Summary
Aims
A longitudinal study investigated the remitted geriatric depression (RGD) patients' persistent cognitive impairment and potential correlation with their PCC functional connectivity network.
Methods
A total of 14 RGD patients and 18 matched controls were recruited. All subjects finished the neuropsychological tests and functional magnetic resonance imaging scan at baseline and follow‐up. A spherical region of interest was placed in PCC to calculate the functional connectivity, and further analysis was employed to detect correlations between longitudinal changes in the brain regions and neuropsychological data.
Results
There were significant cognitive declines in RGD patients at baseline and follow‐up. Altered patterns of functional connectivity were detected within the RGD group showing correlations with neuropsychological tests. The longitudinal change in functional connectivity between PCC and cerebellum posterior lobe was correlated with longitudinal changes in auditory verbal memory test‐recall (r = 0.550, P = 0.042). The longitudinal change in functional connectivity between PCC and right parahippocampal gyrus was correlated with Trail Making Test‐A (r = 0.631, P = 0.015). The longitudinal change in functional connectivity between PCC and supramarginal_R was correlated with Mini‐Mental State Examination (r = −0.630, P = 0.016).
Conclusions
RGD patients performed worse cognitive function, and altered PCC functional connectivity network might have a role in these cognitive declines.
Keywords: Cognitive impairment, Functional connectivity, Magnetic resonance imaging, Posterior cingulate cortex, Remitted geriatric depression
Introduction
Known as one of most common mental disorders in the elderly, depression brings significant decline in both well‐being and daily functioning. It also shows a high risk of functional impairment, mortality, and a high rate of service utilization 1. Geriatric depression, defined as a major depressive episode occurring in older adults (usually after the age of 65 years), shows a potential association with cognitive impairment 2. It has been shown by previous studies that there was generally significant cognitive impairment along with affective symptoms in geriatric depression 3, 4, 5. Furthermore, even after remission of mood symptoms, cognitive deficits will persist 6, 7, 8. Particularly, episodic memory and executive function were found to be much worse in geriatric depression patients than in controls 9. The mechanism behind these persistent cognitive deficits is not clear, and many hypotheses have been raised to address this problem.
Although remitted geriatric depression (RGD) patients often suffer from cognitive impairments, they are usually not so serious as in Alzheimer's disease (AD) or other cognitive disorders in the geriatric population. Interestingly, despite the accumulating reports claiming that RGD patients might have a high rate of conversion into AD, our previous study also found that episodic memory, executive function, and attentional processes were particularly disturbed in RGD patients 9. This pattern of cognitive deficits is similar to that found in mild cognitive impairment (MCI) and AD patients 10, which strongly suggests that RGD patients might have a similar structural or functional brain network disturbance.
Increasing evidence from pathological, structural, and functional imaging research suggests that posterior cingulate cortex (PCC) is a key structure in the pathophysiology of cognitive impairment 11, 12, 13. At the same time, it is also considered as the structural and functional core of default mode network (DMN), indicating its broad connection with other brain regions. The DMN, including PCC, medial prefrontal cortex, medial temporal lobes, dorsolateral prefrontal cortex and inferior parietal lobe, plays a significant role in cognitive function. Previous studies revealed DMN to mediate internally generated thought process, episodic memory, and attention 14, 15. Additionally, the disruption of functional connectivity within the DMN has been reported in various mental disorders, such as schizophrenia 16, anxiety disorder 17, social phobia 18, and major depressive disorder 19, 20. These results indicate that the DMN and its in‐network connections may be particularly important in both affective and cognitive function. Considering most of these previous analyses involved the relationship between PCC and specific regions of interest (ROI) at a single time point, a DMN‐wide longitudinal study might shed further light on the detailed mechanisms underlying the cognitive impairments of RGD patients.
Due to the advance of technology, it is possible to investigate brain function and draw the functional connectivity network with the help of fMRI, especially the resting‐state functional imaging 21, 22, 23, 24. The valuable approach is safe and only requires the participants to remain relaxed and still with their eyes closed during scanning. The aim of this study was to investigate the changes in cognitive functions and PCC functional connectivity patterns in a longitudinal study of RGD patients and controls. We hypothesize that the altered longitudinal changes in the functional connectivity network might provide a neuroimaging marker of cognitive impairments in RGD patients.
