only tyrosine hydroxylase impairment? – A study using PET imaging, autoradiography and immunohistochemistry in the cat. CNS Neurosci Ther 2012;18:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zimmer L, Luxen A. PET radiotracers for molecular imaging in the brain: past, present and future. NeuroImage 2012;61:363–370. [DOI] [PubMed] [Google Scholar]
- 4. Ogren SO, Eriksson TM, Elvander‐Tottie E, et al. The role of 5‐HT(1A) receptors in learning and memory. Behav Brain Res 2008;195:54–77. [DOI] [PubMed] [Google Scholar]
- 5. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS‐ADRDA criteria. Lancet Neurol 2007;6:734–746. [DOI] [PubMed] [Google Scholar]
- 6. Kepe V, Barrio JR, Huang SC, et al. Serotonin 1A receptors in the living brain of Alzheimer's disease patients. Proc Nat Acad Sci USA 2006;103:702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lanctot KL, Hussey DF, Herrmann N, et al. A positron emission tomography study of 5‐hydroxytryptamine‐1A receptors in Alzheimer disease. Am J Geriatr Psychiatry 2007;15:888–898. [DOI] [PubMed] [Google Scholar]
- 8. Truchot L, Costes N, Zimmer L, et al. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer's disease. NeuroImage 2008;40:1251–1256. [DOI] [PubMed] [Google Scholar]
- 9. Truchot L, Costes SN, Zimmer L, et al. Up‐regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology 2007;69:1012–1017. [DOI] [PubMed] [Google Scholar]
- 10. Sato S, Mizukami K, Asada T. A preliminary open‐label study of 5‐HT1A partial agonist tandospirone for behavioural and psychological symptoms associated with dementia. Int J Neuropsychopharmacol 2007;10:281–283. [DOI] [PubMed] [Google Scholar]
- 11. Hall MD, El Mestikawy S, Emerit MB, Pichat L, Hamon M, Gozlan H. [3H]8‐hydroxy‐2‐(di‐n‐propylamino)tetralin binding to pre‐ and postsynaptic 5‐Hydroxytryptamine sites in various regions of the rat brain. J Neurochem 1985;44:1685–1696. [DOI] [PubMed] [Google Scholar]
- 12. Mongeau R, Welner SA, Quirion R, Suranyi‐Cadotte BE. Further evidence for differential affinity states of the serotonine 1A receptor in rat hippocampus. Brain Res 1992;590:229–238. [DOI] [PubMed] [Google Scholar]
- 13. Nénonéné EK, Radja F, Carli M, Grondin L, Reader TA. Heterogeneity of cortical and hippocampal 5‐HT1A receptors: reappraisal of homogenate binding with 8‐[3H]hydroxydipropylaminotetralin. J Neurochem 1994;62:1822–1834. [DOI] [PubMed] [Google Scholar]
- 14. Polter AM, Li X. 5‐HT1A receptor‐regulated signal transduction pathways in brain. Cell Signal 2010;22:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thathiah A, De Strooper B. The role of G protein‐coupled receptors in the pathology of Alzheimer's disease. Nat Rev Neurosci 2011;12:73–87. [DOI] [PubMed] [Google Scholar]
- 16. Aznavour N, Rbah L, Leger L, et al. A comparison of in vivo and in vitro neuroimaging of 5‐HT 1A receptor binding sites in the cat brain. J Chem Neuroanat 2006;31:226–232. [DOI] [PubMed] [Google Scholar]
- 17. Alafuzoff I, Arzberger T, Al‐Sarraj S, et al. Staging of neurofibrillary pathology in Alzheimer's disease: a study of the BrainNet Europe Consortium. Brain Pathol 2008;18:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braak H, Braak E. Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 19. Lemoine L, Verdurand M, Vacher B, et al. [18F]F15599, a novel 5‐HT1A receptor agonist, as a radioligand for PET neuroimaging. Eur J Nucl Med Mol Imaging 2010;37:594–605. [DOI] [PubMed] [Google Scholar]
- 20. Mai JK, Paxinos G. Atlas of the human brain. San Diego, CA: Academic Press, 1997. [Google Scholar]
- 21. Newman‐Tancredi A, Martel JC, Assié MB, et al. Signal transduction and functional selectivity of F15599, a preferential post‐synaptic 5‐HT1A receptor agonist. Br J Pharmacol 2009;156:338–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kung HF, Stevenson DA, Zhuang ZP, Kung MP, Frederick D, Hurst SD. New 5‐HT1A receptor antagonist: [3H]p‐MPPF. Synapse 1996;23:344–346. [DOI] [PubMed] [Google Scholar]
- 23. Burnet PWJ, Eastwood SL, Harrison PJ. [3H]WAY‐100635 for 5‐HT1A receptor autoradiography in human brain: a comparison with [3H]8‐OH‐DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int 1997;30:565–574. [DOI] [PubMed] [Google Scholar]
- 24. Hall H, Lundkvist C, Halldin C, et al. Autoradiographic localization of 5‐HT1A receptors in the post‐mortem human brain using [3H]WAY‐100635 and [11C]way‐100635. Brain Res 1997;745:96–108. [DOI] [PubMed] [Google Scholar]
- 25. Costes N, Merlet I, Ostrowsky K, et al. A 18F‐MPPF PET normative database of 5‐HT1A receptor binding in men and women over aging. J Nucl Med 2005;46:1980–1989. [PubMed] [Google Scholar]
- 26. Cidis Meltzer C, Drevets WC, Price JC, et al. Gender‐specific aging effects on the serotonin 1A receptor. Brain Res 2001;895:9–17. [DOI] [PubMed] [Google Scholar]
- 27. Garcia‐Jimenez A, Cowburn RF, Ohm TG, et al. Loss of stimulatory effect of guanosine triphosphate on [(35)S]GTPgammaS binding correlates with Alzheimer's disease neurofibrillary pathology in entorhinal cortex and CA1 hippocampal subfield. J Neurosci Res 2002;67:388–398. [DOI] [PubMed] [Google Scholar]
- 28. Weinstein D, Magnuson D, Lee J. Altered G‐protein coupling of a frontal cortical low affinity [3H]8‐hydroxy‐N, N‐dipropyl‐2‐aminotetralin serotonergic binding site in Alzheimer's disease. Behav Brain Res 1996;73:325–329. [DOI] [PubMed] [Google Scholar]
- 29. Elliott MS, Ballard CG, Kalaria RN, Perry R, Hortobagyi T, Francis PT. Increased binding to 5‐HT1A and 5‐HT2A receptors is associated with large vessel infarction and relative preservation of cognition. Brain 2009;132:1858–1865. [DOI] [PubMed] [Google Scholar]