Materials and methods
Subjects
This study was approved by Affiliated Brain Hospital of Nanjing Medical University. Firstly, diagnostic evaluation was carefully taken on all the participants, which included clinical interview, focused neurological and mental status examination, review of medical history, and demographic inventory. The participants met the following inclusion criteria: (1) the participants were acquired from the in‐patients of the department of geriatric psychiatry in Affiliated Brain Hospital of Nanjing Medical University. All of patients met the major depressive disorder in Diagnostic and Statistical Manual of Mental Disorders IV criteria and remitted for more than 6 months before the enrollment, (2) the age of first onset was over 60 years, (3) Hamilton Depression Rating Scale (HDRS) scores were lower than 7 and Mini‐Mental State Examination (MMSE) scores were higher than 24, (4) durations of illness were less than 5 years, (5) absence of other major psychiatric disorder, including abuse or dependence on psychoactive substances; (6) absence of primary neurological illness including dementia or stroke, (7) absence of medical illness impairing cognitive function, (8) no history of receiving electroconvulsive therapy, and (9) T2‐weighted MRI of the participants did not show any major white matter changes such as infarction or other vascular lesions. Secondly, participants with head motion more than 3 mm maximum displacement in any direction of x, y, and z or 3° of any angular motion throughout the course of scan, or with poor image quality, were removed at baseline and follow‐up fMRI scan. Finally, 14 RGD patients and 18 well‐matched controls completed two neuropsychological tests and two resting‐state fMRI scans at around 21 months (12–32 months).
Neuropsychological Tests
All the participants received a neuropsychological examination consist of MMSE 25, Auditory Verbal Memory Test‐recall (AVLT) 26, Digit Span Test (DST) 27, Symbol Digit Modalities Test (SDMT) 28, Trail Making Test‐A and B (TMT‐A & B) 29. Table 1 contains demographic and neuropsychological data for the two groups at baseline and follow‐up.
Table 1.
Demographic and neuropsychological data between RGD group and healthy controls group
| Item | Baseline | P value | Follow‐up | P value | ||
|---|---|---|---|---|---|---|
| RGD (n = 14) | Controls (n = 18) | RGD (n = 14) | Controls (n = 18) | |||
| Age (years) | 68.2 ± 3.93 | 70.5 ± 3.24 | 0.061a | – | – | – |
| Education levels (years) | 14.2 ± 2.10 | 15.2 ± 2.8 | 0.185a | – | – | – |
| Gender (male: female) | 7: 7 | 10: 8 | 0.755b | 7: 7 | 10: 8 | 0.755b |
| Mini‐Mental State Examination | 28.6 ± 1.9 | 28.3 ± 1.3 | 0.168a | 27.8 ± 4.0 | 28.6 ± 1.8 | 0.858a |
| Auditory verbal memory test ‐ delayed recall | 5.9 ± 2.5 | 8.1 ± 1.9 | 0.007a | 5.0 ± 2.8 | 8.6 ± 2.7 | 0.002a |
| Trail making test‐A | 133.5 ± 96.7 | 70.0 ± 28.7 | 0.007a | 70.4 ± 25.5 | 75.7 ± 25.5 | 0.595a |
| Trail making test‐B | 237.7 ± 138.4 | 139.3 ± 39.2 | 0.074a | 157.7 ± 36.9 | 138.8 ± 55.5 | 0.106a |
| Symbol digit modalities test | 25.0 ± 15.0 | 34.3 ± 8.7 | 0.074a | 27.1 ± 15.8 | 33.2 ± 12.8 | 0.143a |
| Digit span test | 11.7 ± 2.1 | 13.2 ± 1.8 | 0.032a | 11.7 ± 2.1 | 13.6 ± 2.6 | 0.019a |
Independent‐samples t‐test.
Fisher's exact test.
Magnetic Resonance Imaging Procedures
The participants were scanned using a General Electric 1.5 Tesla scanner (General Electric Medical Systems, Buckinghamshire, UK) with a homogeneous birdcage head coil. The participants were told to lie supine with their head snugly fixed by a belt and foam pads to minimize the head motion. An echo‐planar imaging sequence was set up to acquire the resting‐state images, which was set at following parameters: 30 axial slices, thickness/skip = 4/0 mm, in‐plane resolution = 64 × 64, repetition time = 3000 ms, echo time = 40 ms, flip angle = 90°, field of view = 240 × 240 mm, 240 volumes (7 min 6 s).
Functional Image Preprocessing
SPM5 (http://www.fil.ion.ucl.ac.uk/spm) and REST (http://resting-fmri.sourceforge.net) were used for the data analyses. The first ten volumes of the scanning session were discarded to allow for T1 equilibration effects. The remaining were corrected for timing differences between each slice. The images were then corrected for motion effects (six‐parameter rigid body), where the reference volume was in the center of the run. The resulting images were spatially normalized into a standard stereotaxic space using a 12‐parameter affine approach and an EPI template image, and resampling to 3 × 3 × 3 mm3 voxels, and smoothed with a Gaussian kernel of 4 × 4 × 4 mm (full‐width half‐maximum FWHM). The resulting fMRI data were filtered (0.01 < f < 0.08 Hz) to reduce the low‐frequency drift and high frequency physiological respiratory and cardiac noise. Any linear trend was then removed.
Functional Connectivity Analyses
To characterize the functional connectivity of the PCC, a spherical ROI (radius = 10 mm) was set at the coordinates (−5, −49, 40) within the PCC structure 30, 31, 32.
For each subject, a mean time series for ROI was computed for reference time course. Pearson linear correlation analysis supported by SPM5 and REST tool kit 33 was then carried out between the mean signal change in the PCC and the time series of every voxel of whole brain to assess the functional connectivity 34, 35.
The formula for this calculation 33 is:
where is the Pearson correlation coefficient, and are the mean values of time course x and y, respectively.
A Fisher's z‐transform was applied to improve the normality of the correlation coefficients 36 after the Pearson correlation coefficient maps were created. To remove possible effects of head motion, global, white matter and cerebrospinal fluid signals on the results, six head motion parameters, and mean time series of global, white matter and cerebrospinal fluid signals were introduced as covariates into a random effect.
Comparisons within and between the groups were then made based on the z‐transform maps. Firstly, a one‐sample t‐test in a voxel‐wise manner was used to determine the brain regions showing significant connectivity to PCC, and voxels with P < 0.005 and a minimum cluster size of 324 mm3. This yielded a corrected threshold of P < 0.05, determined by Monte Carlo simulation (see program AlphaSim by D.Ward. Parameters were single voxel P = 0.005, FWHM = 4 mm, with mask. http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). The following calculations were restricted in these results to avoid any possible bias. Secondly, to explore whether longitudinal changes in the functional connectivity network in RGD patients and controls may differ and locate the potential abnormal brain regions, longitudinal changes (the z‐maps at follow‐up subtract those at baseline within each group) were determined using the REST Image Calculator. Then, the longitudinal changes in RGD group and controls were entered a two‐sample t‐test to detect the functional connectivity abnormally changed brain regions also in a voxel‐wise manner with the single voxel threshold of P < 0.005 and minimum cluster sizes of 324 mm3 (the lengths of follow‐up were taken here as covariate).
Statistical Analysis
For the nonnormal distribution of the neuropsychological data, nonparametric Mann–Whitney U‐tests (MWU) were used for group comparisons of demographic and neuropsychological performance (statistical significance was set at P < 0.05). The further correlation analysis between fMRI data and neuropsychological performances was then undertaken. Masks of abnormal brain were extracted. Second, mean z‐values of these abnormal regions within each RGD patient were calculated based on the masks extracted above. The analyses were performed using the REST Extract ROI Series also from REST tool kit. Pearson's correlation analyses were then performed to examine correlations between abnormal brain region's z‐values and neuropsychological performance of RGD patients using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Clinical and Neuropsychological Results
Both groups showed no significant differences in age, education, and gender distributions. Control subjects displayed cognition within the normal range at both baseline and follow‐up. Compared with the controls, the RGD patients showed significant deficits in the performances of AVLT‐delayed recall, TMT‐A and B, symbol digit modalities test and DST at baseline. After the follow‐up, the performance of AVLT‐delayed recall and DST in RGD were still worse than controls, but no significant differences were found in TMT‐A and B and DST. We also found that the RGD patients show significant improvement in TMT‐A and DST, but slightly decline in AVLT‐delayed recall, compared with their performance of baseline (see Table 1).
Functional Connectivity
Reliability
The functional connectivities of the PCC were performed separately, and the patterns were shown in each of four groups (both RGD group and control group at baseline and follow‐up see Figure 1). These patterns were in line with the PCC functional connectivity network detected in previous studies, which generally consisted of posterior and anterior cingulate cortex and medial temporal lobes.
Figure 1.
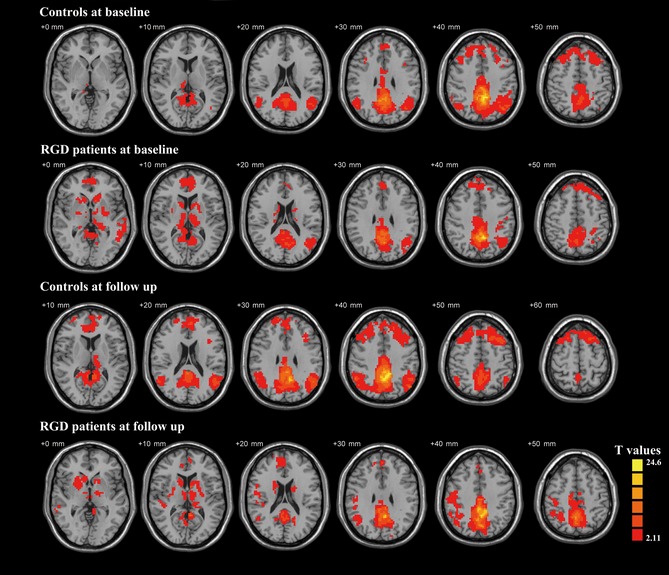
The one‐sample t‐test images show the functional connectivity of PCC in RGD patients and controls at baseline and follow‐up, which indicate the DMN patterns. Thresholds were set at a corrected P < 0.05.
Longitudinal Change in the PCC Functional Connectivity Network
After the follow‐up at around 21 months, the longitudinal change in PCC functional connectivity network in the RGD patient was found significantly altered from those of controls in certain regions (see Figure 2). Furthermore, the longitudinal change between PCC and cerebellum posterior lobe was found positively correlated with the longitudinal change in the AVLT‐recall score (see Figure 2A), longitudinal change in PCC functional connectivity between PCC and right parahippocampal gyrus was found positively correlated with the longitudinal change in TMT‐A score (see Figure 2B), and longitudinal changes in functional connectivity between PCC and supramarginal_R were negatively correlated with longitudinal change in MMSE (see Figure 2C).
Figure 2.
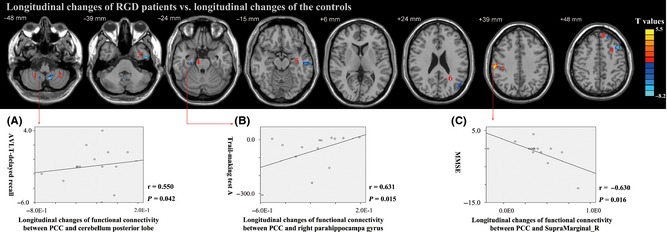
Maps of longitudinal changes in functional connectivity of PCC in RGD vs. controls. Thresholds were set at a corrected P < 0.05 determined by Monte Carlo simulation. L, left; R, right. RGD patients show significant longitudinal changes vs. controls in the following regions: 1. cerebellum posterior lobe, 2. cerebellar tonsil, 3. L inferior temporal gyrus, 4. R parahippocampal gyrus, 5. L middle temporal gyrus, 6. L middle temporal gyrus, 7. supramarginal_R, 8. L middle frontal gyrus, 9. L superior frontal gyrus. (A) Longitudinal changes in functional connectivity between PCC and cerebellum posterior lobe were positively correlated with longitudinal changes in AVLT‐recall in RGD patients (Spearman's ρ = 0.550, P = 0.042, two‐tailed), (B) Longitudinal changes in functional connectivity between PCC and right parahippocampal gyrus were positively correlated with longitudinal changes in trail making test‐A in RGD patients (Spearman's ρ = 0.631, P = 0.015, two‐tailed), (C) Longitudinal changes in functional connectivity between PCC and supramarginal_R were negatively correlated with longitudinal changes in MMSE in RGD patients (Spearman's ρ = −0.630, P = 0.016, two‐tailed).
Discussion
Using the resting‐state fMRI to explore the PCC functional connectivity network and the correlations between altered connectivity and cognitive performance in RGD patients, three major findings were generated.
Firstly, RGD patients still demonstrated a poorer cognitive function than controls after follow‐up, especially in the functions of auditory memory (AVLT‐delayed recall) and attention span (DST). These findings were partly in line with previous studies 7, 37. However, it was notable that the RGD patients also showed a greater improvement in executive function (TMT‐A and B) at follow‐up. The antidepressant treatment might, at least in part, be responsible for this improvement, although it showed no positive effect on auditory memory and attention.
Secondly, the longitudinal changes between PCC and cerebellum posterior lobe, limbic lobe, temporal lobe, and frontal lobe were found to be significantly less in the RGD patients than the controls, while the changes between PCC and parietal lobe were found to be significantly more severe. These patterns of RGD patients indicated a compensatory redistribution and mobilization of the neural resource, particularly in which the parietal lobe was more likely to be affected compared with the controls.
There has been little information available about the strength of functional connectivity in the RGD patients, but it was found that in AD patients, there is increased functional connectivity between frontal cortex and temporal cortex 38 or between frontal cortex and hippocampus 39 in resting state. These results were interpreted as a recruitment of additional neural resources in prefrontal regions to compensate for losses of neural networks in the other regions 40, 41. In our study, the increase in functional connectivity between PCC, right parahippocampal gyrus and right supramarginal gyrus was also considered as a compensation. However, the results showed improvements in processing speed and executive function (TMT‐A and B) in the RGD patients, but at the same time, the increase in functional connectivity was positively correlated with the score of TMT‐A, which indicated that though the recruitment of additional neural resources might help to maintain cognitive function, but at the stage of follow‐up, the increase in functional connectivity would later indicate greater cognitive impairment.
Thirdly, we found that RGD patients tended to have their cerebellum greatly affected at follow‐up. The increased functional connectivity between PCC and posterior cerebellum in RGD patients correlated positively with the AVLT score, which implied this increased connectivity would help to maintain the cognitive function, especially auditory memory. It was believed that the cerebellum was well preserved during the progress of cognitive impairment, while recent studies found that the cerebellum could be subtly altered even at early stages of cognitive impairment 42, 43. Our findings also suggested that the cerebellum was involved in the progress of cognitive deficits in RGD patients, and these involvements might be potential important. At the detected stage when the compensation of cerebral structure is unable to maintain cognitive function, the cerebellum structure would have an important role in maintaining aspects of cognitive function.
Of course, there are technical and biological limitations involved in this study that must be acknowledged. Firstly, this longitudinal study only has a relatively small sample size, and the term of longitudinal follow‐up is also limited. So the findings may not be very categorical and involve some bias due to the sample size. The longitudinal term covered a mean period of 21 months, but it might still not be able to measure the progression of geriatric depression. The longitudinal changes in cognitive deficits along with correlations between cognitive deficits and functional connectivity may serve as markers of progression of geriatric depression to some extent, but further longer follow‐up may be necessary to detect the cognitive dysfunction which can be more severe at later stages. Furthermore, variability in the follow‐up period of each patient might have an effect on the outcome. Secondly, although the participants were asked to rest with their eyes closed, subtle motor or oculomotor movements will inevitably cause cerebellar activities 44. These activities will in turn affect the acquired resting‐state data. Thirdly, a different processing method might be more appropriate. For the most important part, using T1 templates separately for normalization may be more precise than using the EPI templates while conducting the preprogressing, and with the longitudinal follow‐up of 21 months, differences in scanner function may result in subtle differences in image acquisition.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We wish to thank all the participants in this study. This work was partly supported by National Natural Science Foundation of China (30970814 and 81371488, Yonggui Yuan).
References
- 1. Taki Y, Kinomura S, Awata S, et al. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age‐matched normal subjects: A voxel‐based morphometry. J Affect Disord 2005;88:313–320. [DOI] [PubMed] [Google Scholar]
- 2. Butters MA, Young JB, Lopez O, et al. Pathways linking late‐life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci 2008;10:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nebes RD, Butters MA, Mulsant BH, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med 2000;30:679–691. [DOI] [PubMed] [Google Scholar]
- 4. Butters MA, Bhalla RK, Mulsant BH, et al. Executive functioning, illness course, and relapse/recurrence in continuation and maintenance treatment of late‐life depression: Is there a relationship? Am J Geriatr Psychiatry 2004;12:387–394. [DOI] [PubMed] [Google Scholar]
- 5. Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: Relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006;60:58–65. [DOI] [PubMed] [Google Scholar]
- 6. Paradiso S, Lamberty GJ, Garvey MJ, et al. Cognitive impairment in the euthymic phase of chronic unipolar depression. J Nerv Ment Dis 1997;185:748–754. [DOI] [PubMed] [Google Scholar]
- 7. Nebes RD, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: A randomized, double‐blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res 2003;37:99–108. [DOI] [PubMed] [Google Scholar]
- 8. Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. Am J Geriatr Psychiatry 2004;12:50–56. [PubMed] [Google Scholar]
- 9. Yuan Y, Zhang Z, Bai F, et al. Abnormal neural activity in the patients with remitted geriatric depression: A resting‐state functional magnetic resonance imaging study. J Affect Disord 2008;111:145–152. [DOI] [PubMed] [Google Scholar]
- 10. Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting‐state networks across healthy subjects. PNAS 2006;103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiino A, Watanabe T, Maeda K, et al. Four subgroups of Alzheimer's disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. Neuroimage 2006;33:17–26. [DOI] [PubMed] [Google Scholar]
- 12. Liang WS, Reiman EM, Valla J, et al. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. PNAS 2008;105:4441–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pengas G, Hodges JR, Watson P, et al. Focal posterior cingulate atrophy in incipient Alzheimer's disease. Neurobiol Aging 2010;31:25–33. [DOI] [PubMed] [Google Scholar]
- 14. Greicius MD, Srivastava G, Reiss AL, et al. Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. PNAS 2005;101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sorg C, Riedl V, Muhlau M, et al. Selective changes of resting‐state networks in individuals at risk for Alzheimer's disease. PNAS 2007;104:18760–18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ongür D, Lundy M, Greenhouse I, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res 2010;183:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao XH, Wang PJ, Li CB, et al. Altered default mode network activity in patient with anxiety disorders: An fMRI study. Eur J Radiol 2007;63:373–378. [DOI] [PubMed] [Google Scholar]
- 18. Gentili C, Ricciardi E, Gobbini MI, et al. Beyond amygdala: Default Mode Network activity differs between patients with social phobia and healthy controls. Brain Res Bull 2009;79:409–413. [DOI] [PubMed] [Google Scholar]
- 19. Bluhm R, Williamson P, Lanius R, et al. Resting state default‐mode network connectivity in early depression using a seed region‐of‐interest analysis: Decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci 2009;63:754–761. [DOI] [PubMed] [Google Scholar]
- 20. Sheline YI, Barch DM, Price JL, et al. The default mode network and self‐referential processes in depression. PNAS 2009;106:1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mantani T, Okamoto Y, Shirao N, et al. Reduced activation of posterior cingulate cortex during imagery in subjects with high degrees of alexithymia: A functional magnetic resonance imaging study. Biol Psychiatry 2005;57:982–990. [DOI] [PubMed] [Google Scholar]
- 22. Greicius MD, Flores BH, Menon V, et al. Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007;62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 2009;66:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hahn A, Stein P, Windischberger C, et al. Reduced resting‐state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 2011;56:881–889. [DOI] [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 26. Barzotti T, Gargiulo A, Marotta MG, et al. Correlation between cognitive impairment and the Rey auditory‐verbal learning test in a population with Alzheimer disease. Arch Gerontol Geriatr Suppl 2004;9:57–62. [DOI] [PubMed] [Google Scholar]
- 27. Wechsler D. Wechsler Adult Intelligence Scale‐Revised Manual. San Antonio, TX: Psychologcial Corp, 1981. [Google Scholar]
- 28. Watson CG, Gasser B, Schaefer A, et al. Separation of brain‐damaged from psychiatric patients with ability and personality measures. J Clin Psychol 1981;37:347–353. [DOI] [PubMed] [Google Scholar]
- 29. Gordon NG. The Trail Making Test in neuropsychological diagnosis. J Clin Psychol 1972;28:167–169. [DOI] [PubMed] [Google Scholar]
- 30. Long XY, Zuo XN, Kiviniemi V, et al. Default mode network as revealed with multiple methods for resting‐state functional MRI analysis. J Neurosci Methods 2008;171:349–355. [DOI] [PubMed] [Google Scholar]
- 31. Fransson P. Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 2005;26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. PNAS 2001;98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song XW, Dong ZY, Long XY, et al. REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS ONE 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Z, Yuan Y, Bai F, et al. Abnormal default‐mode network in angiotensin converting enzyme D allele carriers with remitted geriatric depression. Behav Brain Res 2012;230:325–332. [DOI] [PubMed] [Google Scholar]
- 35. Guo W, Yao D, Jiang J, et al. Abnormal default‐mode network homogeneity in first‐episode, drug‐naive schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry 2014;49:16–20. [DOI] [PubMed] [Google Scholar]
- 36. Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. Neuroimage 1998;7:511–522. [DOI] [PubMed] [Google Scholar]
- 37. Devanand DP, Pelton GH, Marston K, et al. Sertraline treatment of elderly patients with depression and cognitive impairment. Int J Geriatr Psychiatry 2003;18:123–130. [DOI] [PubMed] [Google Scholar]
- 38. Wang K, Liang M, Wang L, et al. Altered functional connectivity in early Alzheimer's disease: A resting‐state fMRI study. Hum Brain Mapp 2007;28:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang L, Zang Y, He Y, et al. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: Evidence from resting state fMRI. Neuroimage 2006;31:496–504. [DOI] [PubMed] [Google Scholar]
- 40. Gould RL, Arroyo B, Brown RG, et al. Brain mechanisms of successful compensation during learning in Alzheimer disease. Neurology 2006;67:1011–1017. [DOI] [PubMed] [Google Scholar]
- 41. Bai F, Liao W, Watson DR, et al. Mapping the altered patterns of cerebellar resting‐state function in longitudinal amnestic mild cognitive impairment patients. J Alzheimers Dis 2011;23:87–99. [DOI] [PubMed] [Google Scholar]
- 42. Gainotti G, Marra C, Villa G, et al. Sensitivity and specificity of some neuropsychological markers of Alzheimer dementia. Alzheimer Dis Assoc Disord 1998;12:152–162. [DOI] [PubMed] [Google Scholar]
- 43. Johnson SC, Schmitz TW, Moritz CH, et al. Activation of brain regions vulnerable to Alzheimer's disease: The effect of mild cognitive impairment. Neurobiol Aging 2006;27:1604–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haarmeier T, Thier P. The attentive cerebellum ‐ myth or reality? Cerebellum 2007;6:177–183. [DOI] [PubMed] [Google Scholar]


